Vitamin D: navigating what’s new

The vitamin D world is changing. New guidelines balance the adverse effects of prolonged sun exposure with the need for sunlight to synthesise vitamin D. Importantly, the guidelines acknowledge that people with deeply pigmented skin are at low risk of skin cancer and relax the sun exposure recommendations in this group. Adequate vitamin D supplementation is beneficial in older people to help reduce the risk of fractures.
- Vitamin D is essential for calcium homeostasis, as well as bone and muscle function. Guidelines recommend serum levels of vitamin D should exceed 50 nmol/L. Levels lower than 30 nmol/L may and predispose an individual to bone demineralisation, low calcium levels and muscle dysfunction.
- New recommendations on sun safety balance the positive effects of ultraviolet B radiation for vitamin D synthesis against the risks of skin cancer. Guidelines now reflect the ethnic diversity of the Australian population, with advice differing according to the individual risk of skin cancer based on skin pigmentation.
- Testing individuals with specific risk factors for vitamin D deficiency (e.g. deeply pigmented skin, chronic illness and reduced sun exposure) is appropriate and supported by the Medicare Benefits Schedule.
- When considering a dose regimen for vitamin D supplementation, ‘slow and steady’ seems to be the best approach. Moderate doses given daily or weekly (1000 to 4000 IU daily) are safe and effective, compared with mega-doses (e.g. >50,000 IU) given intermittently. High-dose regimens may, however, be necessary in nonadherent individuals or those with malabsorption.
- People with vitamin D deficiency benefit from supplementation, particularly older and at-risk individuals.
- Nearly all osteoporosis trials have included supplementation with calcium (1000 mg daily) and vitamin D (1000 IU daily) along with antiosteoporotic agents.
There is confusing information about vitamin D in the literature that sometimes produces unhelpful headlines in the general media. This article discusses recent randomised controlled trials, and new developments in sun protection guidelines, vitamin D dosing schedules, Medicare Benefits Schedule (MBS) testing guidelines for vitamin D and the impact of vitamin D status on cancer-related mortality. The article mainly addresses issues on vitamin D in adults.
Physiology of vitamin D
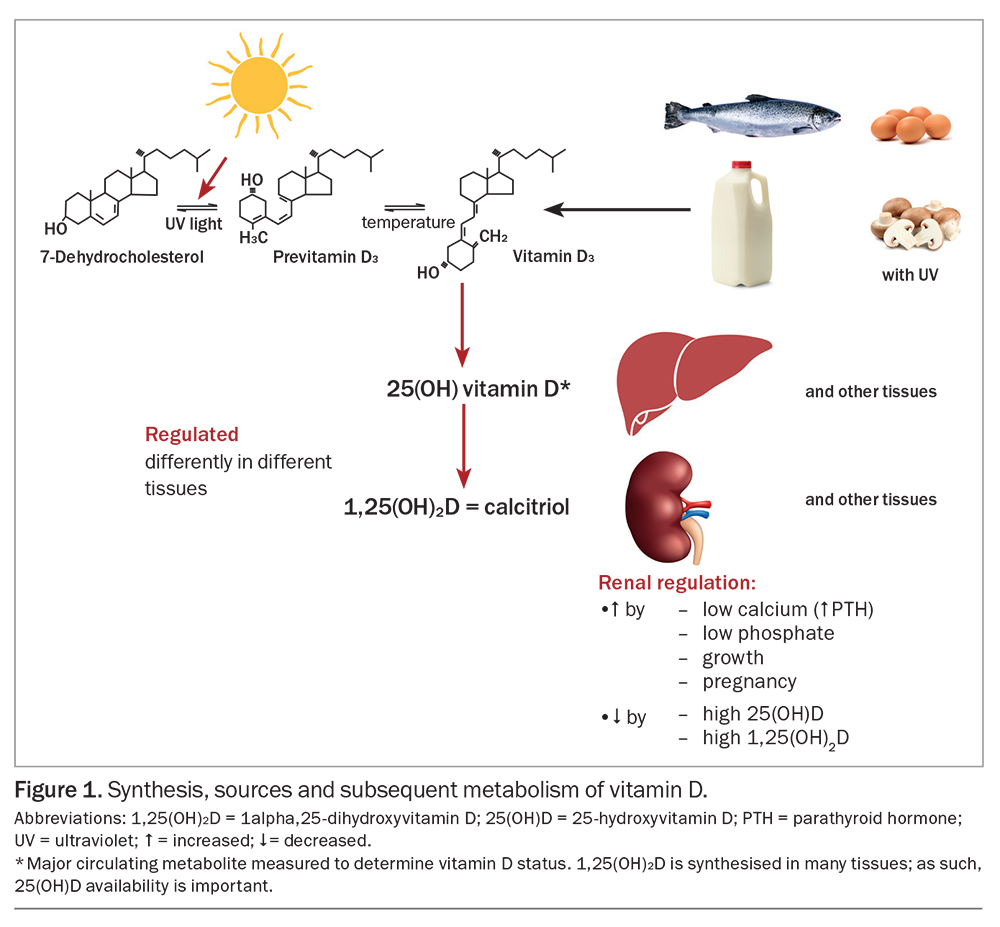
Vitamin D is a precursor to the hormone calcitriol, or 1alpha,25-dihydroxyvitamin D (1,25(OH)2D), which has an important role in increasing calcium and phosphate absorption from the gut and is essential for bone and muscle function.1 Vitamin D, the parent compound, is not strictly a vitamin, as it is made in the skin when a molecule of 7-dehydrocholesterol absorbs a photon of ultraviolet (UV) B (UVB) radiation. Provitamin D3 (7-dehydrocholesterol) accumulates in the skin, and UVB radiation has sufficiently high energy to break its B-ring to form previtamin D, which isomerises into vitamin D at body temperature (Figure 1). This form of vitamin D is cholecalciferol (vitamin D3). The vitamin D made in fungi from ergosterol by exposure to UV radiation is ergocalciferol (vitamin D2).2
Vitamins D2 and D3 are metabolised similarly, and their actions are similar. Vitamin D can be obtained from some foods, although relatively little vitamin D is present in most diets. Fish (with skin and not just oily fish), but not seafood such as shellfish or crustaceans, contains reasonable amounts of vitamin D (80 to 100 IU per 100 g).3 A vitamin D level of 40 IU is equivalent to 1 mcg. A recent Australian study showed that people who ate two or more servings of fish per week had better vitamin D status, particularly in the winter.4 Small amounts of vitamin D are present in eggs, fortified milk and meat.5
