Lipid-lowering therapy for older people: update on prescribing

With the recognition that chronological age alone should not be a barrier to lipid-lowering therapy (LLT), the focus falls on prescribing for older people. LDL-cholesterol targets are similar to those in younger people, but more diligence is needed in monitoring adherence and adverse effects. Initiating LLT with the combination of a lower-dose statin plus ezetimibe can improve adherence and reduce adverse effects in older people.
- Recent studies have shown that lipid-lowering therapy (LLT) achieves similar reductions in cardiovascular disease (CVD) in older compared with younger age groups.
- Age is no longer a bar to initiating LLT in older people who have no contraindicating issues (e.g. comorbidity or problems with adherence).
- Particular considerations in prescribing LLT in older people include potential limited life expectancy and the risk of drug interactions.
- Greater diligence in monitoring adherence and adverse effects is needed in older people.
- Our current recommendation is to initiate LLT using a low-dose statin in combination with ezetimibe rather than high-dose statin monotherapy to achieve LDL-cholesterol targets.
- This strategy results in improved compliance and efficacy in reducing CVD events.
Atherosclerotic cardiovascular disease (CVD) is the major cause of death in older people, usually resulting from plaque rupture or erosion and subsequent mural thrombosis with luminal occlusion. Arterial accumulation of cholesterol derived from plasma LDL cholesterol is the major determinant of atherogenesis initiation and progression. Reducing LDL-cholesterol levels with lipid-lowering therapy (LLT) stabilises plaques and reduces CVD events in proportion to the reduction achieved. For example, a 1 mmol/L lower LDL-cholesterol level is associated with an approximately 23% lower rate of CVD events over a five-year period, independent of age and the means by which LDL-cholesterol is lowered.1 CVD events are reduced further beyond five years of LLT.
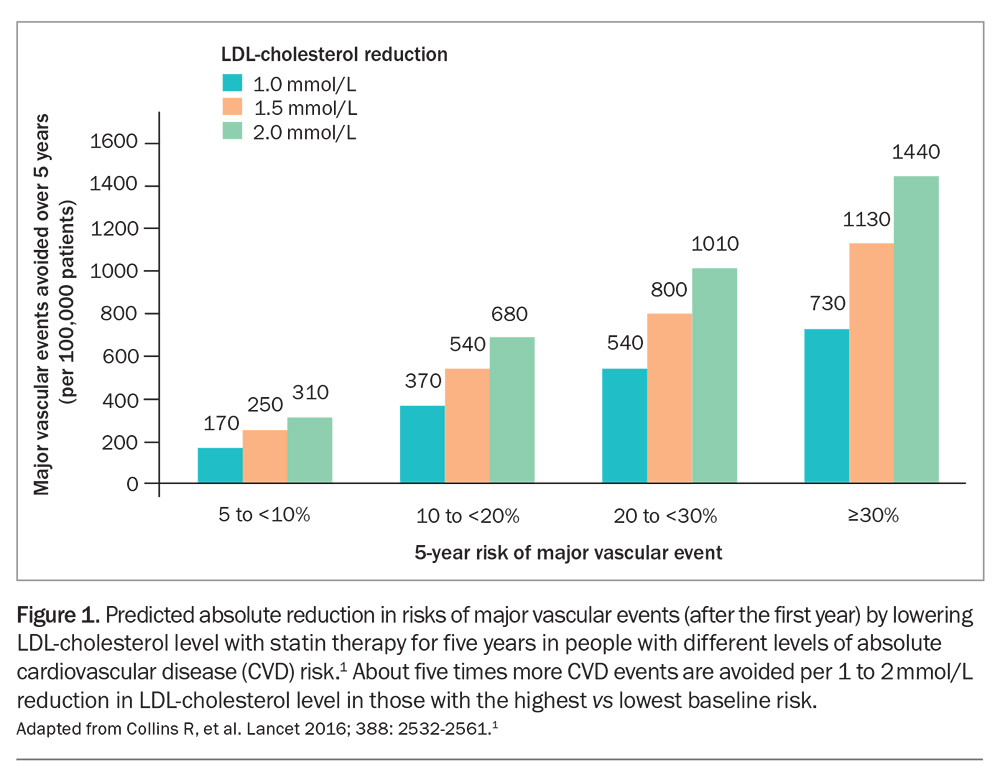
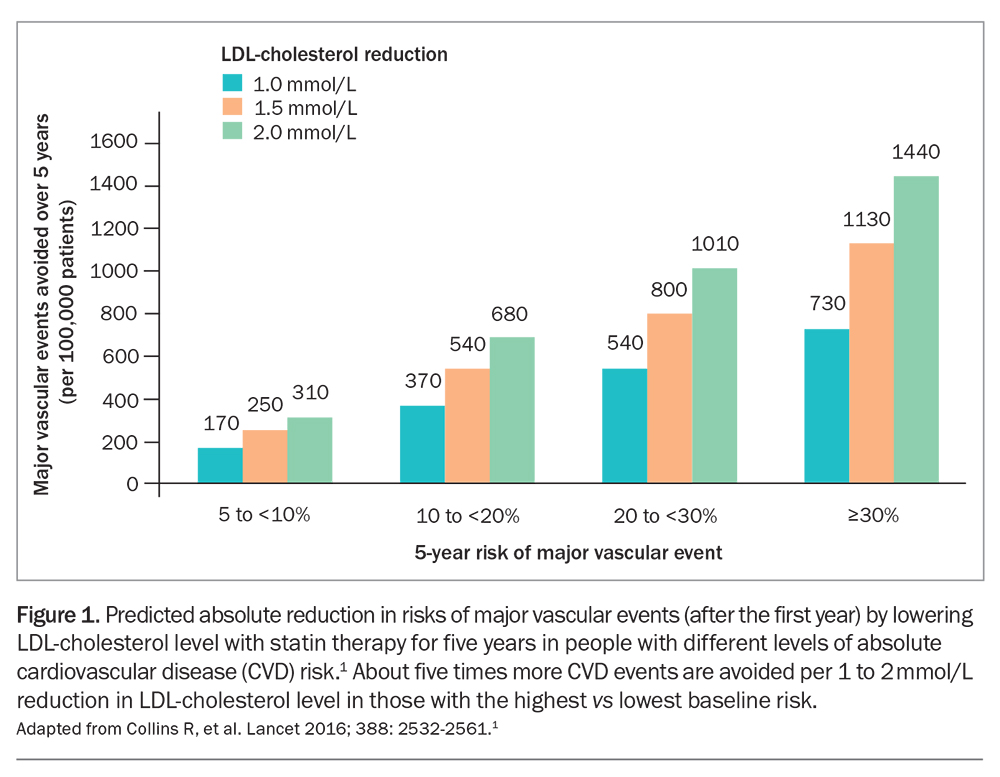
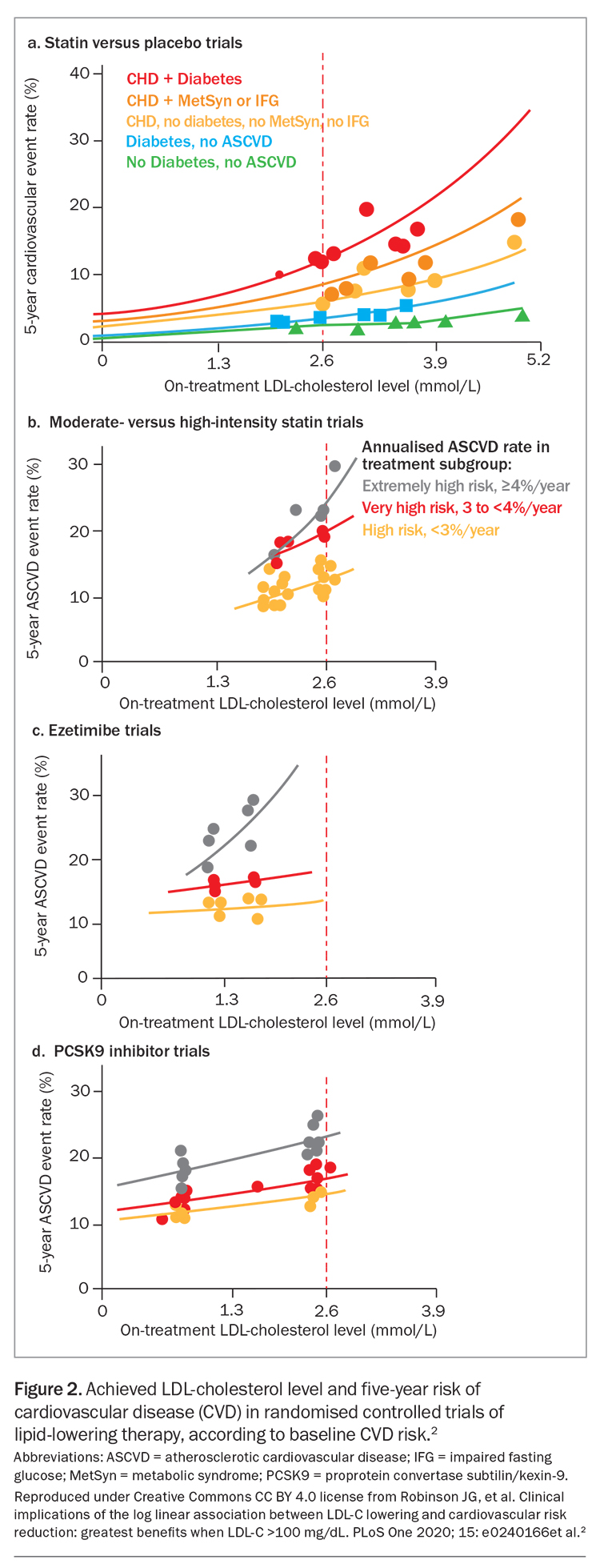
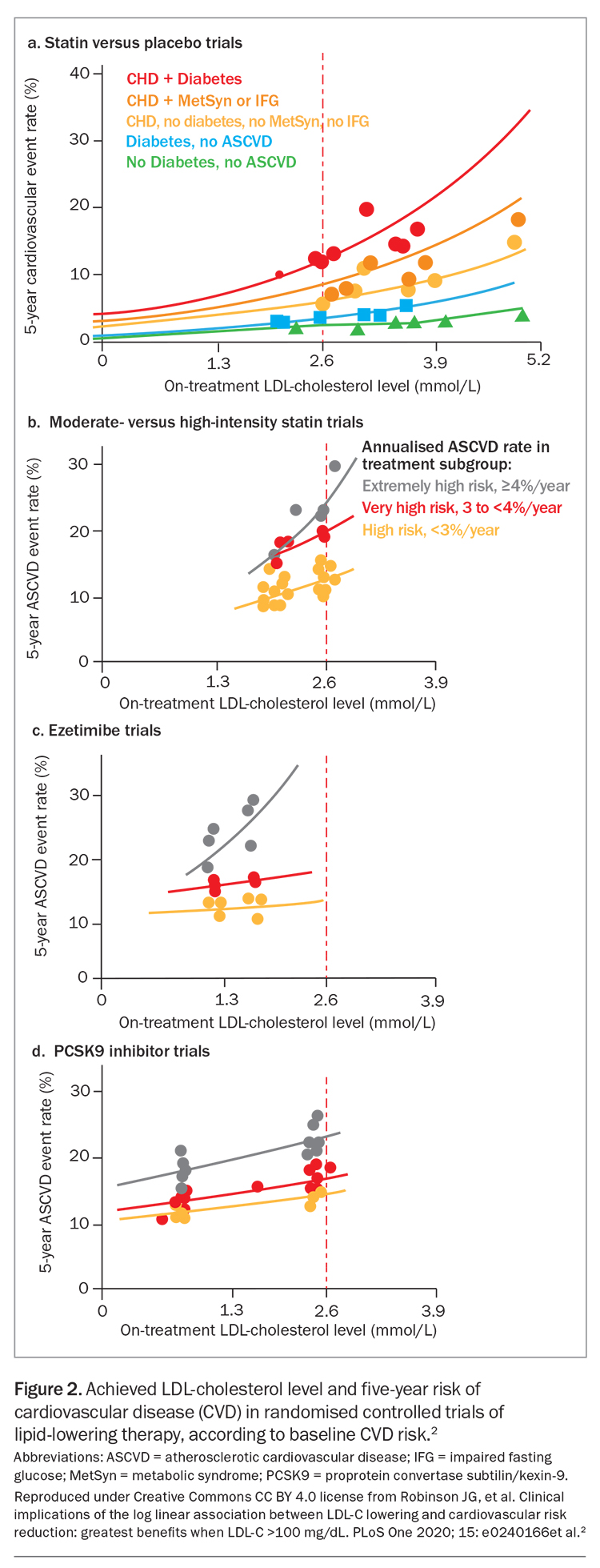
The relative risk reduction in CVD events is independent of baseline LDL-cholesterol level and age; similar benefits are observed for people aged under 70 years and those aged 70 years and over. Absolute risk reduction, and therefore the effectiveness of LLT, increases with increasing baseline CVD risk and age (Figure 1 and Figure 2).1,2
