From Nobel Prize to clinical practice: an update on messenger RNA-based therapies for lipid disorders

The biological mechanism of gene silencing by messenger RNA (mRNA) inhibition has been harnessed to develop therapies to target specific proteins, such as those involved in lipid metabolism. The first of these, inclisiran, is available in Australia for reducing levels of LDL-cholesterol and is PBS listed for people with familial and nonfamilial hypercholesterolaemia.
- Inhibition of messenger RNA (mRNA) and of target protein synthesis (RNA interference) is now possible using either silencing RNA or antisense oligonucleotide therapy.
- These types of therapies currently require subcutaneous injection.
- Inclisiran is the first siRNA proprotein convertase subtilisin– kexin type 9 (PCSK9) inhibitor to be listed on the PBS. It has similar efficacy to available monoclonal antibody PCSK9 inhibitors but a longer duration of action allowing six-monthly subcutaneous injections after completion of basal loading doses.
- Inclisiran, in combination with high-dose statins and ezetimibe, can reduce LDL-cholesterol levels by more than 80%, with a further reduction in cardiovascular (CVD) events likely to occur in the longer term.
- Results from a randomised controlled trial on CVD outcomes with inclisiran are expected in 2026.
On January 6, 2006, the Nobel prize in Physiology or Medicine was awarded to Professor Andrew Fire and Professor Craig Mello for the discovery of RNA interference – using double-stranded RNA to ‘silence’ genes – following publication of their research in Nature on 19 February, 1998.1 The Nobel Prize press release stated: ‘RNA interference is already being widely used in basic science as a method to study the function of genes and it may lead to novel therapies in the future’.2 This prediction has been fulfilled. Since 2006, research on messenger RNA (mRNA) therapy has resulted in the development of several drugs that inhibit the activity of key mRNA and protein targets. This article outlines the mechanisms of RNA interference and mRNA targets involved in lipid metabolism and discusses the first of these lipid-lowering therapies available in Australia.
mRNA interference: targets and mechanisms
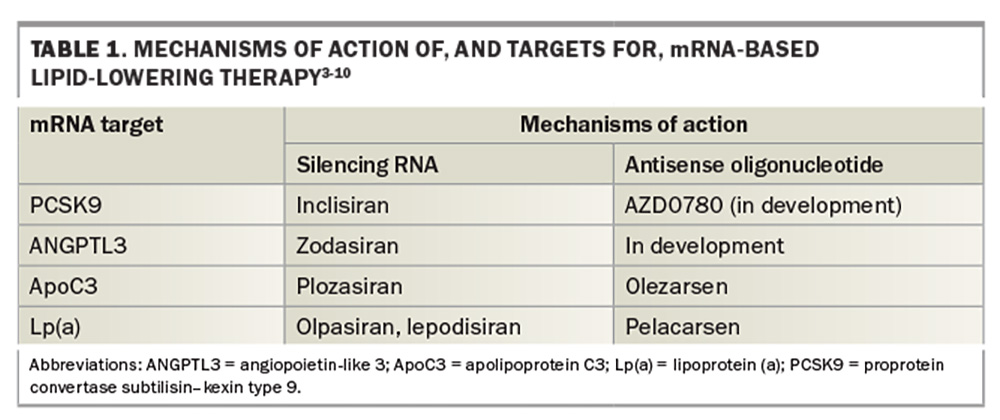
The mRNA targets and drugs currently available or in development and their associated mechanisms of action are outlined in Table 1, and are being studied in several clinical trials, including ACCLAIM-Lp(a) (ClinicalTrials.gov ID NCT06292013), OCEAN(a) (ClinicalTrials.gov ID NCT05581303) and LP(a)HORIZON (ClinicalTrials.gov ID NCT04023552) trials. Targets include the following.3-10
- Proprotein convertase subtilisin–kexin type 9 (PCSK9), which regulates activity of the LDL-receptor in the liver. Inhibiting PCSK9 results in greater clearance of LDL-cholesterol from the plasma and lower LDL-cholesterol levels.
- Angiopoietin-like 3 (ANGPTL3), an enzyme involved in triglyceride (TG) catabolism, the inhibition of which results in lower plasma levels of TG and LDL-cholesterol.