Vitamin D is converted to 25-hydroxyvitamin D (25(OH)D), the major circulating metabolite of vitamin D, predominantly, but not exclusively, in the liver. This conversion is not well regulated, and there is an approximately linear relationship between vitamin D intake and blood 25(OH)D concentrations.6 Unlike most steroids and unlike the hormone 1,25-dihydroxyvitamin D, 25(OH)D has a relatively long half-life in blood (15 to 51 days).7,8 Thus, 25(OH)D concentrations are measured to determine an individual’s vitamin D status. Most consensus statements, including those of Australia and New Zealand, advise a target blood concentration of 25(OH)D of at least 50 nmol/L.1,9
Sun exposure is the main source of vitamin D, specifically UVB radiation in sunlight (wavelength: 290 to 320 nm). In general, more skin exposed to UVB radiation will generate more vitamin D.4,10 A seasonal drop in vitamin D of around 10 to 20 nmol/L occurs in most parts of Australia in the winter, as UVB radiation is low and skin exposure is reduced by clothing.11 To allow for this reduction in vitamin D, a target of 25(OH)D blood concentration of 60 to 70 nmol/L is recommended in mid- or late summer.1,9 People with 25(OH)D concentrations lower than 30 nmol/L are at risk of impaired bone mineralisation, which may result in rickets (if growth plates are present) and osteomalacia, requiring treatment with vitamin D supplementation.12 Even 25(OH)D concentrations lower than 50 nmol/L pose a risk of increased bone loss through secondary hyperparathyroidism.13
The kidneys are the main site where 25(OH)D is converted to the biologically active 1,25(OH)2D for the bloodstream, but many other sites, including the gut, the bone, activated macrophages and the skin, also produce 1,25(OH)2D for local use.12 As such, the supply of 25(OH)D is important. The conversion of 25(OH)D to 1,25(OH)2D is highly regulated, but the nature of the regulation is different in different tissues. In macrophages, 1,25(OH)2D production is increased following the stimulation of toll-like receptors and by interleukin-1 and tumour necrosis factor alpha.14 In the kidneys, 1,25(OH)2D production is increased by parathyroid hormone, which in turn is increased by low blood calcium concentrations, by low blood phosphate levels and under circumstances of bone growth and pregnancy involving skeletal growth.15 The increased circulating 1,25(OH)2D acts in the gut, along with other mechanisms, to increase the fraction of ingested calcium that is absorbed, providing mineral for the growing skeleton. Somewhat surprisingly, 1,25(OH)2D production is not increased during lactation.15 The result is that mineral is lost from the bone during lactation to maintain blood calcium concentrations, despite losses of calcium through milk production. The bone mineral loss is fully restored within three to six months after lactation ceases.15 Degradation of vitamin D metabolites is also increased by some antibiotics, such as rifampicin, and some older antiepileptic agents.8
Sun exposure: an update to the guidelines
Use of sunscreen and other factors affecting vitamin D synthesis
Sun safety guidelines recommend the regular use of sunscreen. When applied at the standard concentration of 2 mg/cm2 (about one teaspoon for each arm) to the whole body, sunscreen blocks vitamin D synthesis, which is not surprising as the action spectrum for vitamin D production is almost identical to that for DNA damage or sunburn, at least in the UVB range.10,16 Surprisingly, several studies have shown that the use of sunscreen in the community makes little difference to an individual’s vitamin D status.4,10 Apart from evidence of less-than-ideal sunscreen application in general, people who use sunscreen often tend to have greater sun exposure than those who do not use sunscreen often.4,10