This is the second in a two-part series on LLT in older people. The first part, in the January/February 2024 issue of Medicine Today, described evidence that strongly supports the need to consider LLT in every suitable older person. Chronological age alone should not be a barrier to LLT.3 Here we describe how to prescribe LLT for older people, updating our previous publications on the topic.4,5 We highlight recent calls for the use of low-dose statins combined with ezetimibe for more effective and tolerable LDL-cholesterol reduction in older people.6,7
Principles of lipid-lowering therapy
High-intensity LDL reduction therapy is now the recommended strategy for LLT, rather than high-intensity statin therapy, as the main determinant of cardiovascular benefit is the extent of LDL-cholesterol reduction (Figure 1).1 As greater benefits accrue with longer duration of therapy, ‘lower, longer, earlier’ has become the mantra for LLT in patients of all ages.
Combination lipid-lowering therapy
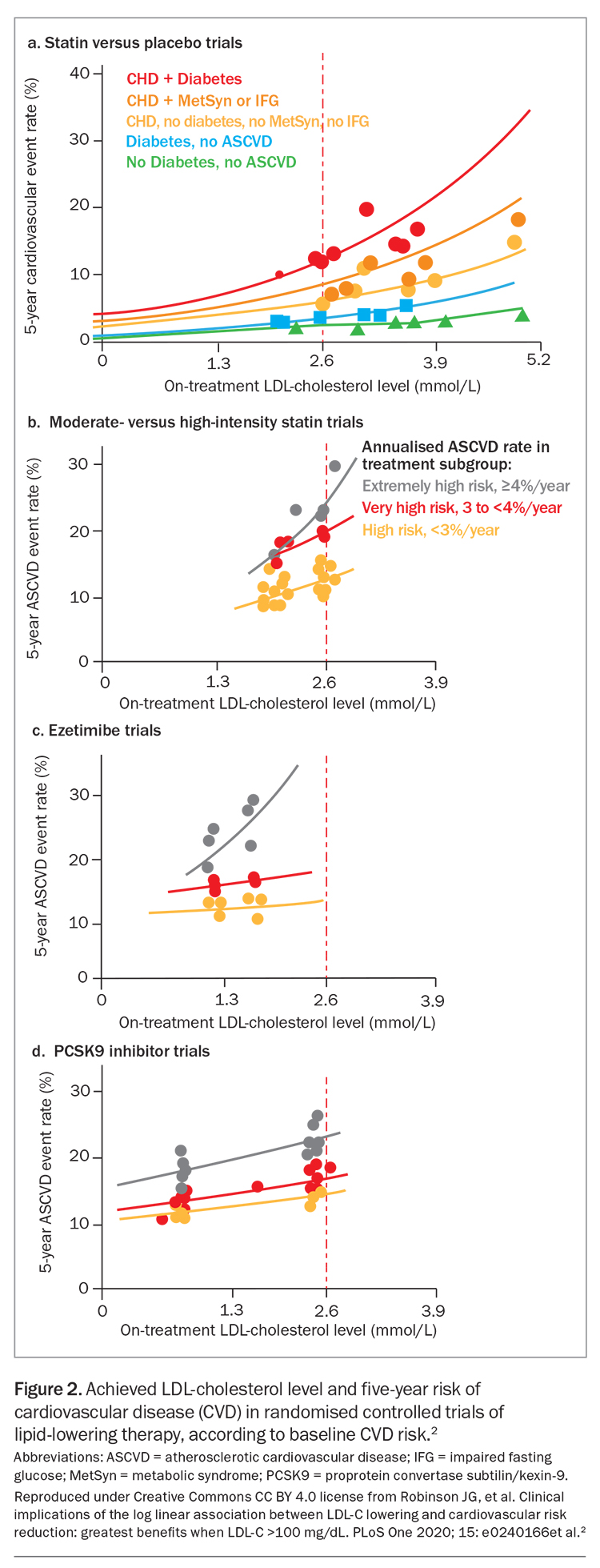
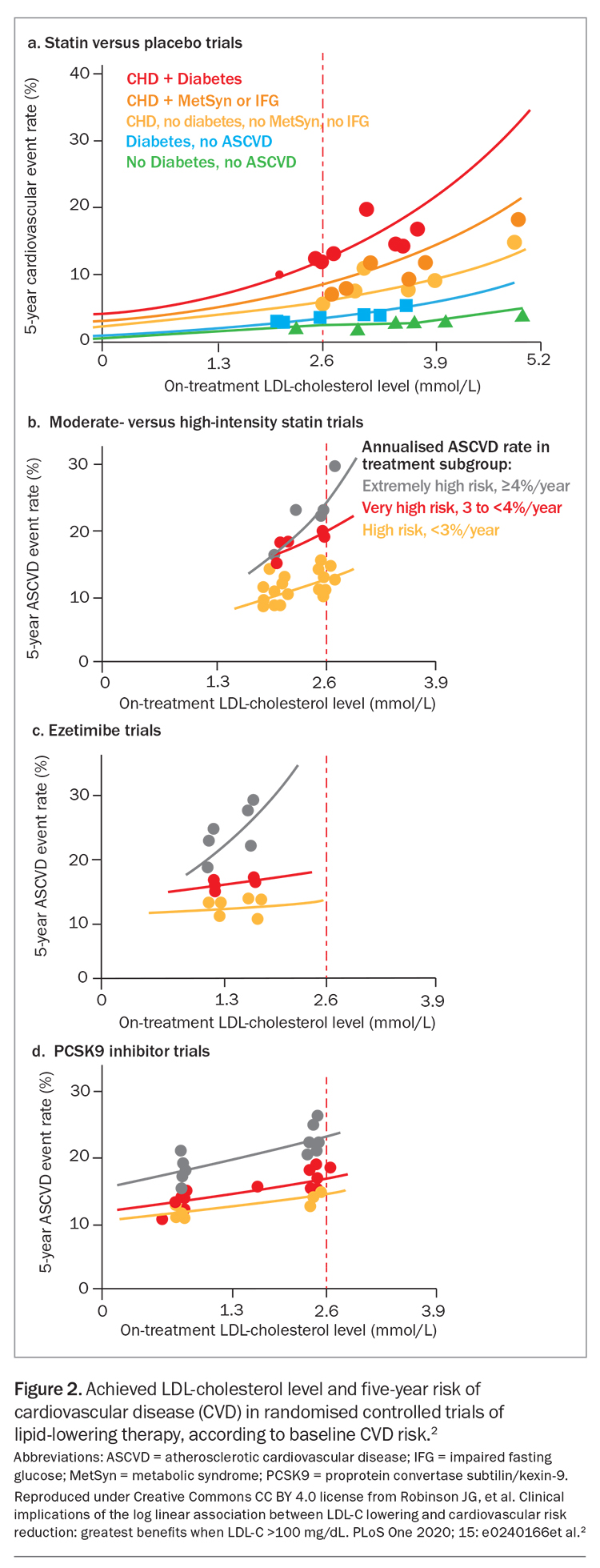
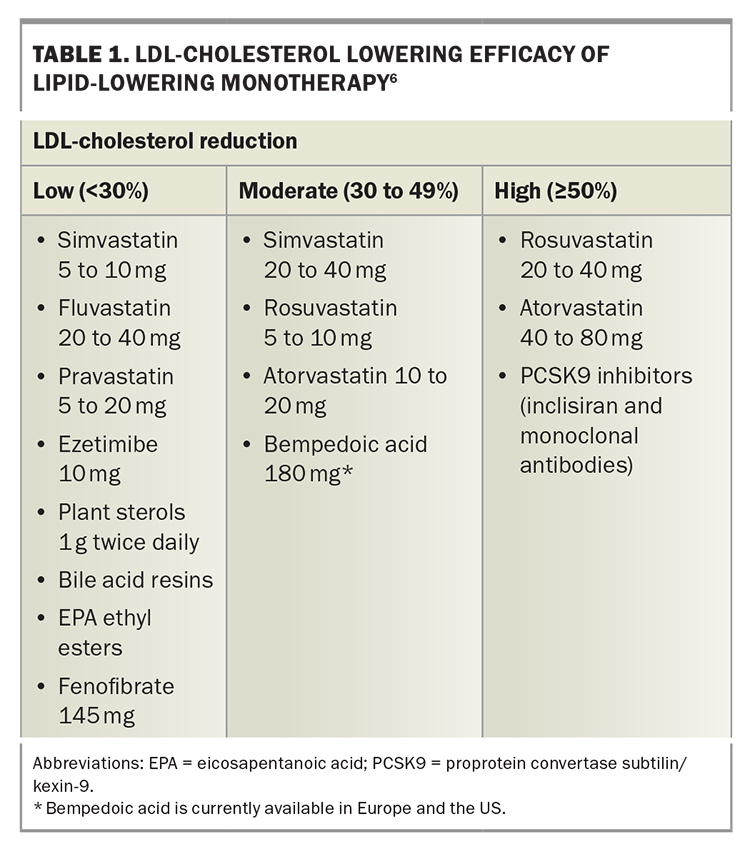
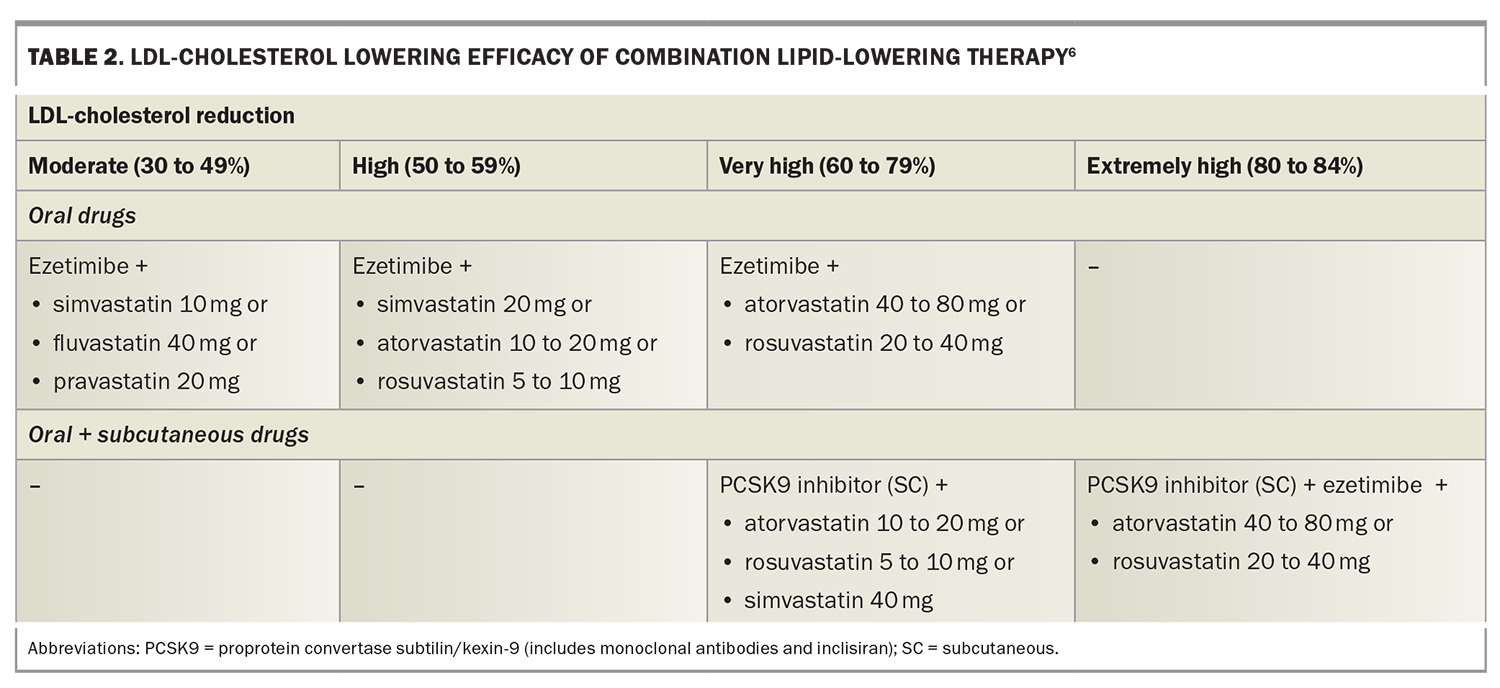
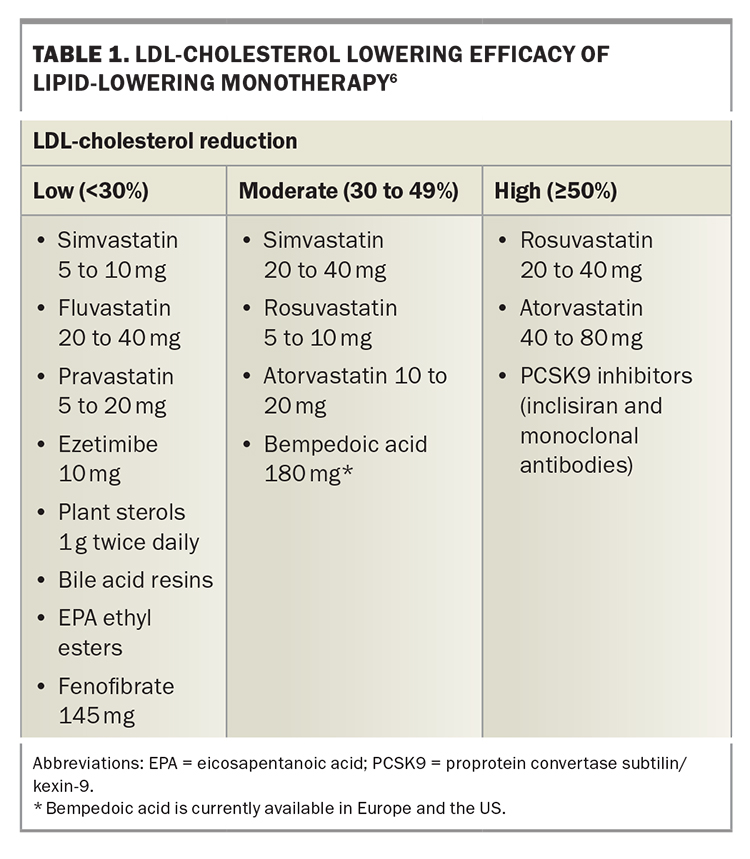
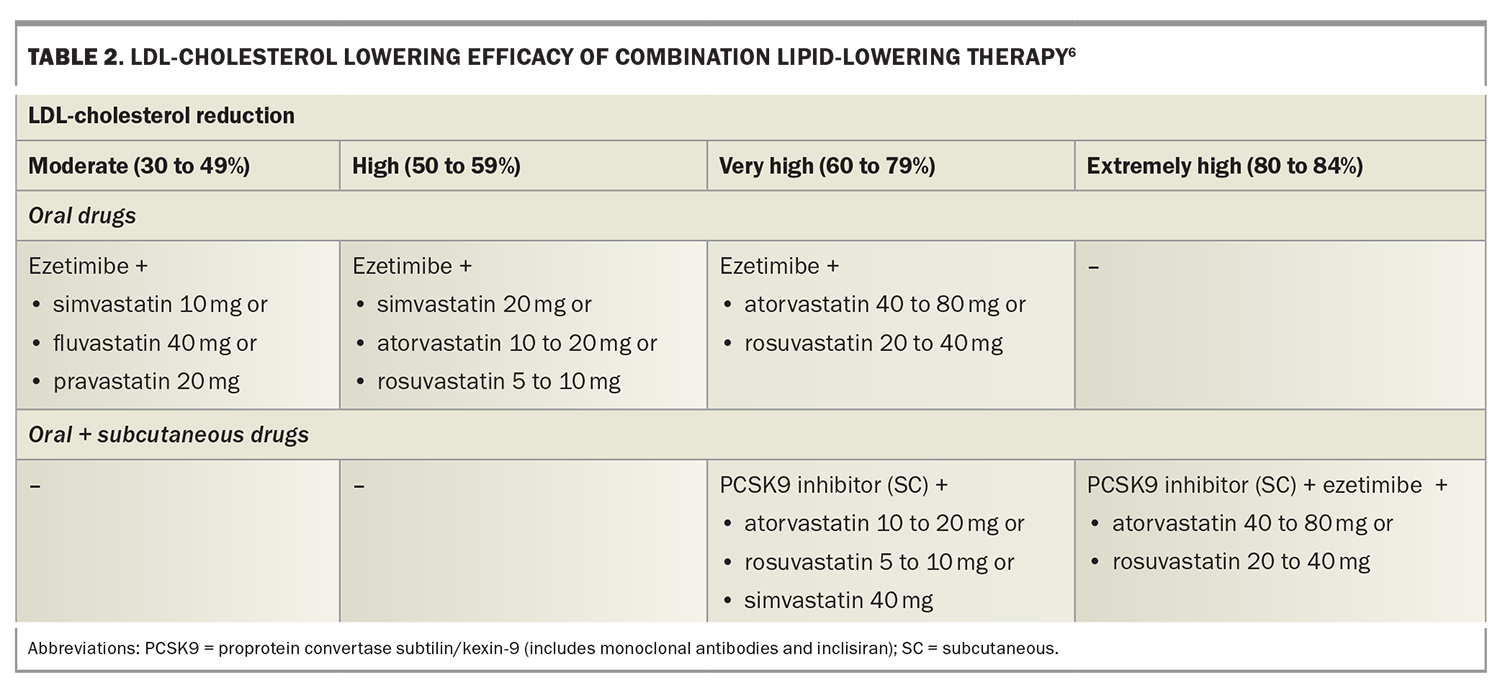
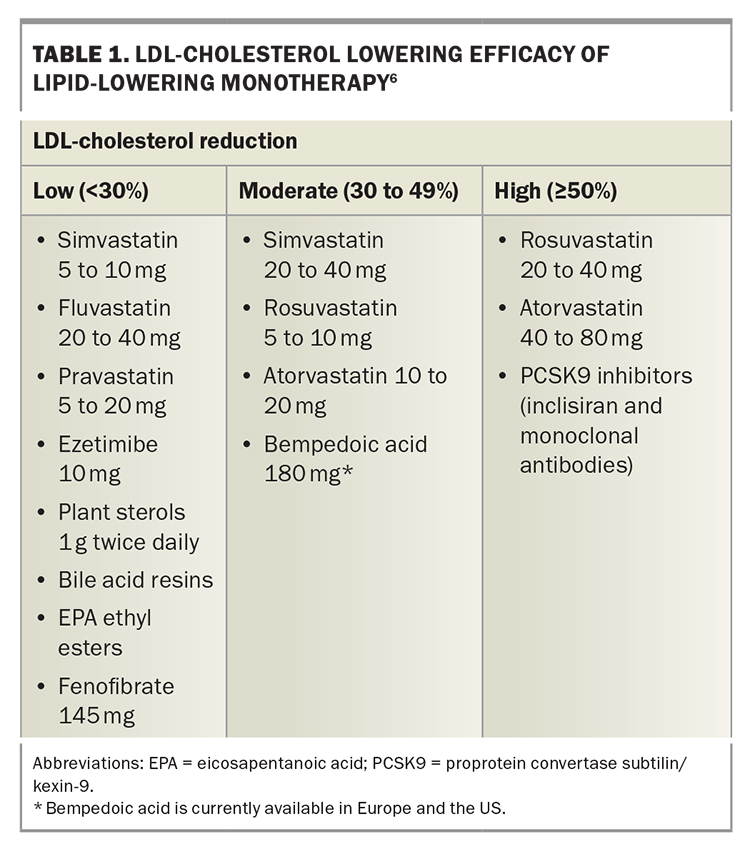
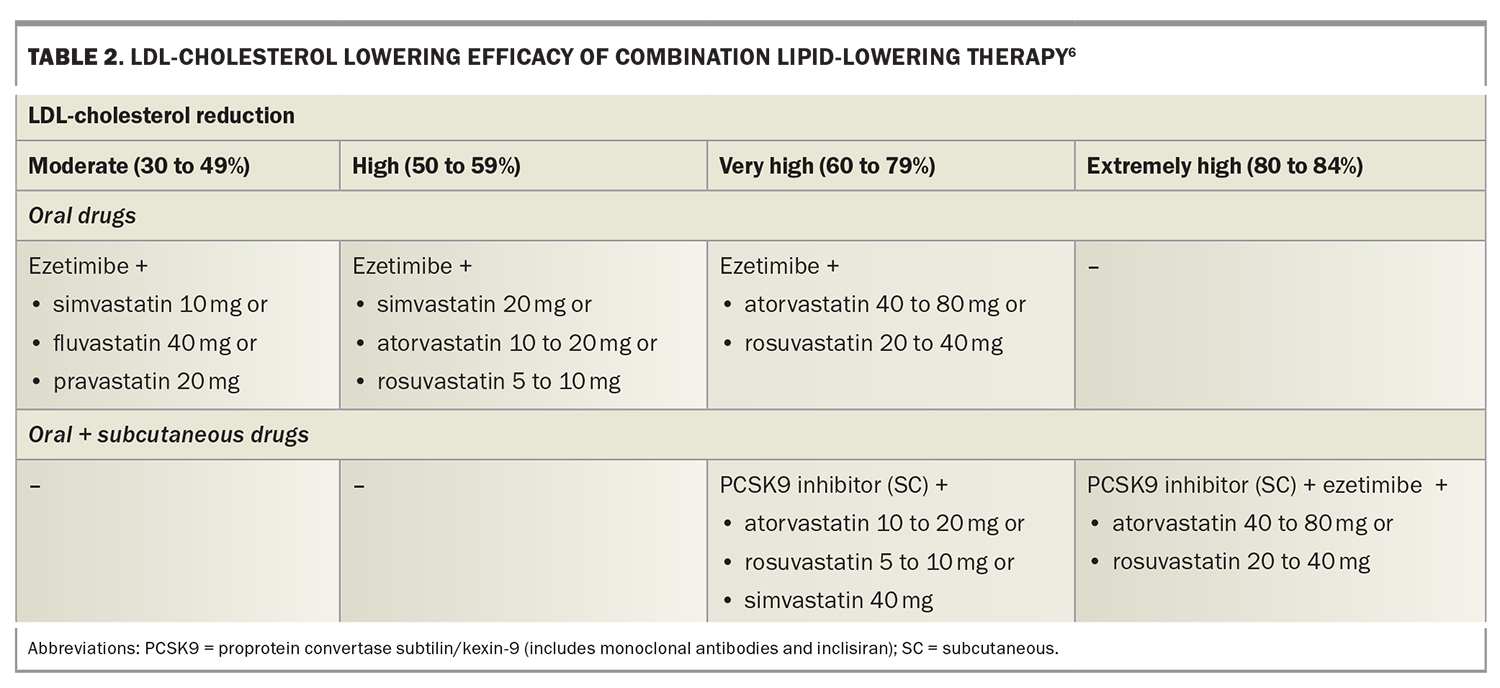
