Diabetic kidney disease: the four pillars of therapy

Diabetic kidney disease (DKD) is a serious threat to public health in Australia and globally, as a major cause of end-stage kidney disease, rising healthcare costs and premature mortality. The advent of several highly efficacious therapies over the last decade has dramatically altered the landscape of DKD management. In addition to lifestyle modification, the management of DKD secondary to type 2 diabetes now centres on four ‘pillars’ of treatment: renin-angiotensin system blockade (with ACE inhibitors or angiotensin receptor blockers), sodium-glucose cotransporter-2 inhibitors, nonsteroidal mineralocorticoid receptor antagonists and glucagon-like peptide-1 receptor agonists. Early implementation of these therapies is likely to confer substantial long-term gains in survival, free from cardiovascular and kidney disease.
- The management of diabetic kidney disease (DKD) secondary to type 2 diabetes now centres on four pillars of treatment: renin-angiotensin system blockade (with ACE inhibitors or angiotensin receptor blockers), sodium-glucose cotransporter-2 inhibitors, nonsteroidal mineralocorticoid receptor antagonists and glucagon-like peptide-1 receptor agonists.
- These pillars have a wide range of cardiorenal benefits, reducing the risk of progression of kidney disease, cardiovascular events and death.
- Initiating these therapies is dependent on the detection of DKD, by screening for reduced estimated glomerular filtration rate and albuminuria, which both independently predict the risk of cardiovascular disease as well as the risk of chronic kidney disease progression.
- These pillars of therapy have different mechanisms of action, addressing the abnormalities in haemodynamics (glomerular hyperfiltration), metabolism, inflammation and fibrosis that characterise the development and progression of DKD.
- Emerging evidence suggests that using the four pillars of therapy in combination can be safe and confer substantial additive benefits. Sodium-glucose cotransporter-2 inhibitors can ameliorate the risk of hyperkalaemia caused by ACE inhibitors and angiotensin receptor blockers.
- Strategies in routine clinical practice can match the intensity of combination therapy to a patient’s overall cardiovascular-kidney-metabolic risk profile.
Over the last two decades, the prevalence of type 2 diabetes in Australia has doubled, affecting at least 1.5 million people in Australia.1 Diabetic kidney disease (DKD) is a common complication of diabetes, affecting an estimated 330,000 people with diabetes in Australia, and is associated with higher rates of hospitalisation and mortality and significantly poorer health-related quality of life.2,3 Many people with type 2 diabetes are unaware of their kidney disease, in part because of low rates of testing for albuminuria.4 Early screening for kidney disease, including the detection of albuminuria, is therefore crucial to stratify a patient’s cardiovascular and kidney risk, personalise risk prediction and facilitate the uptake of proven therapies to improve cardiorenal outcomes.
Kidney failure requiring dialysis or transplantation is associated with a substantially increased morbidity and mortality.5 DKD remains the leading cause of kidney failure in Australia, responsible for about 40% of cases of kidney failure.6 Moreover, chronic kidney disease (CKD) itself is associated with 33% of all cardiovascular deaths in Australia, reflecting the multidirectional relationship between kidney and cardiovascular disease.7,8 Preventing cardiovascular events is a critical aspect of managing CKD, as most deaths in patients with CKD are caused by cardiovascular disease before the patients reach kidney failure.
The landscape of DKD treatment has dramatically changed in the last 10 years. There are now four proven therapies that can reduce the risk of kidney disease progression in people with type 2 diabetes:
- renin-angiotensin aldosterone system (RAAS) blockage, with either angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs)
- sodium-glucose cotransporter-2 (SGLT-2) inhibitors
- nonsteroidal mineralocorticoid receptor antagonists (MRAs)
- glucagon-like peptide-1 (GLP-1) receptor agonists.
Individuals at high risk of disease progression may benefit from an accelerated initiation of multiple therapies within a compressed timeframe, rather than a stepwise sequential approach. Emerging evidence suggests that the pillars of DKD therapy confer additive benefits and can be used in combination safely, with targeted education and appropriate sick day medication guidance. Evidence for these therapies is currently limited to type 2 diabetes, although trials in patients with type 1 diabetes are underway.
This article focuses on the mechanisms of action and evidence for these four ‘pillars’ of DKD therapy, how to stratify and personalise cardiorenal risk in patients with DKD and practical recommendations for the potential use of these therapies in combination safely.
Pathophysiology of diabetic kidney disease
The development of DKD shares many risk factors with established cardiovascular disease, including obesity, hypertension, and dyslipidaemia.9 Glomerulopathy tends to be an early feature of DKD, characterised by hyperfiltration and activation of pro-inflammatory and fibrotic pathways. This in turn leads to albuminuria and subsequently potentiates a cascade of progressive fibrosis. Yet heterogeneity is seen on a cellular level: up to half of patients with DKD have been found to exhibit classic diabetic glomerulopathy, although many others have been found to demonstrate severe tubulointerstitial or arteriolar or vascular changes in the presence of mild glomerular lesions.10 This heterogeneity underscores the need for multiple therapeutic agents with different mechanisms of action to reduce the decline of kidney function.