Having deeply pigmented skin (i.e. Fitzpatrick’s classification V and VI) is a major risk factor for low vitamin D status.1,4 Melanin absorbs UVB radiation, competing with 7-dehydrocholesterol.10 There is also some evidence suggesting that people with deeply pigmented skin tend to limit sun exposure to avoid further darkening of the skin.10 In addition, wearing clothing with more coverage, for cultural or religious reasons, reduces the skin area exposed to sunlight and reduces the amount of vitamin D produced.10 This poses a problem for women from some ethnic groups and their babies, who acquire their vitamin D from the mother via the placenta.17 There is very little vitamin D in breast milk, even if the mother has normal levels of 25(OH)D.15
Sun damage and skin cancer
Both UVB and UVA radiation cause sun damage, the latter having lower energy but comprising 95% of the UV energy in sunlight.18 This sun damage includes direct and indirect DNA damage, immune suppression and photoageing.18 If DNA damage is not adequately repaired, mutations may result, which, together with immune suppression, are key to the development of skin cancers.18 Australia and New Zealand have some of the highest skin cancer rates in the world, given the tropical or subtropical environment and that large proportions of the population have light-coloured skin.18 Even small amounts of sun exposure can cause small amounts of DNA; however, this damage can be fully repaired within a day or so in most people.19 Fortunately, small amounts of exposure to UVB radiation are also more efficient at generating vitamin D.20 Continued exposure to sunlight results in the conversion of pre-vitamin D or vitamin D to ‘over-irradiation products’, such as lumisterol and tachysterol, which are further metabolised in the skin.21 Although these over-irradiation compounds have little classic vitamin D activity, they may contribute, along with skin-derived 1,25(OH)2D, to improved repair of sun-induced DNA damage.22
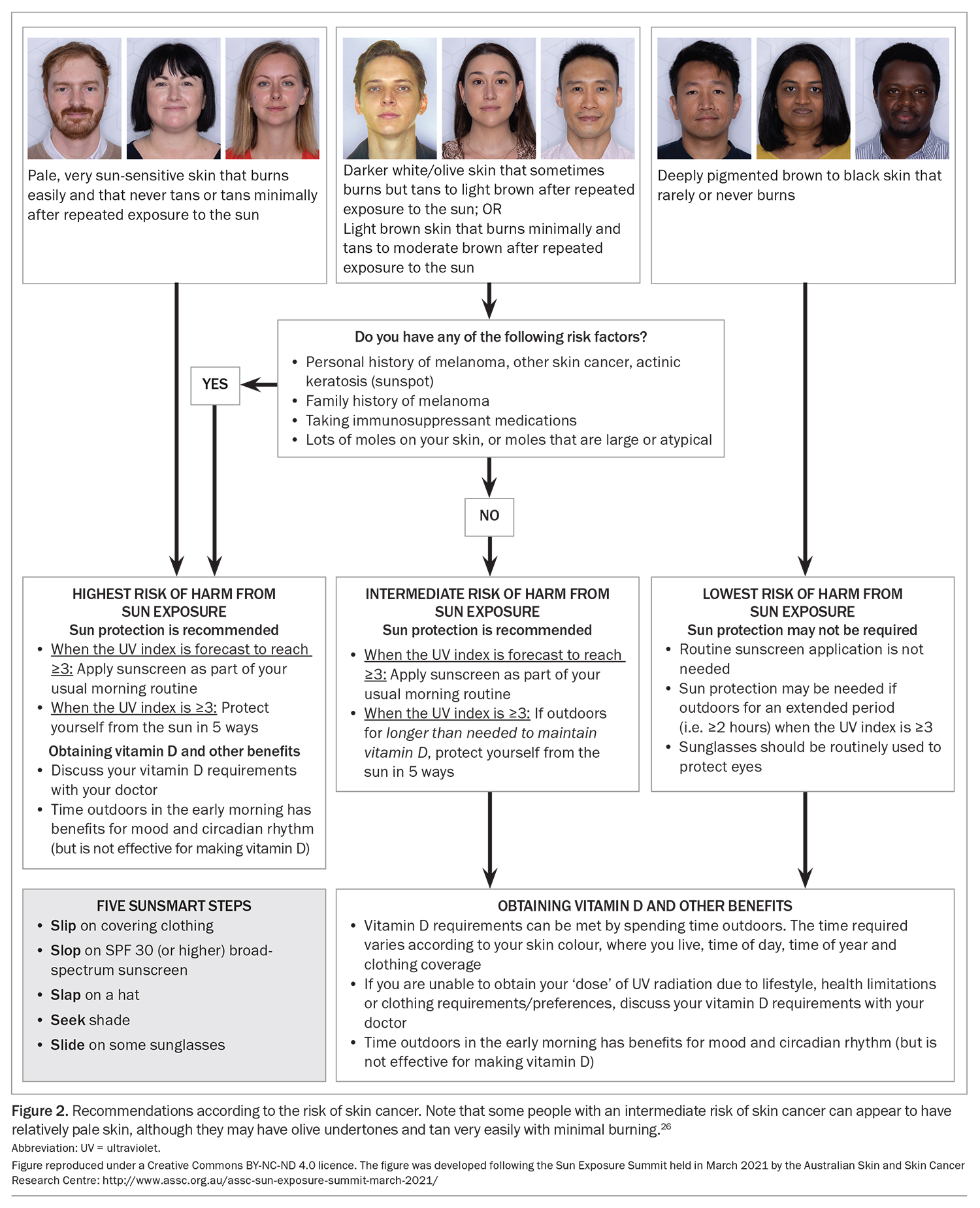
Given the high sun-related skin cancer rate in Australia, extensive skin cancer prevention programs have been designed to reduce sun exposure.23 In the past 20 years or so, sun exposure has been officially recognised to confer positive benefits, including vitamin D synthesis, as well as its effects on mood and the circadian rhythm (the latter being influenced primarily by visible light). Although UV-induced systemic immune suppression facilitates the development of skin cancers, it also dampens overactive adaptive immune systems, resulting in a lower incidence of autoimmune diseases, such as multiple sclerosis, at latitudes closer to the equator.18 These observations led to the publication of guidelines on the risks and benefits of sunlight exposure in 2005 and 2016.24,25 These guidelines for adults recommend small amounts of sun exposure to generate vitamin D and for other benefits, at least in people with a low risk of skin cancer. These guidelines also advise the implementation of ‘Slip’ (on some covering clothing), ‘Slop’ (on some SPF30+ sunscreen), ‘Slap’ (on a hat), ‘Seek’ (shade) and ‘Slide’ (on some sunglasses) if the UV index is 3 or higher. People with a high risk of skin cancer are advised to protect themselves from the sun at all times when the UV index is 3 or higher. Risk factors of skin cancer include:
- pale skin that burns easily and tans minimally
- a personal history of melanoma or other skin cancers, even with somewhat more pigmented skin
- a family history of melanoma
- taking immunosuppressive medications
- the presence of many moles or moles that are large or atypical.25
The new guidelines