LLT that combines drugs with different mechanisms of action can achieve additive reductions in LDL-cholesterol level. These drugs include statins, ezetimibe, plant sterols, bile-acid sequestrants, fibrates, bempedoic acid, icosapent ethyl, nicotinic acid and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (Figure 2).2,7,8 Each drug type is independently able to lower LDL-cholesterol level, depending on dosage, allowing an LDL-cholesterol reduction of more than 80% with triple combinations of the most potent drugs. Target LDL-cholesterol levels can therefore be achieved in most patients.9 The efficacy of lipid-lowering monotherapy and combination therapy is shown in Table 1 and Table 2, respectively.6
Diet and nutraceuticals
Nutraceuticals are dietary supplements of plant or microbial origin that can modify lipid profiles and have potential benefits in CVD prevention when added to LLT and lifestyle changes. They include plant sterols, red yeast rice, olive oil, almonds, omega-3 (fish) oils, garlic and various vitamins and minerals.1,10,11 Nutraceuticals may be particularly helpful for statin-intolerant patients. In the PREDIMED RCT, a Mediterranean diet supplemented with extra virgin olive oil or nuts reduced the incidence of CVD compared with a reduced-fat diet.12
Our practice is to routinely recommend plant sterols with the Mediterranean diet, in addition to omega-3 supplements for those not eating fish regularly.