- Apolipoprotein C3 (ApoC3), a potent inhibitor of lipoprotein lipase (LPL), the enzyme promoting TG catabolism to free fatty acids. Inhibition of apoC3 results in LPL activation and lower plasma TG levels.
- Apolipoprotein (a) [Apo(a)], a protein attached to LDL to form lipoprotein (a) [Lp(a)], an atherogenic and prothrombotic lipoprotein that is an important risk factor for premature CVD.
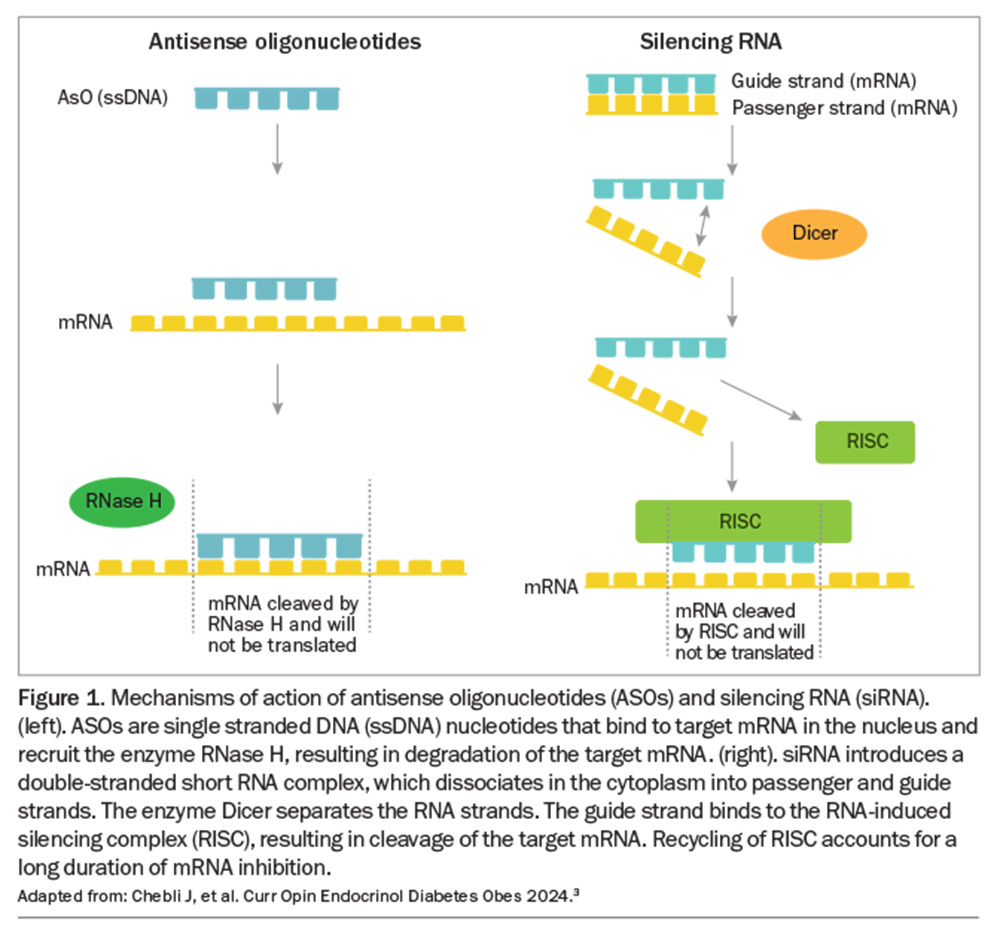
The two mechanisms of targeting mRNA involved in lipid metabolism are antisense oligonucleotides (ASOs) and silencing RNA (siRNA), summarised in Figure 1. These techniques promise a significant benefit in reducing cardiovascular (CVD) events through improved control of lipid-related CVD risk factors.3-10
ASOs are small single-stranded nucleic acid sequences that bind with complementary target mRNA within the nucleus, resulting in degradation and inactivity of the mRNA (Figure 1).3
siRNA uses a 20 to 25 nucleotide sequence of double-stranded RNA, of which one strand (the guide strand) is complementary with and binds to the mRNA sequence to be silenced, while the other strand (the passenger strand) is eventually degraded in the cytoplasm (Figure 1).3 The enzyme Dicer separates each strand of the double-stranded RNA. The guide RNA then complexes with the RNA-induced silencing complex (RISC), resulting in degradation of the target mRNA sequence, thereby preventing synthesis of the target protein.3 Recycling of RISC and its attached guide strand account for the prolonged (three to six months) duration of activity of a single dose of siRNA.