Screening and risk stratification
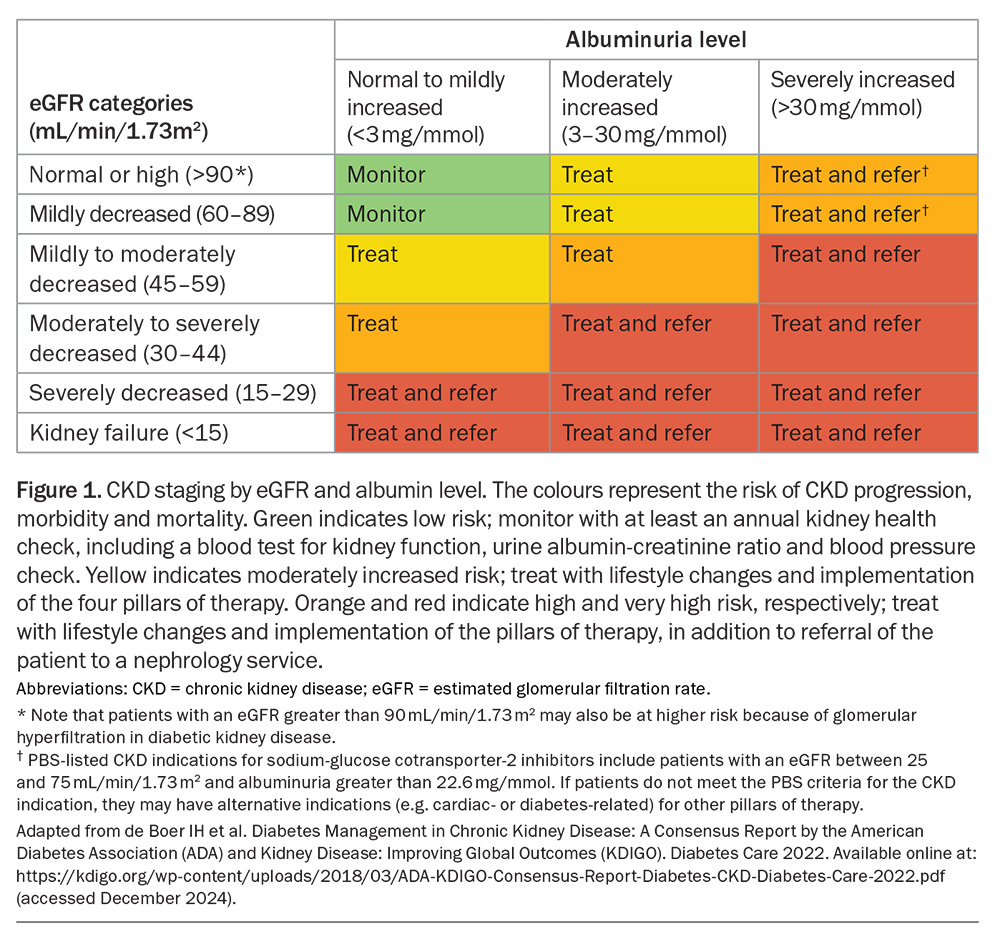
CKD is diagnosed as either a persistent reduction in estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 or evidence of kidney damage (including albuminuria) over at least three months.11 Detection of CKD is the first step towards initiating appropriate treatment. Screening for CKD with an annual measurement of kidney function (serum creatinine level and eGFR) and at least one urinary albumin- creatinine ratio (UACR) measurement to assess for the presence of albuminuria is recommended for all patients with diabetes.12 Measurement of the UACR is a readily accessible, noninvasive screening test that is available for the detection of CKD and remains underused in clinical practice.4 Causes of CKD other than DKD should also be considered, although this is beyond the scope of this article. Further guidance can be found in the Chronic Kidney Disease Handbook, available online at: https://assets.kidney.org.au/resources/KHA-CKD-Handbook-5th-Ed-July2024.pdf.
CKD is itself a risk multiplier for cardiovascular disease, with reduced eGFR and albuminuria both independently associated with cardiovascular morbidity and mortality.13 Since 2012, Kidney Disease Improving Global Outcomes (KDIGO) CKD staging has rephrased what has been known as ‘normo, micro- and macro-albuminuria’ to ‘mildly, moderately and severely increased albuminuria’, respectively, in recognition that albuminuria of any level carries an increased risk of cardiovascular disease (Figure 1). Accordingly, the presence of albuminuria is incorporated in the updated Australian Cardiovascular Disease Risk Calculator (https://www.cvdcheck.org.au/calculator).14 It reclassifies people with moderate-to-severe CKD (patients with an eGFR of less than 45mL/min/1.73 m2) as at high risk for cardiovascular disease, irrespective of other risk factors.