New nonstatin cholesterol-lowering drugs
Several new nonstatin cholesterol-lowering drugs are on the horizon. These include bempedoic acid, which inhibits an enzyme in the cholesterol synthesis pathway one step before HMG coenzyme A (the action site of statins). A trial of bempedoic acid in statin-intolerant patients found a 13% significant reduction in CVD events resulting from a 21% LDL-cholesterol reduction over 41 months.13 Bempedoic acid is expected to be useful in combination with ezetimibe in statin-intolerant patients, as the combination has a low rate of myalgia with an expected reduction in LDL-cholesterol level of about 50%.14 Bempedoic acid is currently available in Europe and the US.
Icosapent ethyl, an ethyl ester of the omega-3 fatty acid eicosapentanoic acid, derived from fish oil, was shown to reduce CVD events in the recent REDUCE-IT trial in high-risk patients taking a statin with triglyceride levels between 1.5 and 5.6 mmol/L.15 Icosapent ethyl will hopefully be available in Australia later in 2024 for selected statin-treated patients.8
LDL-cholesterol targets
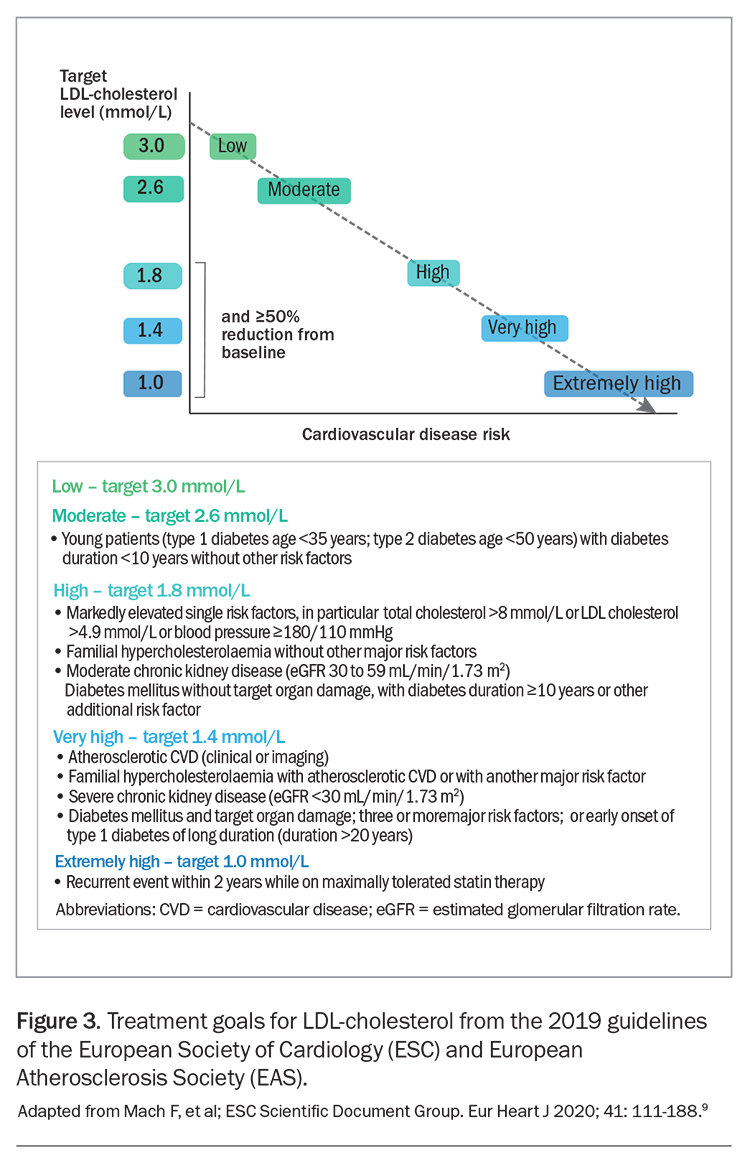
The differing efficacy of specific LDL-lowering drugs and drug combinations allows regimens to be chosen to achieve specific LDL-cholesterol targets (Table 1 and Table 2).6 Recommended LDL-cholesterol targets vary between guidelines. The 2019 guidelines of the European Society of Cardiology and European Atherosclerosis Society recommend targets from 3.0 mmol/L down to 1.0 mmol/L, depending on baseline risk (Figure 3).9 These targets also include a 50% or greater reduction in baseline LDL-cholesterol level. Current Australian LDL-cholesterol targets are less than 2.0 mmol/L for primary prevention and less than 1.8 mmol/L for secondary prevention.16,17
Lowering the LDL-cholesterol level by 2 mmol/L with an effective low-cost statin such as atorvastatin 40 mg daily in 10,000 patients with pre-existing CVD for five years would typically prevent about 1000 CVD events.1 This is a 10% absolute benefit, with a number needed to treat (NNT) of 10 to prevent one event (secondary prevention). The same LDL-cholesterol lowering in people at increased CVD risk with no prior CVD would prevent about 500 events, providing a 5% absolute benefit, with a NNT of 20 (primary prevention). More prolonged therapy would produce even larger absolute benefits.1
Recent randomised controlled trials (RCTs) of statins in combination with nonstatin lipid-lowering drugs (including ezetimibe, PCSK9 inhibitors and bempedoic acid) have shown benefit from LDL-cholesterol lowering to levels below 0.5 mmol/L.18,19 There appears to be no lower limit for benefit from LDL reduction, and there is a log-linear relationship between achieved LDL-cholesterol levels and CVD outcomes (Figure 2).2 Very low LDL-cholesterol levels were found to be safe in the ODYSSEY-OUTCOMES and FOURIER RCTs of PCSK-9 inhibitors, which achieved mean LDL-cholesterol levels of 0.98 mmol/L and 0.78 mmol/L, respectively, with a continuous reduction in CVD events down to LDL-cholesterol levels less than 0.5 mmol/L (Figure 2).2,18,19 The current Australian LDL-cholesterol targets may therefore be regarded as conservative.16,17
Case scenario
Roger is a previously healthy 70-year-old man who has come for the results of a risk assessment check. His blood pressure and thyroid, renal and hepatic function are in the reference range, as are levels of creatine kinase, glucose and vitamin D. His ratio of total cholesterol to HDL cholesterol is 6 and his LDL-cholesterol level is 4.5 mmol/L.