In recent years, a working group consisting of representatives of major stakeholders has convened to update these guidelines, taking into account the culturally diverse population of Australia, and the resulting diversity in the degrees of sun sensitivity and sun resilience in the form of higher levels of pigmentation (Figure 2).26 In the new guidelines, people with deeply pigmented skin that never or rarely burns (i.e. Fitzpatrick’s classification V or VI) are noted to be at low risk of skin cancer. In general, skin cancers, including melanoma and squamous cell carcinoma, which do develop in people with deeply pigmented skin, tend to occur on nonsun-exposed sites. The same group of people, including adults and their children, are at significantly greater risk of having vitamin D deficiency.1,4,9 The new advice indicates that, for people with deeply pigmented skin, routine sun protection for skin is not needed, unless the person is expected to be outdoors for more than two hours when the UV index is 3 or higher. Nevertheless, sunglasses should still be worn to protect the eyes. The hope is that this new advice will help improve the vitamin D status in this group, without adverse effects.
MBS testing for vitamin D status
Although skin type, UVB availability, skin area exposed and length of time spent in the sun are all factors affecting vitamin D synthesis, there is considerable biological variability in the 25(OH)D concentrations achieved as a result of sun exposure.10 A study of 93 surfers in Hawaii with high levels of sun exposure showed that their blood 25(OH)D concentrations varied from 37 to 155 nmol/L.28
Given the difficulty of predicting an individual’s vitamin D status, which is based on 25(OH)D concentrations in blood, there is some justification for the desire to test for this. The laboratory test for 25(OH)D is complex and expensive for the MBS, so there have been efforts to rein in what was once large numbers of vitamin D tests. Apart from a letter campaign to doctors deemed to be requesting ‘higher than average’ numbers of vitamin D tests, a workshop of major stakeholders was held in 2013, leading to the development of clinical indications for routine vitamin D testing with a reasonable level of consensus. The MBS criteria for a rebate were launched in November 2014.29 These included the expected criteria of any indications of osteoporosis or osteomalacia; calcium or phosphate abnormalities; malabsorption; chronic renal failure or being a transplant recipient; or for a child or infant, having a mother or sibling with suspected vitamin D deficiency. Having deeply pigmented skin is a clinical indication for testing. Importantly, ‘chronic and severe lack of sun exposure for cultural, medical, occupational or residential reasons’ was included in the criteria for rebate, which potentially covers most people who might benefit from vitamin D testing.
Can vitamin D reduce the risk of fractures?
People reading media headlines about some vitamin D studies might be forgiven for feeling that there was little point in obtaining vitamin D from sunlight or supplements, as it would do little good anyway. This was particularly the case with the media coverage of a secondary outcome of the VITamin D and OmegA-3 TriaL (VITAL).30 In this large-scale study, more than 25,000 participants were given 2000 IU vitamin D3 per day with or without omega 3, or placebo. Nearly 2000 fractures were reported in around 1550 participants followed up for a median of 5.3 years. Overall, there was no effect of taking vitamin D supplements on the total incidence of fractures or hip fractures regardless of age, sex, race (20% Black), body mass index or serum 25(OH) D concentrations. The authors concluded that ‘vitamin D3 supplementation did not result in a significantly lower risk of fractures than placebo among generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass, or osteoporosis’. The media, not entirely unreasonably, reported this, more or less, as ‘vitamin D supplements are useless even in people with low vitamin D’. The media reports caused many patients at the time to question their GPs about their intake of vitamin D supplements.