The ‘pillars’ of therapy for diabetic kidney disease
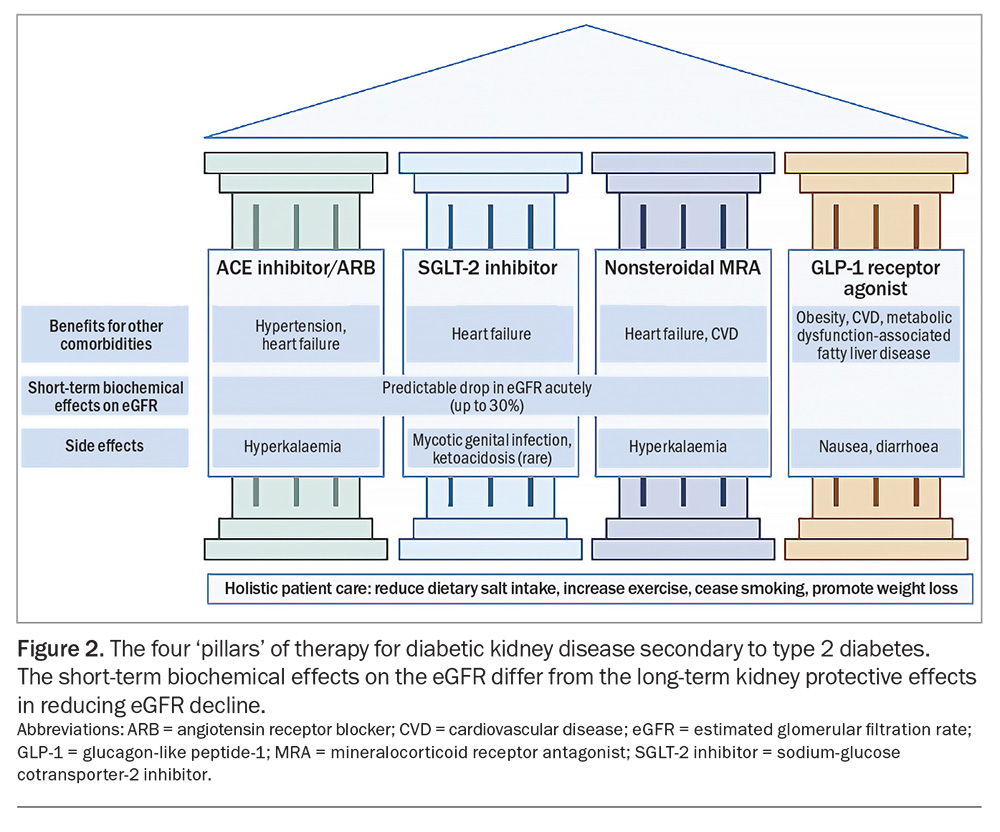
Despite conventional treatment for DKD that is focused on glycaemic and blood pressure control with RAAS inhibition, high-risk patients with DKD and increased albuminuria may still lose kidney function at up to 5 mL/min/1.73m2 per year.15,16 Even with the addition of SGLT-2 inhibitor therapy, the residual risk of kidney function decline remains high (at 2 to 3 mL/min/1.73 m2 per year) in people with severely increased albuminuria.17 This underscores the need for additional therapies to further reduce the rate of kidney function decline to be able to achieve one that is similar to normal healthy ageing (about 1 mL/min/1.73 m2 per year). Combination therapy with multiple agents offers the opportunity to address the haemodynamic, inflammatory and metabolic abnormalities that characterise the development and progression of DKD (Figure 2).
Pillar 1: Renin-angiotensin aldosterone system blockade
The evidence for RAAS blockade in DKD treatment has been established for over 30 years. Captopril was first shown to reduce DKD progression in patients with insulin-dependent diabetes in 1993.18 Two landmark trials followed, establishing RAAS blockade as the standard of care for preventing DKD. The effects of ACE inhibitors and ARBs on DKD are independent of their blood pressure lowering effects and are achieved by reducing intraglomerular pressure (which can lead to mesangial cell proliferation, activation of proinflammatory responses, fibrosis and albuminuria).19,20
Beyond their role in DKD, ACE inhibitors and ARBs continue to be part of the mainstay of treatment for cardiovascular disease, including hypertension and heart failure.
Practical tips for the initiation and maintenance of RAAS inhibition
- When initiated, ACE inhibitors and ARBs can cause a reduction in glomerular blood flow, thereby leading to an acute decline in eGFR of up to 30%. In this context, these kidney-protective agents should be continued, provided the acute reduction in eGFR is less than 30%.