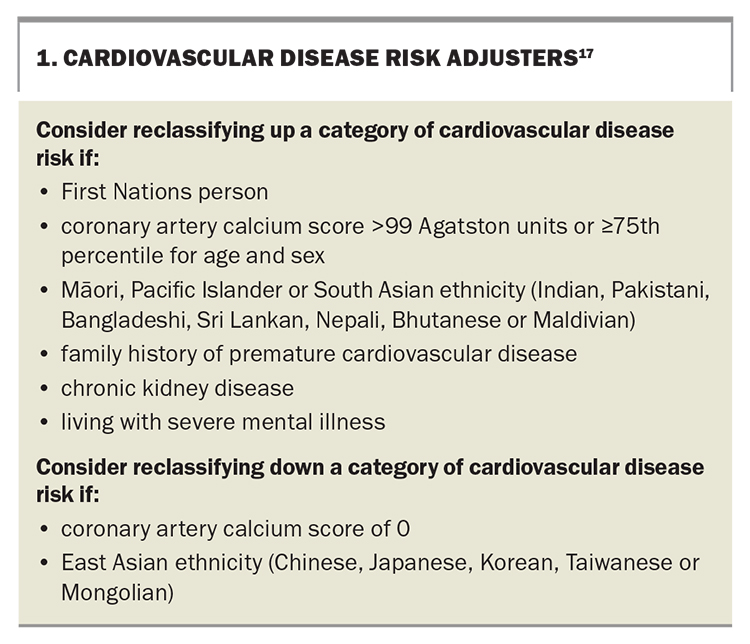
According to the 2023 Australian CVD risk assessment tool, Roger’s five-year CVD risk is 8%, in the intermediate-risk category, for which LLT may be considered if sufficient changes in lifestyle have not improved the lipid profile.16,17 This risk category can be upgraded or downgraded according to other risk factors (Box 1).17
Assessment
A CT scan shows that Roger has a coronary artery calcium score of 455 Agatston units, which is at the 71st percentile for his age and sex.20 His CVD risk is reclassified to high (>10% over five years). Australian guidelines recommend statin therapy for patients aged 70 years and over with a high CVD risk, starting at a low dose if there is significant renal impairment or the potential for drug interactions.16,17
Management
You explain to Roger that he can reduce his risk of future CVD events by reducing his LDL-cholesterol level. You describe the mechanisms by which this occurs, showing images of atherosclerotic plaque formation and rupture causing CVD events, and images of plaque regression with LLT.3 As older people are often more concerned about stroke than heart attack, because of the ensuing disability, you explain his likelihood of having a stroke can also be reduced to a similar degree as that of having a heart attack.21
As a high-risk patient with no previous CVD, Roger’s target LDL-cholesterol level is less than 2.0 mmol/L, requiring a 55% reduction in LDL-cholesterol level. This will require either a high dose of a potent statin (rosuvastatin 20 to 40 mg or atorvastatin 40 to 80 mg) or a lower dose of a statin in combination with ezetimibe (Table 1 and Table 2). In view of Roger’s age, with an increased likelihood of side effects on high-dose statin therapy, you prescribe low-dose statin plus ezetimibe combination therapy (atorvastatin 10 mg and ezetimibe 10 mg single tablet formulation). You also prescribe over-the-counter plant sterols 1 g twice daily, which have additive effects on LDL-cholesterol reduction to ezetimibe as they inhibit cholesterol absorption at a different site.22
Outcome
Two months later, Roger has had no adverse effects of the LLT. His biochemistry test results, including creatine kinase level, are within the reference range, and his LDL-cholesterol level is 1.9 mmol/L. You plan to check his lipid levels, symptoms and general biochemistry test results annually thereafter.
Discussion
Roger’s 55% reduction in LDL-cholesterol level is likely to result in a 55% lower rate of CVD over five years (about 23% lower CVD rate for every 1 mmol/L reduction in LDL-cholesterol level). There will be a further reduction in events with continuing therapy over succeeding decades.
Prescribing lipid-lowering therapy in older people
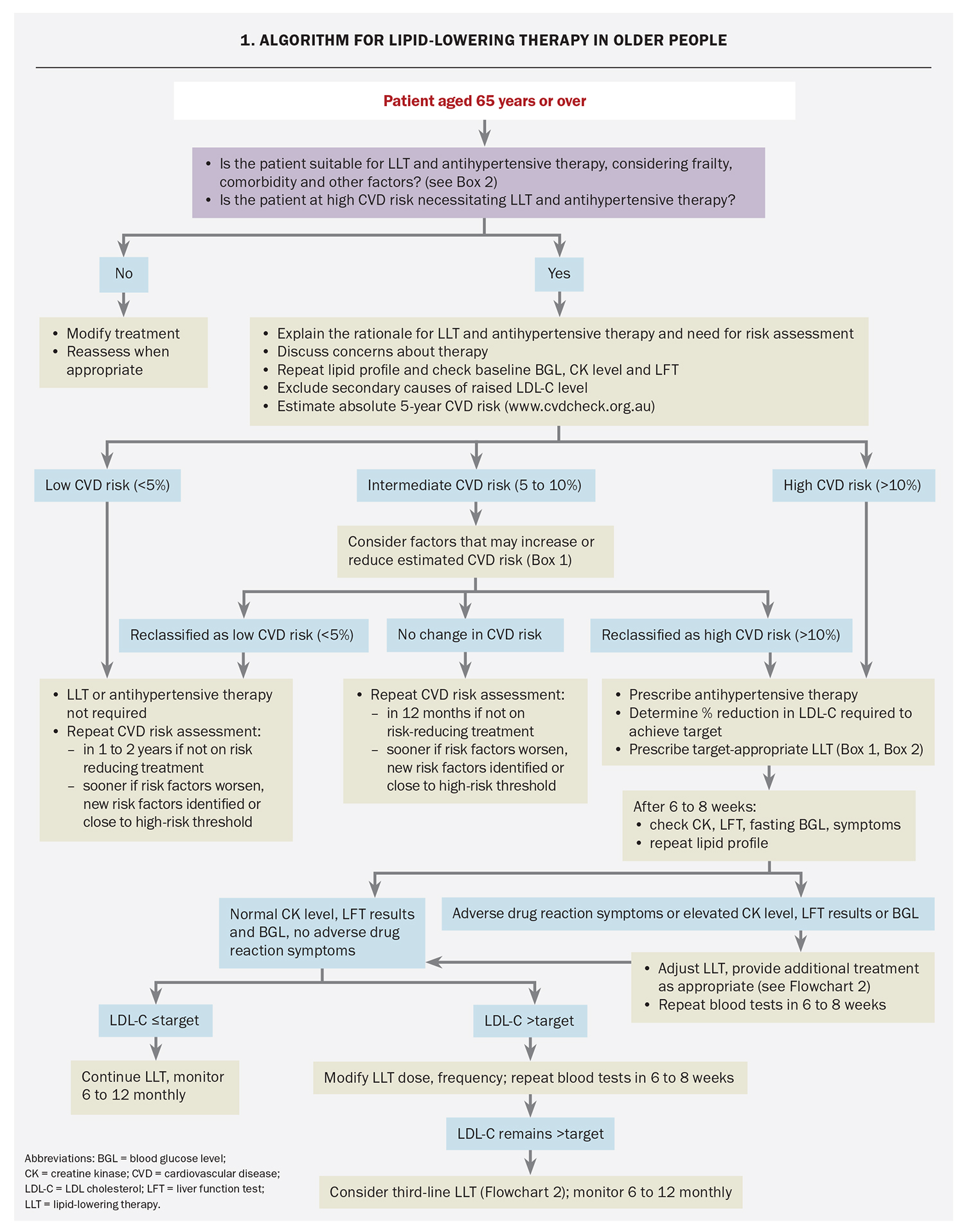
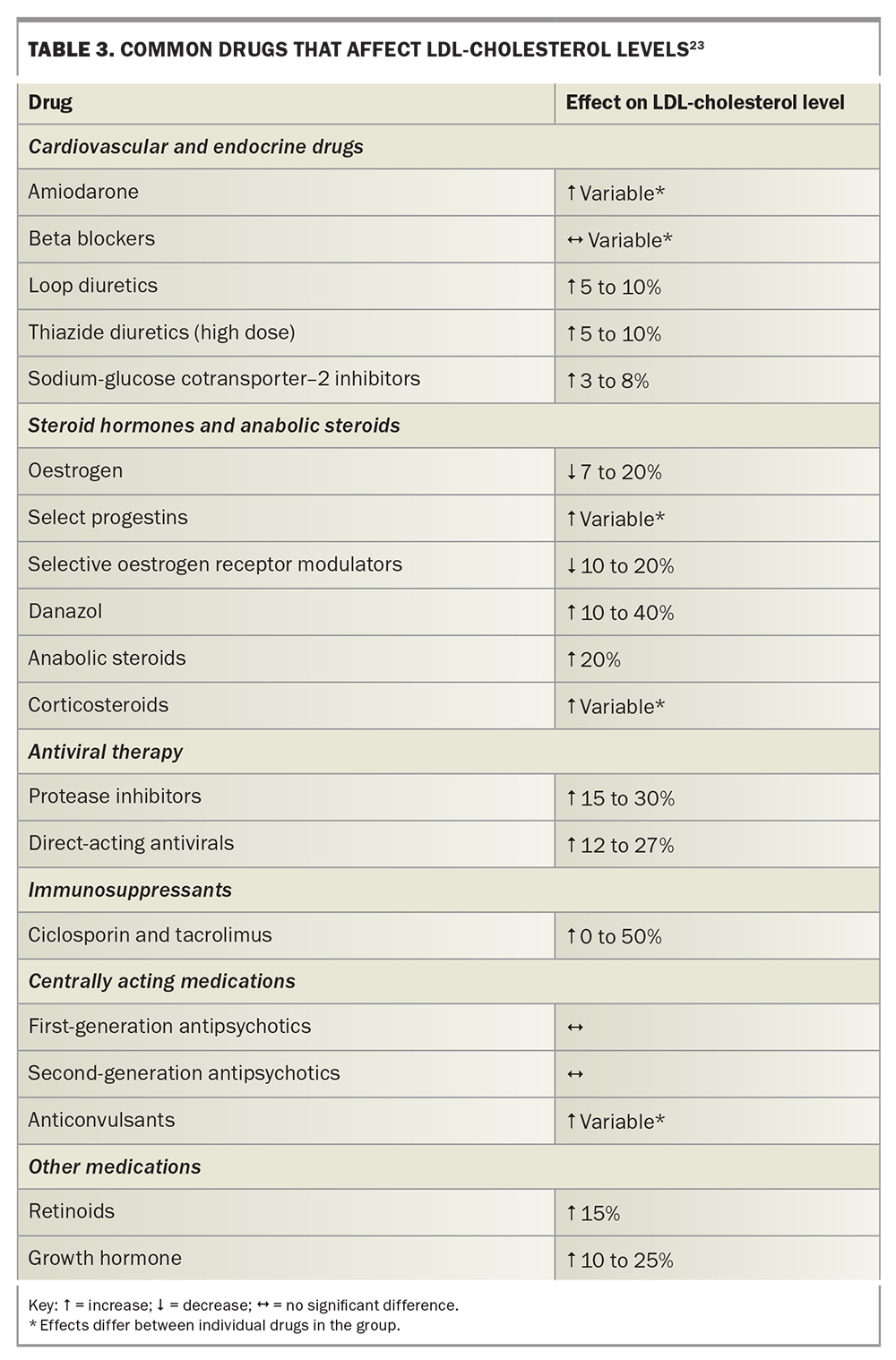
An algorithm for prescribing LLT in older people based on the protocol in our lipid clinic is shown in Flowchart 1. Questions to consider when assessing an older person’s suitability for LLT are shown in Box 2 and medications that can affect LDL-cholesterol level in Table 3.23
In people aged 80 years or over, Australian guidelines recommend not using the Australian CVD Risk Calculator to assess and manage CVD risk, but rather applying individual clinical decision-making.17 Other risk calculators are available to estimate CVD risk in people aged over 80 years but have not been validated in an Australian population.24
Considerations in older people
Considerations about LLT in older patients include possible limited life expectancy, as the event curves for placebo and active treatments in RCTs generally do not diverge until 12 to 24 months after starting therapy. Other considerations include the higher risk of drug interactions because of polypharmacy and the risk of adverse effects such as new-onset diabetes and statin-associated muscle symptoms, particularly with high-intensity statin use and statin use in women with low muscle mass.