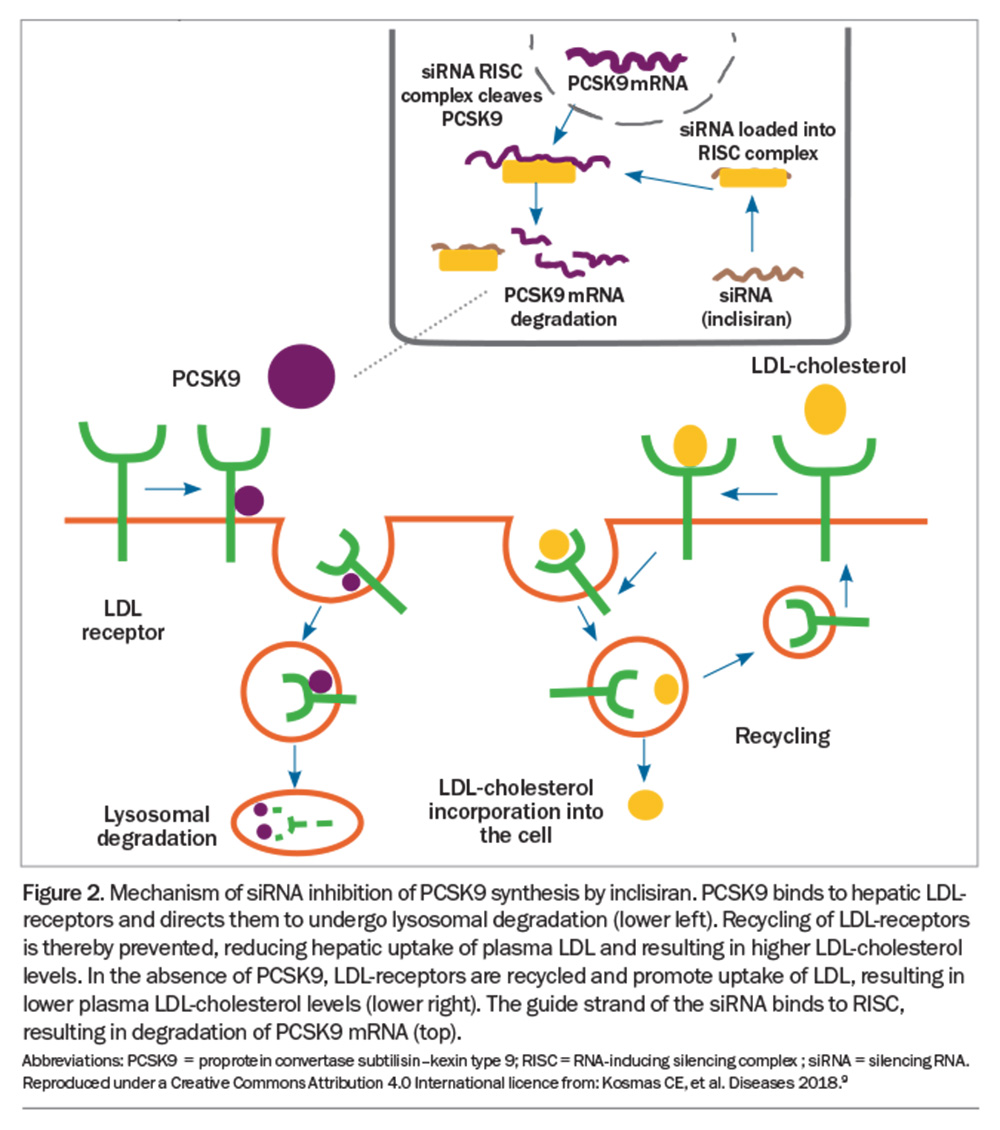
Therapy with apoC3 siRNA restores LPL activity, resulting in a significant reduction of plasma TG levels. Silencing PCSK9 mRNA with inclisiran uses similar mechanisms, involving the actions of Dicer and RISC, resulting in upregulation of hepatic LDL-receptors, increased uptake of LDL-cholesterol into the liver, and about a 50% further reduction in lower plasma LDL-cholesterol levels than that achieved with high-dose statins and ezetimibe (Figure 2).9,10
The potency and long duration of action of siRNA therapies and their potential for reducing CVD events is shown by the close to 100% inhibition of plasma Lp(a) levels over a 12-month period with a single injection of the siRNA lepodisiran.6 