- The risk of hyperkalaemia should be recognised if ACE inhibitors or ARBs are initiated in patients with high-normal serum potassium levels, which can be managed effectively with dietary and medical interventions.
- RAAS inhibition should ideally be uptitrated to the maximum tolerated dose, balanced with the risk of side effects (e.g. hypotension and electrolyte abnormalities, such as hyperkalaemia), and recommenced following episodes of recovered acute kidney injury.
- Using ACE inhibitor and ARB therapies in combination should be avoided because of the increased risk of acute kidney injury and hyperkalaemia.
Pillar 2: Sodium-glucose cotransporter-2 inhibitors
Originally developed as glucose-lowering agents, SGLT-2 inhibitors have also demonstrated cardiac and kidney protective effects, even in people without diabetes. By inhibiting sodium and glucose reuptake in the proximal tubule, SGLT-2 inhibitors increase distal sodium delivery, thereby restoring tubuloglomerular feedback. This leads to afferent arteriolar vasoconstriction and a reduction in intraglomerular pressure, manifesting clinically as an acute ‘dip’ in eGFR. This mechanism parallels and is complementary to that of RAAS blockade, which also reduces intraglomerular pressure. SGLT-2 inhibitors also modestly lower blood pressure (comparable to the introduction of a thiazide diuretic), reduce albuminuria and induce modest weight loss.21-24
Three landmark kidney outcome trials have established SGLT-2 inhibitors as foundational therapy for CKD, alongside RAAS blockade. In patients with type 2 diabetes and CKD, CREDENCE first established that canagliflozin could reduce the risk of end-stage kidney disease, progressive CKD or death from cardiovascular kidney causes by about 30%.16 The DAPA-CKD trial then demonstrated that dapagliflozin reduced the risk of decline in eGFR, end-stage kidney disease or death because of cardiovascular or kidney causes in patients with CKD, irrespective of diabetes.25 Further, the EMPA-KIDNEY trial confirmed that empagliflozin also reduced the risk of progressive CKD or cardiovascular death by 28% in a broad population of patients with CKD with or without diabetes.26
SGLT-2 inhibitors have been found to reduce the risk of cardiovascular events, particularly heart failure, irrespective of the presence of established atherosclerotic cardiovascular disease, diabetes or the level of kidney function, solidifying their clinical benefits in improving cardiorenal outcomes as a class of treatment.27 They are broadly approved in Australia for the treatment of not only diabetes and CKD, but also heart failure, irrespective of ejection fraction or diabetes.
Practical tips for the initiation and maintenance of SGLT-2 inhibition
- Empagliflozin is indicated in adults with CKD Stages 2 and 3A with UACR 30 mg/g or greater (≥22.6 mg/mmol per PBS criteria), or CKD Stages 3B, 4 and 5 irrespective of UACR (albuminuria). Dapagliflozin’s indication is limited to adults with proteinuric CKD Stages 2, 3 or 4 and UACR 30 mg/g or greater (≥22.6 mg/mmol per PBS criteria). Dapagliflozin and empagliflozin should not be initiated if the starting eGFR is less than 25 mL/min/1.73 m2 or 20 mL/min/1.73 m2, respectively; however, the current PBS criteria include a starting eGFR of 25 mL/min/1.73 m2 or greater for both drugs.
- Similar to ACE inhibitors and ARBs, SGLT-2 inhibitors can cause a predictable acute and reversible reduction in a patient’s eGFR because of changes in glomerular haemodynamics. Clinicians may expect up to a 30% short-term decrease in eGFR but should stay the course by continuing therapy to derive long-term benefits. For example, patients with an eGFR of 60 mL/min/1.73 m2 would need to reduce their eGFR by 18 mL/min/1.73 m2 to reach the 30% decline threshold.
- As the eGFR declines to below 45 mL/min/1.73 m2, the glucose-lowering effects of SGLT-2 inhibitors are modest and other glucose-lowering medications may need to be introduced. However, the kidney and cardiovascular benefits of SGLT-2 inhibitors are still maintained at lower eGFRs, at least to a starting eGFR of 20 mL/min/1.73m2.
- SGLT-2 inhibition in combination with other therapies is not only safe but can enhance adherence to other therapies. They reduce the risk of serious hyperkalaemia and acute kidney injury, which can help maintain the use of RAAS inhibition and nonsteroidal MRAs.28
- Potential adverse effects from SGLT-2 inhibitors include genital mycotic infection as well as euglycemic diabetic ketoacidosis; therefore, patients with diabetes must be cautioned to temporarily cease SGLT-2 inhibitors during periods of illness, dehydration or fasting.