Were the media headlines reasonable? The trial participants were community dwelling and well enough to participate in a clinical trial that involved multiple visits to trial centres. Many of them were relatively young, as the eligibility criteria included ages older than 55 years for women and older than 50 years for men; the participants’ mean age was only 67 years. Furthermore, the median baseline 25(OH)D concentration was 77 nmol/L, with concentrations lower than 60 nmol/L comprising 25% of participants. The consensus vitamin D position statement for Australia and New Zealand includes a 25(OH)D target of 50 nmol/L all year.1 Only 401 participants (2.4%) had 25(OH)D concentrations lower than 30 nmol/L; their ages were not specified in the study and they experienced only 15 fractures in five years. In the trial, vitamin D was given to healthy, relatively young people at low risk of sustaining fractures and who typically had an adequate vitamin D status and found no effect on the fracture risk. Even the authors stated, ‘the trial was not designed to test the effects of vitamin D supplementation in those who are vitamin D deficient’.30
This brings up some of the issues related to trials of vitamin D. There is a generally accepted view that vitamin D is a threshold nutrient – if people have ‘enough’ vitamin D, giving more yields no further benefit.12 A 25(OH)D threshold for the suppression of parathyroid hormone through the intake of vitamin D with calcium was determined to be around
50 nmol/L; for co-ordinated muscle activity in older individuals, the threshold was found to be 40 to 50 nmol/L; and for a vitamin D effect on bone mineral density, a threshold 30 to 40 nmol/L has been proposed.12,13,31,32
A classic randomised controlled trial enrolled 3270 women with a mean age of 84 years (the youngest participant was 69 years of age) living in aged care facilities and apartments, who had a baseline 25(OH)D concentration of 40 ± 25 nmol/L and a baseline calcium intake of 511 ± 172 mg per day.33 Based on these features, the participant pool had a relatively high fracture risk. They were given vitamin D3 (800 IU per day) plus calcium (1200 mg per day), or double placebo, and followed up for 18 months. Over half (54%) of the participants completed the study, with similar percentages in the treatment and placebo groups. On trial completion, the vitamin D and calcium group had 32% fewer nonvertebral fractures (66 vs 97, p=0.015) and 43% fewer hip fractures (21 vs 37, p=0.043) compared with the placebo group. When expressed as a cumulative probability of sustaining a fracture, the curves for hip fracture started to diverge at 10 months, whereas those for other nonvertebral fractures diverged from two months.33 Although the trial was approved by the local ethics committee and the participants provided informed consent, it is unlikely that a trial of this nature would be approved today as it is unethical to withhold vitamin D treatment from people with known vitamin D deficiency. Furthermore, in the VITAL study, as in other similar trials, participants were permitted to take their own vitamin D supplements at a dose of up to 800 IU per day (around 40% were doing so at baseline) and calcium at a dose of up to 1200 mg per day (around 20% did so at baseline).34
The importance of adequate calcium (and protein) intake
Local production of 1,25(OH)2D in bone has been linked to the promotion of bone formation.35 Overall, key mechanisms in reductions in fracture risk through vitamin D treatment are increased calcium absorption from the intestine, which reduces secondary hyperparathyroidism and thus decreases bone turnover.36 For this increased calcium absorption to confer these beneficial actions, adequate intake of calcium is needed. Many meta-analyses of randomised controlled trials of vitamin D for fracture risk reduction have concluded that a modest beneficial effect of vitamin D plus calcium on the fracture risk exists, with no benefit of vitamin D alone.37,38 A recent meta-analysis of systematic reviews and earlier meta-analyses supported these conclusions and pointed to the combination of vitamin D and calcium in reducing body sway and fall risk.39 This analysis indicated that groups most likely to benefit from a vitamin D and calcium combination are older individuals and those living in aged care facilities, with not much proven benefit for those who are generally healthy and living in the community.39 A recent study in Australia showed no overall benefit of monthly doses of vitamin D alone in reducing fractures in healthy, community-dwelling individuals between 69 and 84 years of age, although no adverse effects and a trend to decreased fractures after 3.5 years were observed.34
The importance of adequate calcium and protein intake, factors that tend to be forgotten despite their key role in bone and muscle function, was illustrated in a trial of more than 7000 adults, with a mean age of 86 years, across 60 aged care facilities in Victoria.40 In this cluster randomised trial, the facility kitchens increased the amounts of milk, yoghurt and cheese present in the daily diets of the intervention group, thus increasing their dietary calcium intake from around 700 mg per day to 1142 mg per day and protein intake from around 58 g per day (0.9 g/kg) to 69 g per day (1.1 g/kg). All the participants were taking vitamin D supplements, with a mean baseline 25(OH)D concentration of 72 nmol/L. After 24 months, there was a 33% risk reduction for all fractures (p<0.02) and a 46% risk reduction in hip fractures (p<0.005) in the intervention group. The difference in the cumulative incidence of fractures between the groups achieved significance at five months. The incidence of falls also decreased in the intervention group with a relative risk reduction of 11% (p<0.04) after 24 months, with a small but significant decrease at three months.40
The participants in this study were provided additional dietary calcium and protein, predominantly from dairy products, which can be low fat or lactose free. Dietary calcium can be found in canned fish (with bones) and some nuts and vegetables.41 Without calcium-rich foods, most diets provide around 300 mg of calcium. Additional dietary calcium is potentially beneficial for bones and not harmful to the cardiovascular system – and may even be helpful in this context.42-44
Calcium supplements, however, are somewhat controversial. Some analyses of bone health trials that studied calcium supplements indicated an increase in nonfatal strokes and heart attacks in people treated with calcium supplements,42,44 whereas other analyses indicated a beneficial effect on cardiovascular and nonbone health outcomes.43,44 The advice from the Australian and New Zealand Bone and Mineral Society and Healthy Bones Australia is to aim for total calcium intake of 1000 to 1200 mg per day, depending on age and sex, through dietary intake of calcium-rich foods. If this is not feasible and additional calcium is needed for fracture risk reduction, calcium supplements containing 500 to 600 mg can be considered. Similar advice was given by the American Society for Bone and Mineral Research.