Zilebesiran, an siRNA drug for inhibiting angiotensinogen, has also been shown to reduce levels of its target protein by almost 100% over 12 weeks after a single injection.6,11
Inclisiran
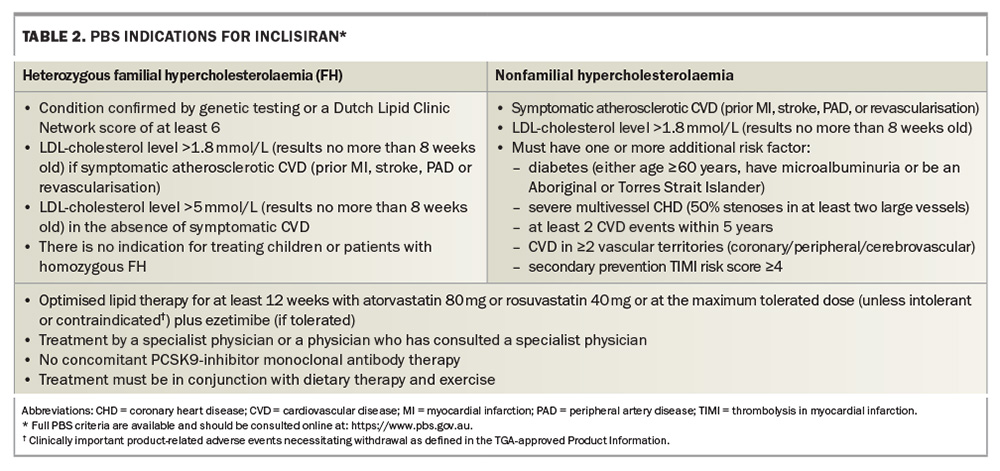
Inclisiran has undergone seven phase 3 clinical trials (the ORION clinical development program) to determine its LDL-cholesterol-lowering efficacy and potential side effects.12-14 This innovative lipid-lowering mRNA therapy inhibits PCSK9 and is indicated for the treatment of patients with symptomatic CVD and elevated LDL-cholesterol levels while taking maximally tolerated oral lipid-lowering therapy or for patients who are statin intolerant. It is available as an injectable therapy and PBS-listed (since 1 April, 