- Despite their use in a broad range of conditions, SGLT-2 inhibitors are not approved for use in patients with type 1 diabetes (because of the potential risk of ketoacidosis), and they are currently considered off-label for patients receiving immunosuppression for kidney disease (e.g. lupus nephritis, vasculitis and patients with an organ transplant). Their benefits and potential harms have not yet been studied in autosomal dominant polycystic kidney disease.
Pillar 3: Nonsteroidal MRAs
Despite maximally tolerated ACE or ARB therapy and SGLT-2 inhibition, people with DKD are still at heightened cardiorenal risk, related to elevated aldosterone activity.29 Increased mineralocorticoid receptor activation by aldosterone activates downstream proinflammatory and profibrotic pathways within the kidneys, contributing to DKD progression.30
Steroidal MRAs (e.g. spironolactone and eplerenone) can decrease albuminuria; however, data on clinical outcomes are limited and the risk of hyperkalaemia has hindered their widespread use. In comparison, nonsteroidal MRAs have greater selectivity for the mineralocorticoid receptor, cause less hyperkalaemia and gynaecomastia, have more balanced distribution in cardiac and kidney tissue, and may have more pronounced anti-inflammatory or antifibrotic effects.31
Two companion trials, FIDELIO DKD and FIGARO DKD, have collectively studied the impact of finerenone on clinical outcomes in almost 13,000 patients with DKD.32,33 Like the SGLT-2 inhibitor trials, all participants were taking the maximum tolerated or labelled dose of RAAS blockade. In the pooled FIDELITY analysis of both trials, finerenone reduced the risk of CKD progression (a 57% or greater reduction in eGFR, kidney failure or death due to kidney failure) by 23% and reduced the risk of major adverse cardiovascular events by 14%.34 These effects were observed irrespective of SGLT-2 inhibitor use.35
Practical tips for the initiation and maintenance of nonsteroidal MRAs
- The current PBS criteria for finerenone include patients with CKD and type 2 diabetes, residual albuminuria (UACR ≥22.6 mg/mmol) and an eGFR 25 mL/min/1.73 m2 or greater despite treatment with RAAS blockade, and an SGLT-2 inhibitor unless medically contraindicated or intolerant.
- The main adverse effect of finerenone is hyperkalaemia, but the absolute risk is low. Finerenone can be commenced in patients with a baseline serum potassium level of up to 5.0 mmol/L, with routine monitoring of potassium.
- Like RAAS or SGLT-2 inhibitors, finerenone can cause a predictable drop in eGFR, which typically recovers and stabilises.
- Finerenone should not be used in combination with steroidal MRAs, including spironolactone or eplerenone.
Pillar 4: GLP-1 receptor agonists
GLP-1 is an incretin that is produced following a meal, which acts to stimulate insulin release and inhibit glucagon release. GLP-1 receptor agonists were developed as a potent glucose-lowering agent but have also been shown to lead to substantial weight loss and reduce cardiorenal outcomes.36
The recently published FLOW (Evaluate Renal Function with Semaglutide Once Weekly) trial was the first to examine the long-term kidney protective effects of a GLP-1 receptor agonist in patients with DKD. Semaglutide reduced the risk of CKD progression or cardiovascular death (kidney failure, at least 50% reduction in eGFR, or death from kidney or cardiovascular causes) by 24%.37 Outcomes from large cardiovascular outcome trials have also shown that GLP-1 receptor agonists significantly reduce major adverse cardiovascular events in patients with type 2 diabetes by 14%.38
Furthermore, there is emerging evidence from the SELECT trial that semaglutide reduces the risk of cardiovascular events and may improve CKD outcomes in overweight and obese patients without diabetes.39 A post-hoc analysis of the SELECT trial also suggested that semaglutide might reduce the risk of adverse kidney outcomes (death from kidney disease, initiation of renal replacement therapy, onset of eGFR <15 mL/min/1.73 m2, persistent reduction in eGFR of greater than 50% or onset of macroalbuminuria) by 22%, driven largely by reductions in albuminuria.40 Recently, semaglutide was also shown to substantially reduce albuminuria in patients who are overweight or obese in the setting of nondiabetic CKD.41
These cardiorenal protective benefits appear to be only partially mediated by reductions in body weight, blood pressure and HbA1c, suggesting there are potentially vascular- and kidney-specific mechanisms underlying their clinical benefits.42 The mechanism of action of GLP-1 receptor agonists is also entirely distinct to that of SGLT-2 inhibitors and nonsteroidal MRAs, indicating that there are likely to be additive benefits to their use in combination. Indeed, a prespecified analysis of the FLOW trial and meta-analyses showed that the benefits of GLP-1 receptor agonists in patients with type 2 diabetes and CKD were consistent, irrespective of SGLT-2 inhibitor use, and vice versa.43-45
Practical tips for the initiation and maintenance of GLP-1 receptor agonists
- GLP-1 receptor agonists are currently PBS listed for use in patients with type 2 diabetes in combination with at least one of: metformin, a sulfonylurea and/or insulin. Patients must also not have achieved a clinically meaningful glycaemic response with an SGLT-2 inhibitor, or have a contraindication to, or intolerance requiring treatment discontinuation of an SGLT-2 inhibitor.