It has been speculated that any vascular problems associated with calcium may arise from a slight spike in blood calcium concentrations after taking calcium supplementation.42 If this occurs, taking limited doses of calcium with food may reduce these spikes. Most forms of calcium are absorbed to a similar extent given adequate gastric acidity. If not, the more easily absorbed calcium citrate may be preferable to calcium carbonate supplements. The problems of nonadherence persist, attributed at least in part to the size of the tablets, their cost and their tendency to cause constipation.
Vitamin D dosing schedules
Nonadherence with vitamin D treatment is a frequent problem in clinical trials and in general practice. Given the long half-life of 25(OH)D, intermittent bolus doses can be given to maintain blood 25(OH)D concentrations above the target level.45 Daily, weekly or monthly equivalent doses of vitamin D produce similar increases in blood 25(OH)D concentrations, achieving a stable level after around three months.46,47 Although the average increase in blood 25(OH)D concentrations is around 13 to 17 nmol/L per 1000 IU (25 mcg) per day, there is considerable variation in the concentration of 25(OH)D achieved. Larger increases in 25(OH)D are often observed in people who have a lower baseline 25(OH)D concentration.46,47 It is even possible to achieve vitamin D adequacy, based on blood 25(OH)D concentrations, without causing hypercalcaemia, with annual doses of vitamin D around 500,000 IU (12,500 mcg) per dose.45
However, this approach is not advised to achieve favourable functional outcomes, particularly in older individuals. In a classic trial conducted in Victoria, menopausal women were given vitamin D3 at a dose of 500,000 IU annually for four years. Paradoxically, the incidences of both falls and fractures significantly increased during this time, with a subanalysis showing that the incidence of falls significantly increased in the first three months after each dose.48 This accords with other analyses of musculoskeletal outcomes, such as falls when subjects were given high or intermittent doses of vitamin D.49,50
For other functional outcomes of vitamin D supplementation, including respiratory infections and cancer-related mortality, daily supplementation appears to be more useful than infrequent, large bolus supplements.50,51 These results are not entirely surprising when the physiology of vitamin D is considered. Vitamin D in excess can cause toxicity, and is even an ingredient of rat poison.6,52 Many mechanisms have evolved to reduce the toxic effects of excess vitamin D. Production of the active hormone 1,25(OH)2D is reduced by the product itself and by high 25(OH)D concentrations.8 High 25(OH)D concentrations also activate degradation pathways for itself, 1,25(OH)2D and other metabolites.53
Are there different thresholds for different outcomes?
Although the reported thresholds for observing a benefit of vitamin D supplementation for musculoskeletal effects appear to be in the 30 to
50 nmol/L range, there may be different thresholds for different outcomes. For example, an analysis of the findings of the VITAL study revealed a benefit on the incidence of autoimmune disease, particularly after three years of treatment.54 A recent meta-analysis showed that vitamin D supplements reduced cancer-related mortality after around three years in trials in which more than half the participants had baseline 25(OH)D concentrations higher than 50 nmol/L.55 Another analysis was also consistent in showing an effect of daily vitamin D supplementation and a need for extended treatment periods longer than four years to observe an effect of vitamin D on cancer-related mortality.12,56 Thus, current target levels of 25(OH)D of at least 50 nmol/L all year seem adequate for musculoskeletal benefits, but there remains a possibility that higher targets for 25(OH)D may be appropriate to observe nonskeletal benefits, although considerably more research is warranted.