2024) for patients with familial heterozygous hypercholesterolaemia and nonfamilial hypercholesterolaemia (Table 2).
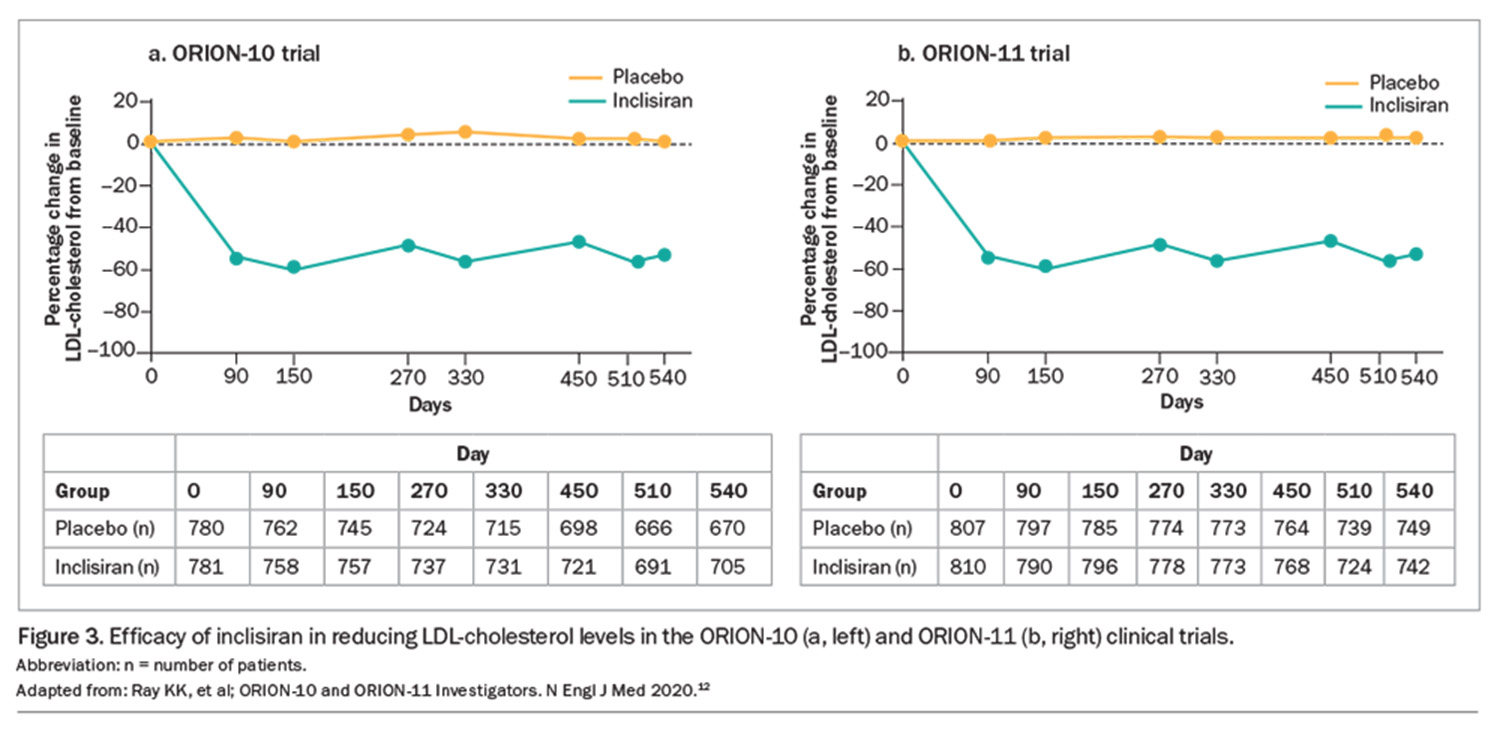
Inclisiran has a prolonged duration of action, which differentiates if from the injectable monoclonal antibody inhibitors of PCSK9. About 50% LDL-cholesterol reduction can be achieved with an initial dose of inclisiran, a second dose after three months and subsequent six-monthly dosing intervals over 18 months, compared with placebo (Figure 3).12
The efficacy of 180 days of inclisiran therapy compared with placebo in individual patients is shown in Figure 4.12 Patients taking inclisiran had a consistent reduction in LDL-cholesterol levels (mean, 52.6%; maximum, 80.9%) compared with