- At present, unlike SGLT-2 inhibitor therapy or finerenone, there is no PBS-listed CKD indication for a GLP-1 receptor agonist.
- In Australia, GLP-1 receptor agonists can be used in combination with SGLT-2 inhibitors if the SGLT-2 inhibitor is used for a PBS-listed indication outside of diabetes (i.e. CKD or heart failure). GLP-1 receptor agonists are not currently PBS-listed for indications outside of type 2 diabetes.
- The most common adverse effects with GLP-1 receptor agonists are gastrointestinal, including nausea and diarrhoea, and patients should have their dose initiated and uptitrated slowly with appropriate counselling to increase the likelihood of treatment persistence.
Implementation of the four pillars
Co-prescribing therapies
Given the availability of several cardio-renoprotective drugs, the next question to address is whether using them in combination is safe and confers additional benefits. Emerging evidence from pooled analyses of trial subgroups including individuals on combinations of therapies have shown that the benefit of SGLT-2 inhibitors was consistent in patients with or without GLP-1 receptor agonist therapy, and vice versa.43-45 This suggests that the benefits of these pillars of therapies, which have different mechanisms of action, are additive. A further analysis showed that if individuals were using all four pillars of therapies, then this approach may delay progression of CKD by 5.5 years compared with conventional care (i.e. addressing risk factors and RAAS inhibition).46
There may also be important safety advantages with combination therapy as SGLT-2 inhibitors can ameliorate increases in serum potassium levels caused by RAAS inhibition or nonsteroidal MRAs.28 Furthermore, the simultaneous initiation of SGLT-2 inhibitors and finerenone is currently under investigation in the multicentre double- blinded CONFIDENCE trial.47 Further data from ongoing trials will help to substantiate the benefits and safety of combination therapy. We envisage that additional evidence regarding the implementation of these pillars of therapy will be forthcoming in future studies.
Matching the intensity of treatment to a patient’s risk
It is important to select the right drugs for the right patient at the right time. To tailor the implementation of therapy for DKD, clinicians should build a profile of each patient’s cardiovascular and kidney risk. There are multiple proposed approaches to implementing the four pillars of DKD therapy. The KDIGO guidelines for diabetes management in CKD describes first-line treatment that comprises RAAS inhibition and SGLT-2 inhibitors, with the addition of a nonsteroidal MRA for patients with residual albuminuria (in line with current PBS prescribing criteria) and GLP-1 receptor agonists for additional glycaemic and cardiovascular benefits.
There is emerging evidence that selected patients with the highest cardiovascular- kidney-metabolic risk should have accelerated implementation of these therapies, whereas low- or moderate-risk individuals need not be on all four pillars of therapies.48,49 This method of implementation has drawn parallels with the pillars of guideline-directed medical therapy for heart failure. Indeed, treating one condition can delay or prevent the onset of the other. Early referral to a nephrologist should be considered as part of a multidisciplinary team approach, particularly for high-risk individuals with severely increased albuminuria (UACR of ≥30 mg/mmol) regardless of the level of their eGFR, as they may gain greater absolute benefits from early combination therapy.
Patients with advanced CKD who have been commenced on SGLT-2 inhibitors, nonsteroidal MRAs and/or GLP-1 receptor agonists should continue these medications to maintain their kidney protective benefits until they reach dialysis.
Beyond initiating medication, educating patients regarding appropriate use of medications is of utmost importance. Sick day medication guidance should be provided (such as the Sick Day Action Plan from Kidney Health Australia, available online at https://assets.kidney.org.au/resources/KHA-How-To-Sick-Day-Action-Plan-FINAL.pdf), so that patients with diabetes are aware to temporarily withhold SGLT-2 inhibitors when unwell or not eating or drinking normally.50
Lastly, addressing lifestyle factors, such as smoking cessation, promoting weight loss and dietary salt reduction, remains the foundation of holistic patient-centred care. Implementing these strategies alongside medications with targeted education can give patients a greater sense of agency.