Conclusion
Despite the narrowing of indications for vitamin D testing based on measurements of 25(OH)D concentrations in blood, most people for whom this test might be useful remain included. New sun exposure guidelines are now designed to be appropriate for our diverse population. Strict adherence to the guidelines is important for people at high risk of skin cancers. The risk of sun-associated skin cancers is low, but the risk of vitamin D deficiency is high, for people with deeply pigmented skin. Thus, sun protection measures are relaxed for this group, at least when considering less than two hours of exposure, except for eye protection with sunglasses. Clinical data support vitamin D treatment for all individuals with 25(OH)D concentrations lower than 30 nmol/L, which poses a risk of rickets (if young) and osteomalacia, and when levels are lower than 50 nmol/L for a sustained period. Inadequate dietary calcium intake and vitamin D deficiency also contribute to the development of osteoporosis and fragility fractures. Trial data support the provision of vitamin D at a dose of 800 to 1000 IU per day (daily preferred over large intermittent doses), with calcium intake of 1000 to 1200 mg per day for people with vitamin D levels lower than 50 nmol/L or low calcium intake, particularly if they have a high risk of sustaining fractures, such as older people, especially in residential aged care facilities. MT
COMPETING INTERESTS: Associate Professor Girgis chairs the Clinical Practice Committee of the ANZ Bone and Mineral Society; and is a working group member for the Risks and Benefits of Sun Exposure, Cancer Council Australia. Professor Mason has received speaker fees from Sanofi; was a Board Member for Healthy Bones Australia from 2002 to 2022; has co-chaired the ANZ Bone & Mineral Society Research Committee since 2022; and has been a working group member for the Risks and Benefits of Sun Exposure update, Cancer Council Australia, since 2020.
References
1. Nowson CA, McGrath J, Ebeling PR, et al. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust 2012; 196: 686-687.
2. Cashman KD, Kiely M, Seamans KM, Urbain P. Effect of ultraviolet light-exposed mushrooms on vitamin d status: liquid chromatography-tandem mass spectrometry reanalysis of biobanked sera from a randomized controlled trial and a systematic review plus meta-analysis. J Nutr 2016; 146: 565-575.
3. Padula D, Greenfield H, Cunningham J, Kiermeier A, McLeod C. Australian seafood compositional profiles: A pilot study. Vitamin D and mercury content. Food Chem 2016; 193: 106-111.
4. Fayet-Moore F, Brock KE, Wright J, et al. Determinants of vitamin D status of healthy office workers in Sydney, Australia. J Steroid Biochem Mol Biol 2019; 189: 127-134.
5. Dunlop E, James AP, Cunningham J, et al. Vitamin D composition of Australian foods. Food Chem 2021; 358: 129836.
6. Mason RS, Lissner D, Grunstein HS, Posen S. A simplified assay for dihydroxylated vitamin D metabolites in human serum: application to hyper- and hypovitaminosis D. Clinical Chemistry 1980; 26: 444-450.
7. Mason RS, Lissner D, Posen S, Norman AW. Blood concentrations of dihydroxylated vitamin D metabolites after an oral dose. Br Med J 1980; 280: 449-450.
8. Clements MR, Davies M, Hayes ME, et al. The role of 1,25-dihydroxyvitamin D in the mechanism of acquired vitamin D deficiency. Clin Endocrinol (Oxf) 1992; 37: 17-27.
9. Paxton GA, Teale GR, Nowson CA, Mason RS, McGrath JJ, Thompson MJ, Siafarikas A, Rodda CP, Munns CF; Australian and New Zealand Bone and Mineral Society; Osteoporosis Australia. Vitamin D and health in pregnancy, infants, children and adolescents in Australia and New Zealand: a position statement. Med J Aust 2013; 198: 142-143.
10. Neville JJ, Palmieri T, Young AR. Physical determinants of vitamin D photosynthesis: a review. JBMR Plus 2021; 5: e10460.
11. Daly RM, Gagnon C, Lu ZX, et al. Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: a national, population-based study. Clin Endocrinol (Oxf) 2012; 77: 26-35.
12. Bouillon R, Manousaki D, Rosen C, Trajanoska K, Rivadeneira F, Richards JB. The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol 2022; 18: 96-110.
13. Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet 1998; 351: 805-806.
14. Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006; 311: 1770-1773.
15. Kovacs CS. The role of vitamin D in pregnancy and lactation: insights from animal models and clinical studies. Annu Rev Nutr 2012; 32: 97-123.
16. Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab 1987; 64: 1165-1168.
17. Clements MR, Fraser DR. Vitamin D supply to the rat fetus and neonate. J Clin Invest 1988; 81: 1768-1773.
18. Neale RE, Lucas RM, Byrne SN, et al. The effects of exposure to solar radiation on human health. Photochem Photobiol Sci 2023; 22: 1011-1047.
19. Felton SJ, Cooke MS, Kift R, et al. Concurrent beneficial (vitamin D production) and hazardous (cutaneous DNA damage) impact of repeated low-level summer sunlight exposures. Br J Dermatol 2016; 175: 1320-1328.
20. Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab 1989; 68: 882-887.
21. Slominski RM, Raman C, Elmets C, Jetten AM, Slominski AT, Tuckey RC. The significance of CYP11A1 expression in skin physiology and pathology. Mol Cell Endocrinol 2021; 530: 111238.