Muscle symptoms
A review of the effect of statin therapy on muscle symptoms was included in a recently published meta-analysis from the Cholesterol Treatment Trialists’ (CTT) Collaboration.25 It concluded that statins caused a small excess of muscle pain that is mostly mild, and that more than 90% of reported muscle symptoms were not due to statin therapy. Creatine kinase levels were increased by 0.02 times the upper limit of the reference range, a clinically insignificant rise. Over four years, muscle symptoms were reported by 26.6% of participants in the placebo group and 27.1% receiving statin therapy. Severe statin-associated muscle symptoms (SAMS) such as myositis and rhabdomyolysis were rare.25
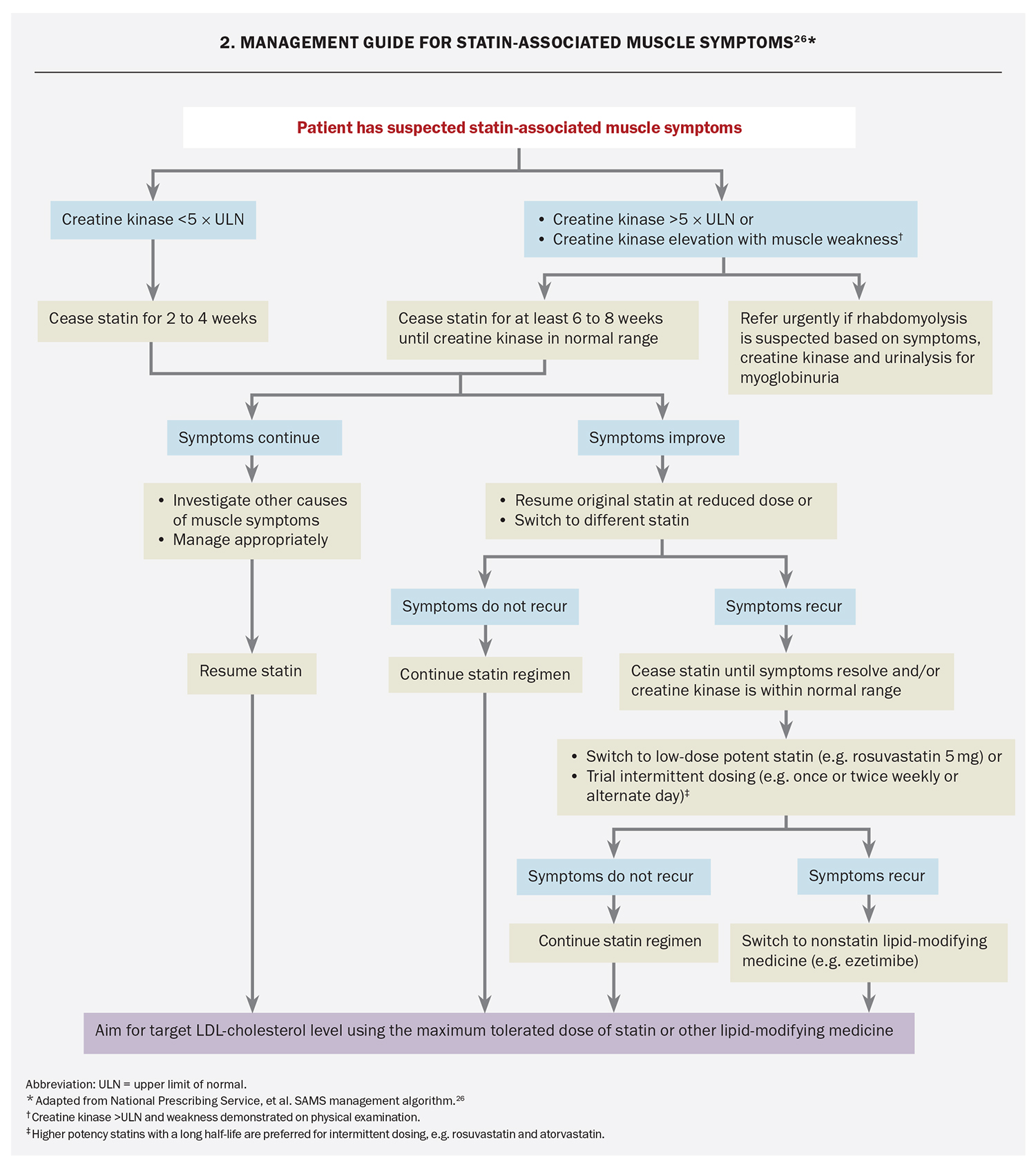
Before initiating a statin, it is important to exclude hypothyroidism and low vitamin D levels, which can increase the risk of SAMS. Recommended management of statin-associated myalgia and myopathy is shown in Flowchart 2.26
An increase in hepatic alanine aminotransferase or aspartate aminotransferase levels to more than three times the upper limit of normal (ULN) requires withdrawal or dose reduction of the statin, checking of alcohol intake and other drug therapy and monitoring until levels are less than three times the ULN. At this time, statin therapy can be resumed at a lower dose or with a different statin.27
New-onset diabetes mellitus
Statin therapy is associated with an increased incidence of new-onset diabetes mellitus in people with fasting hyperglycaemia (pre-diabetes), central abdominal obesity and insulin resistance.28 Multiple meta-analyses of studies in older patients have shown a 10% increase in the rate of new-onset diabetes mellitus, the rate increasing in proportion to dose and duration of statin therapy.28 Glycaemic status should therefore be assessed in older people before and during statin therapy, and preventive measures taken as necessary.
Other potential adverse effects
Some RCTs have reported a small and statistically insignificant increased incidence of cerebral haemorrhage, whereas a recent Danish nationwide case-control study showed a lower risk of cerebral haemorrhage with current statin use and a longer duration of statin therapy.29 Meta-analyses have shown no evidence for increased cancer incidence or cognitive impairment. Contemporary LLT has not been associated with cognitive impairment in RCTs, and low LDL-cholesterol levels are not associated with an adverse effect on cognitive disorders or global cognitive performance.30
Statin-ezetimibe combination therapy
Evidence supporting combination therapy
Evidence from three clinical trials and secondary analyses supports treating high-risk older patients with the combination of ezetimibe and a lower-dose statin rather than high-intensity statin therapy. IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) showed that ezetimibe-simvastatin cotherapy led to greater reductions in CVD than moderate-intensity statin monotherapy.31 The greatest absolute CVD risk reduction was in people aged over 75 years.31 The reduction in CVD events was proportional to the reduction in LDL-cholesterol level, with the regression line for statin-ezetimibe cotherapy being continuous with that for statin alone, as found in other RCTs.