Conclusion
DKD is a common and clinically important complication of type 2 diabetes that portends increased dual risk of cardiovascular disease and kidney failure. Since the advent of RAAS blockade, the landscape of DKD treatment has changed dramatically with additional therapies: SGLT-2 inhibitors, nonsteroidal MRAs and GLP-1 receptor agonists. Together, these form four pillars of current DKD management in conjunction with comprehensive lifestyle modification. These therapies offer the opportunity to address the multiple distinct pathways of injury that underlie the development and progression of DKD. Primary care plays a pivotal role in ensuring early detection, stratifying risk of future cardiorenal complications, initiating treatments to match this risk and preventing serious long-term complications in patients with DKD. MT
COMPETING INTERESTS: Associate Professor Neuen is supported by an Australian National Health and Medical Research Council Emerging Leader Investigator Grant (grant number 2026621) and a Ramaciotti Foundation Health Investment Grant (grant number 2023HIG69); has received fees for travel support, advisory boards and scientific presentations from AstraZeneca, Bayer, Boehringer Ingelheim, Cambridge Healthcare Research, Cornerstone Medical Education, the Limbic, Janssen, Medscape, Novo Nordisk and Travere Therapeutics; and serves on clinical trial committees for studies sponsored by AstraZeneca, Bayer and CSL Behring, with all honoraria paid to The George Institute for Global Health. Dr Ha was supported by an Australian Government Research Training Program Scholarship and a University Postgraduate Award from UNSW Sydney, Sydney; has received payment to his institution for his role as faculty member for The George Institute for Global Health’s Quantitative Research Training Program; has received conference travel support from the Australian and New Zealand Society of Nephrology and the American Society of Nephrology; and has an unpaid role as an Australasian Kidney Trials Network Chronic Kidney Disease Working Group member. Dr Zheng, Dr Mather and Dr Roxburgh: None.
References
1. Australian Institute of Health and Welfare (AIHW). Diabetes: Australian facts. Canberra: AIHW; 2023. Available online at: https://www.aihw.gov.au/reports/diabetes/diabetes/contents/summary (accessed December 2024).
2. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017; 12: 2032-2045.
3. Gorodetskaya I, Zenios S, Mcculloch CE, et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int 2005; 68: 2801-2808.
4. Jun M, Wick J, Neuen BL, et al. The prevalence of CKD in Australian primary care: analysis of a national general practice dataset. Kidney Int Rep 2024; 9: 312-322.
5. McDonald SP, Russ GR, Kerr PG, Collins JF. ESRD in Australia and New Zealand at the end of the millennium: a report from the ANZDATA registry. American J Kidney Dis 2002; 40: 1122-1131.
6. Irish G, Davies C, Au E, et al. Chapter 1: Incidence of kidney failure with replacement therapy. Adelaide, Australia: Australia and New Zealand Dialysis and Transplant Registry; 2023.
7. AIHW. Chronic kidney disease: Australian facts. Canberra: AIHW; 2023. Available online at: https://aihw.gov.au/reports/chronic-kidney-disease/chronic-kidney-disease/contents/summary (accessed December 2024).
8. Ndumele CE, Rangaswami J, Chow SL, et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation 2023; 148: 1606-1635.
9. Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers 2015; 1: 15018.
10. Fiorentino M, Bolignano D, Tesar V, et al. Renal biopsy in patients with diabetes: a pooled meta-analysis of 48 studies. Nephrol Dial Transplant 2017; 32: 97-110.
11. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int 2024; 105: S117-s314.
12. Australia KH. Chronic kidney disease (CKD) management in primary care. Kidney Health Australia Melbourne; 2020.
13. Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073-2081.
14. Department of Health and Aged Care. Australian CVD risk calculator 2024. Available online at: https://www.cvdcheck.org.au/calculator (accessed December 2024).
15. Naaman SC, Bakris GL. Slowing diabetic kidney disease progression: where do we stand today? In: Chronic kidney disease and type 2 diabetes. Arlington (VA): American Diabetes Association; 2021. p. 28-32.
16. Perkovic V, Jardine Meg J, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 2019; 380: 2295-2306.
17. Duo Y, Gao J, Yuan T, Zhao W. Effect of sodium-glucose cotransporter 2 inhibitors on the rate of decline in kidney function: A systematic review and meta-analysis. J Diabetes 2023; 15: 58-70.
18. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329: 1456-1462.
19. Keane WF, Brenner BM, De Zeeuw D, et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63: 1499-1507.
20. Berl T, Hunsicker LG, Lewis JB, et al. Cardiovascular outcomes in the irbesartan diabetic nephropathy trial of patients with type 2 diabetes and overt nephropathy. Ann Int Med 2003; 138: 542-549.
21. Beal B, Schutte AE, Neuen BL. Blood pressure effects of SGLT2 Inhibitors: mechanisms and clinical evidence in different populations. Curr Hypertens Rep 2023; 25: 429-435.
22. Sternlicht H, Bakris GL. Blood pressure lowering and sodium-glucose co-transporter 2 inhibitors (SGLT2is): more than osmotic diuresis. Curr Hypertens Rep 2019; 21: 12.