22. De Silva WGM, Han JZR, Yang C, et al. Evidence for involvement of nonclassical pathways in the protection from UV-induced DNA damage by vitamin D-related compounds. JBMR Plus 2021; 5: e10555.
23. Walker H, Maitland C, Tabbakh T, Preston P, Wakefield M, Sinclair C. Forty years of Slip! Slop! Slap! A call to action on skin cancer prevention for Australia. Public Health Res Pract 2022; 32: 31452117.
24. Sinclair C. Risks and benefits of sun exposure: implications for public health practice based on the Australian experience. Prog Biophys Mol Biol 2006; 92: 173-178.
25. Position statement - Sun exposure and vitamin D - risks and benefits. Sydney: Cancer Council Australia; 2016. Available online at: https://wiki.cancer.org.au/policy/Position_statement_-_Risks_and_benefits_of_sun_exposure (accessed August 2023).
26. Position statement: balancing the harms and benefits of sun exposure. Brisbane: Australian Skin and Skin Cancer Research Centre; 2021. Available online at: http://www.assc.org.au/assc-sun-exposure-summit-march-2021/ (accessed August 2023).
27. Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol 2006; 55: 741-760; quiz 761-764.
28. Binkley N, Novotny R, Krueger D, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 2007; 92: 2130-2135.
29. MBS item number changes for vitamin B12, folate and vitamin D tests. Sydney: NPS MedicineWise; 2015. Available online at: https://www.nps.org.au/radar/articles/mbs-item-number-changes-for-vitamin-b12-folate-and-vitamin-d-tests (accessed August 2023).
30. LeBoff MS, Chou SH, Ratliff KA, et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med 2022; 387: 299-309.
31. Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr 2004; 80: 752-758.
32. Macdonald HM, Reid IR, Gamble GD, Fraser WD, Tang JC, Wood AD. 25-hydroxyvitamin D threshold for the effects of vitamin D supplements on bone density: secondary analysis of a randomized controlled trial. J Bone Miner Res 2018; 33: 1464-1469.
33. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992; 327: 1637-1642.
34. Waterhouse M, Ebeling PR, McLeod DSA, et al. The effect of monthly vitamin D supplementation on fractures: a tertiary outcome from the population-based, double-blind, randomised, placebo-controlled D-Health trial. Lancet Diabetes Endocrinol 2023; 11: 324-332.
35. Anderson PH, Lam NN, Turner AG, et al. The pleiotropic effects of vitamin D in bone. J Steroid Biochem Mol Biol 2013; 136: 190-194.
36. Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev 2019; 40: 1109-1151.
37. Avenell A, Gillespie WJ, Gillespie LD, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev 2005; 3: CD000227.
38. Yao P, Bennett D, Mafham M, et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw Open 2019; 2: e1917789.
39. Chakhtoura M, Bacha DS, Gharios C, et al. Vitamin D supplementation and fractures in adults: a systematic umbrella review of meta-analyses of controlled trials. J Clin Endocrinol Metab 2022; 107: 882-898.
40. Iuliano S, Poon S, Robbins J, et al. Effect of dietary sources of calcium and protein on hip fractures and falls in older adults in residential care: cluster randomised controlled trial. BMJ 2021; 375: n2364.
41. Calcium & Bone Health. Sydney: Healthy Bones Australia; 2023. Available online at: https://healthybonesaustralia.org.au/your-bone-health/calcium/ (accessed August 2023).
42. Reid IR, Bolland MJ, Avenell A, Grey A. Cardiovascular effects of calcium supplementation. Osteoporos Int 2011; 22: 1649-1658.
43. Lewis JR, Radavelli-Bagatini S, Rejnmark L, et al. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. J Bone Miner Res 2015; 30: 165-175.
44. Li K, Wang XF, Li DY, et al. The good, the bad, and the ugly of calcium supplementation: a review of calcium intake on human health. Clin Interv Aging 2018; 13: 2443-2452.
45. Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int 2009; 20: 1407-1415.
46. Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab 2008; 93: 3430-3435.
47. Aloia JF, Patel M, Dimaano R, et al. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr 2008; 87: 1952-1958.
48. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010; 303: 1815-1822.
49. Smith LM, Gallagher JC, Suiter C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: A randomized clinical trial. J Steroid Biochem Mol Biol 2017; 173: 317-322.
50. Mazess RB, Bischoff-Ferrari HA, Dawson-Hughes B. Vitamin D: bolus is bogus-a narrative review. JBMR Plus 2021; 5: e10567.
51. Martineau AR, Jolliffe DA, Greenberg L, et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess 2019; 23: 1-44.
52. Gascon-Barré M, Côté MG. Effects of phenobarbital and diphenylhydantoin on acute vitamin D3 toxicity in the rat. Toxicol Appl Pharmacol 1978; 43: 125-135.
53. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008; 88: 582s-586s.
54. Hahn J, Cook NR, Alexander EK, et al. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022; 376: e066452.
55. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ 2019; 366: l4673.
56. Keum N, Chen QY, Lee DH, Manson JE, Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality by daily vs. infrequent large-bolus dosing strategies: a meta-analysis of randomised controlled trials. Br J Cancer 2022; 127: 872-878.