The RACING trial compared ezetimibe- statin co-therapy with statin monotherapy in patients at high CVD risk.32 In patients aged 75 years or over, there was no significant difference in the primary endpoint in the co-therapy group (10.6%) versus the high-intensity statin group (12.3%). The cotherapy group achieved a lower median LDL-cholesterol level (1.5 mmol/L vs 1.6 mmol/L), a two-thirds lower rate of therapy discontinuations and 50% lower rate of new-onset diabetes.
EWTOPIA 75 (Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerotic Cardiovascular Disease in 75 or Older), enrolled Japanese adults aged 75 years or over without a history of CVD.33 Those treated with ezetimibe had reduced CVD events (a composite of sudden cardiac death, myocardial infarction, coronary revascularisation or stroke).
Two RCTs of atorvastatin 40 mg daily are currently underway in older people: PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-lowering in Older Adults) and STAREE (A Clinical Trial of Statin Therapy for Reducing Events in the Elderly).34,35 In STAREE, 56% of participants are aged 70 to 74 years, 27% are 75 to 79 years, 12% are 80 to 84 years and 5% are 85 years or over. With treatment adherence, atorvastatin 40 mg daily is expected to reduce CVD rates by 25%, and improve the rate of disability- free survival by 18%.
Prescribing statin-ezetimibe combination therapy
Based on this evidence, we recommend initiating LLT in older people with a low-dose statin in combination with ezetimibe, rather than a high-dose statin. This strategy is also being used currently for people with familial hypercholesterolaemia.36
Current PBS eligibility requirements for prescribing ezetimibe are:
- inadequate control of cholesterol levels after three months of statin therapy in conjunction with dietary therapy and exercise (streamlined authority item 7996) or
- statin intolerance (item 7966).37
Unlike statins and fibrates, ezetimibe is not listed among drugs that can be prescribed at any cholesterol level and without a previous trial of dietary therapy in patients at high CVD risk.
The PBS restrictions on prescribing ezetimibe, based on a postmarket review submitted to the Pharmaceutical Benefits Advisory Committee in 2017, aim to enhance optimal uptitration of statins. This paradigm is no longer appropriate as CVD benefit depends on LDL-cholesterol reduction, independent of the drug used. We believe combination therapy with lower drug doses is a preferable strategy, as for the treatment of hypertension. Further, there seems little justification to require authority approval for a medication that is widely used and has a robust database supporting its safety and efficacy. We believe the PBS criteria for LLT with ezetimibe need to be modified accordingly.
Conclusion
LLT in older people requires a greater degree of diligence in monitoring adherence and side effects. Single-tablet low-dose statin-ezetimibe combination LLT should be used wherever possible. LLT in older people is supported by robust evidence from RCTs and observational studies. Unless found to be unsuitable, LLT should be considered in every older person. MT
COMPETING INTERESTS: Professor Ian Hamilton-Craig is honorary Chairman of the SA Lipid Group, sponsored by Amgen (Australia). Professor Christian Hamilton-Craig received speaker fees from Amgen and Novartis.
References
1. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 388: 2532-2561. Erratum in: Lancet 2017; 389: 602.
2. Robinson JG, Jayanna MB, Bairey Merz CN, Stone NJ. Clinical implications of the log linear association between LDL-C lowering and cardiovascular risk reduction: greatest benefits when LDL-C >100 mg/dL. PLoS One 2020; 15: e0240166.
3. Hamilton-Craig IR. Lipid-lowering therapy for older people: the changing paradigm. Med Today 2024; 25(1-2): 39-45.
4. Hamilton-Craig I, Hamilton-Craig C, Kostner K. Management of hyperlipidemia in the elderly. In: Davidson M, Toth PP, Maki KC, editors. Therapeutic lipidology. Springer Naturel 2021.
5. Hamilton-Craig I, Colquhoun D, Kostner K, Woodhouse S, d’Emden M. Lipid-modifying therapy in the elderly. Vasc Health Risk Manag 2015; 11: 251-263.
6. Masana L, Ibarretxe D, Plana N. Reasons why combination therapy should be the new standard of care to achieve the LDL-cholesterol targets: lipid-lowering combination therapy. Curr Cardiol Rep 2020; 22: 66.
7. Lee SH, Lee YJ, Heo JH, et al. Combination moderate-intensity statin and ezetimibe therapy for elderly patients with atherosclerosis. J Am Coll Cardiol 2023; 81: 1339-1349.
8. Hamilton-Craig C, Kostner K, Colquhoun D, Nicholls SJ. Omega-3 fatty acids and cardiovascular prevention: is the jury still out? Intern Med J 2023; 53: 2330-2335.
9. Mach F, Baigent C, Catapano AL, et al; ESC Scientific Document Group. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111-188. Erratum in: Eur Heart J. 2020; 41:4255.
10. Cheung B, Sikand G, Dineen EH, et al. Lipid-lowering nutraceuticals for an integrative approach to dyslipidemia. J Clin Med 2023; 12: 3414.
11. Banach M, Catapano AL, Cicero AFG, et al; International Lipid Expert Panel. Red yeast rice for dyslipidaemias and cardiovascular risk reduction: a position paper of the International Lipid Expert Panel. Pharmacol Res 2022; 183: 106370.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018; 378: e34.
13. Nicholls SJ, Nelson AJ, Lincoff AM, et al. Impact of bempedoic acid on total cardiovascular events: a prespecified analysis of the CLEAR outcomes randomized clinical trial. JAMA Cardiol 2024: e235155.
14. Goyal A, Changez MIK, Tariq MD, et al. Efficacy and outcomes of bempedoic acid versus placebo in patients with statin-intolerance: a pilot systematic review and meta-analysis of randomized controlled trials. Curr Probl Cardiol 2024; 49: 102236.
15. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019; 380: 11-22.
16. Heart Foundation. Practical guide to pharmacological lipid management. Available online at: https://www.heartfoundation.org.au/bundles/heart-health-check-toolkit/pharmacological-lipid (accessed March 2024).
17. Australian CVD risk calculator. Canberra: Australian Government Department of Health and Aged Care. Available online at: https://www.cvdcheck.org.au/calculator (accessed March 2024).
18. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713-1722.
19. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018; 379: 2097-2107.
20. McClelland RL, Chung H, Detrano R, et al. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006; 113: 30-37.
21. Stolker JM, Spertus JA, Cohen DJ, et al. Rethinking composite end points in clinical trials: insights from patients and trialists. Circulation 2014; 130: 1254-1261.
22. Jakulj L, Trip MD, Sudhop T, et al. Inhibition of cholesterol absorption by the combination of dietary plant sterols and ezetimibe: effects on plasma lipid levels. J Lipid Res 2005; 46: 2692-2698.
23. Herink M, Ito MK. Medication induced changes in lipid and lipoproteins. In: Feingold KR, Anawalt B, Blackman MR, et al, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com; 2018. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK326739/ (accessed March 2024).
24. SCORE2-OP Working Group and ESC Cardiovascular Risk Collaboration. SCORE2-OP risk prediction algorithms: estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur Heart J 2021; 42: 2455-2467.
25. Cholesterol Treatment Trialists’ Collaboration. Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials. Lancet 2022; 400: 832-845.
26. National Prescribing Service; Colquhoun D, Hamilton-Craig I, Harris M, et al. SAMS management algorithm. Sydney: NPS MedicineWise; 2017. Available online at: https://www.nps.org.au/assets/06f38ae10ff0b5b7-009a670e9bda-SAMS-Management-Algorithm.pdf (accessed March 2024).
27. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation 2002; 106: 1024-1028.
28. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010; 375: 735-742.
29. Rudolph DA, Hald SM, Rodríguezm LAG, et al. Association of long-term statin use with the risk of intracerebral hemorrhage. a Danish nationwide case-control study. Neurology 2022; 99: e711-e719.
30. Ying H, Wang J, Shen Z, Wang M, Zhou B. Impact of lowering low-density lipoprotein cholesterol with contemporary lipid-lowering medicines on cognitive function: a systematic review and meta-analysis. Cardiovasc Drugs Ther 2021; 35: 153-166.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372: 2387-2397.
32. Kim BK, Hong SJ, Lee YJ, et all RACING Investigators. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet 2022; 400: 380-390.
33. Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation 2019; 140: 992-1003.
34. Joseph J, Pajewski NM, Dolor RJ, et al; PREVENTABLE Trial Research Group. Pragmatic evaluation of events and benefits of lipid lowering in older adults (PREVENTABLE): trial design and rationale. J Am Geriatr Soc 2023; 71: 1701-1713.
35. Zoungas S, Curtis A, Spark S, et al; STAREE Investigator Group. Statins for extension of disability-free survival and primary prevention of cardiovascular events among older people: protocol for a randomised controlled trial in primary care (STAREE trial). BMJ Open 2023; 13: e069915.
36. Song C, Rosenson RS. Competing genetic traits and their influence on LDL cholesterol concentration in familial hypercholesterolemia. JACC Case Rep 2023; 29: 102171.
37. Pharmaceutical Benefits Scheme. Ezetimibe. Canberra: Australian Government Department of Health and Aged Care. Available online at: https://www.pbs.gov.au/medicine/item/8757X (accessed March 2024).