23. Dekkers CCJ, Wheeler DC, Sjöström CD, Stefansson BV, Cain V, Heerspink HJL. Effects of the sodium-glucose co-transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and Stages 3b-4 chronic kidney disease. Nephrol Dial Transplant 2018; 33: 2005-2011.
24. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circ 2016; 134: 752-772.
25. Heerspink Hiddo JL, Stefánsson Bergur V, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436-1446.
26. Fletcher RA, Jongs N, Chertow GM, et al. Effect of SGLT2 inhibitors on discontinuation of renin-angiotensin system blockade: a joint analysis of the CREDENCE and DAPA-CKD trials. J Am Soc Nephrol 2023; 34: 1965-1975.
27. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 2022; 400: 1788-1801.
28. Neuen BL, Oshima M, Agarwal R, et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation 2022; 145: 1460-1470.
29. Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension 2003; 41: 64-68.
30. Barrera-Chimal J, Lima-Posada I, Bakris GL, Jaisser F. Mineralocorticoid receptor antagonists in diabetic kidney disease - mechanistic and therapeutic effects. Nat Rev Nephrol 2022; 18: 56-70.
31. Grune J, Beyhoff N, Smeir E, et al. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone’s antifibrotic activity. Hypertension 2018; 71: 599-608.
32. Bakris GL, Agarwal R, Anker SD, Pitt et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383: 2219-2229.
33. Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021; 385: 2252-2263.
34. Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022; 43: 474-484.
35. Rossing P, Anker SD, Filippatos G, et al. Finerenone in patients with chronic kidney disease and type 2 diabetes by sodium-glucose cotransporter 2 inhibitor treatment: the FIDELITY analysis. Diabet Care 2022; 45: 2991-2998.
36. Michos ED, Bakris GL, Rodbard HW, Tuttle KR. Glucagon-like peptide-1 receptor agonists in diabetic kidney disease: a review of their kidney and heart protection. Am J Prev Cardiol 2023; 14: 100502.
37. Perkovic V, Tuttle KR, Rossing P, et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med 2024; 391: 109-121.
38. Giugliano D, Scappaticcio L, Longo M, et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol 2021; 20: 189.
39. Lincoff AM, Brown-Frandsen K, Colhoun Helen M, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023; 389: 2221-2232.
40. Colhoun HM, Lingvay I, Brown PM, et al. Long-term kidney outcomes of semaglutide in obesity and cardiovascular disease in the SELECT trial. Nat Med 2024; 30: 2058-2066.
41. Apperloo EM, Gorriz JL, Soler MJ, et al. Semaglutide in patients with overweight or obesity and chronic kidney disease without diabetes: a randomized double-blind placebo-controlled clinical trial. Nat Med 2024; online ahead of print.
42. Mann JFE, Buse JB, Idorn T, et al. Potential kidney protection with liraglutide and semaglutide: exploratory mediation analysis. Diabetes Obes Metab 2021; 23: 2058-2066.
43. Mann JFE, Rossing P, Bakris G, et al. Effects of semaglutide with and without concomitant SGLT2 inhibitor use in participants with type 2 diabetes and chronic kidney disease in the FLOW trial. Nat Med 2024; 30: 2849-2856.
44. Neuen BL, Fletcher RA, Heath L, et al. Cardiovascular, kidney and safety outcomes with GLP-1 receptor agonists alone and in combination with SGLT2 inhibitors in type 2 diabetes: a systematic review and meta-analysis. Circulation 2024; 150: 1781-1790.
45. Apperloo EM, Neuen BL, Fletcher RA, et al. Efficacy and safety of SGLT2 inhibitors with and without glucagon-like peptide 1 receptor agonists: a SMART-C collaborative meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol 2024; 12: 545-557.
46. Neuen BL, Heerspink HJL, Vart P, et al. Estimated lifetime cardiovascular, kidney, and mortality benefits of combination treatment with SGLT2 inhibitors, GLP-1 receptor agonists, and nonsteroidal MRA compared with conventional care in patients with type 2 diabetes and albuminuria. Circulation 2024; 149: 450-462.
47. Green JB, Mottl AK, Bakris G, et al. Design of the COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using a UACR Endpoint study (CONFIDENCE). Nephrol Dial Transplant 2023; 38: 894-903.
48. Neuen BL, Tuttle KR, Vaduganathan M. Accelerated risk-based implementation of guideline-directed medical therapy for type 2 diabetes and chronic kidney disease. Circulation 2024; 149: 1238-1240.
49. Neuen BL, Tuttle KR, Bakris G, Vaduganathan M. Reframing chronicity with urgency in chronic kidney disease management. Clin J Am Soc Nephrol 2024; 19: 1209-1211.
50. Kidney Health Australia. How to...Sick Day Action Plan. 2022. Available from: https://assets.kidney.org.au/resources/KHA-How-To-Sick-Day-Action-Plan-FINAL.pdf (accessed December 2024).