those on placebo, who had little change in mean LDL-cholesterol levels and considerable variation between individuals. In a pooled analysis of seven ORION trials, inclisiran therapy achieved target LDL-cholesterol levels below 1.8 mmol/L and below 1.3 mmol/L in 87% and 75% of patients, respectively.15
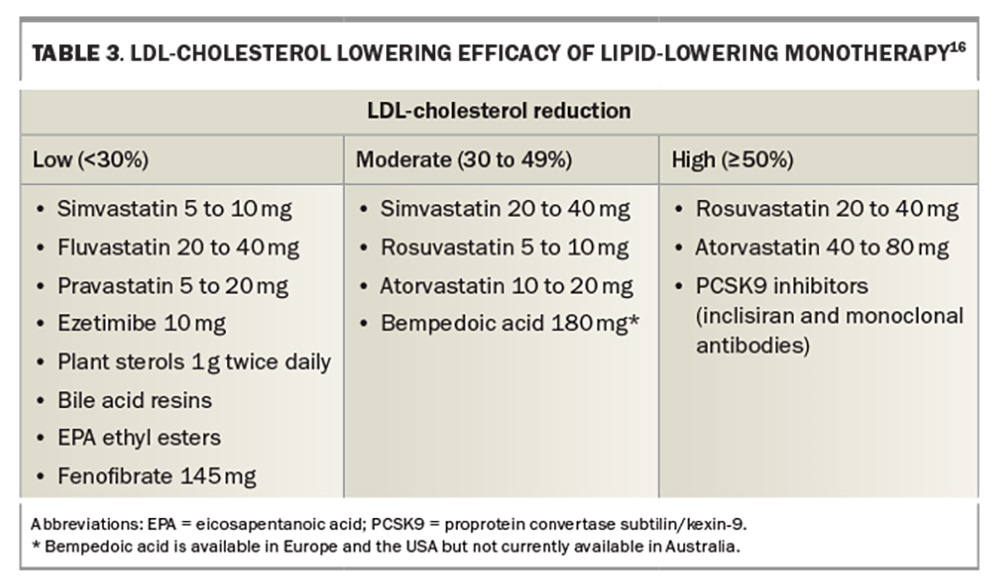
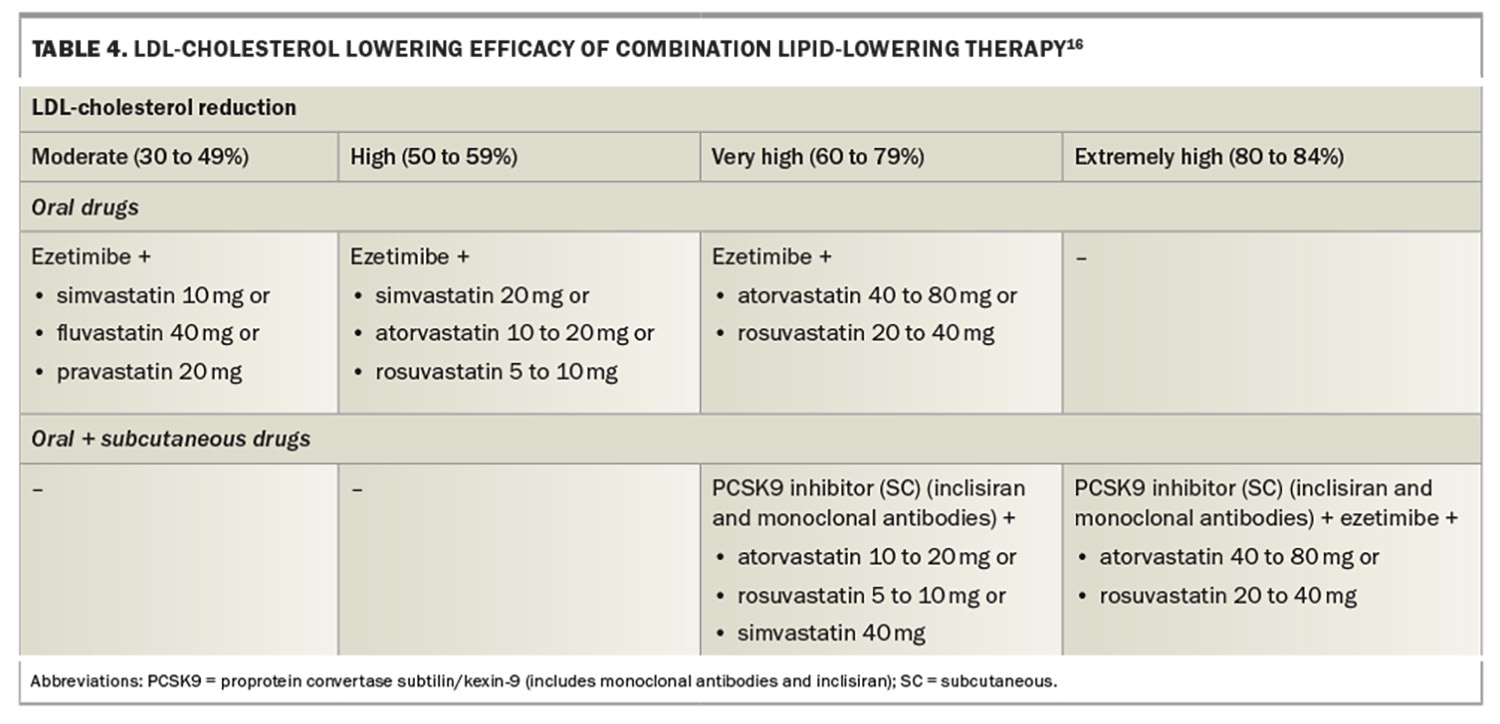
The current paradigm for lipid-lowering therapy is to achieve the lowest possible LDL-cholesterol levels for the longest possible duration, starting at the lowest suitable age. ‘Earlier, lower and longer’ is the current paradigm for LDL-cholesterol reduction regardless of therapy choice, including statins, PCSK9 inhibitors, ezetimibe, bile acid-binding resins, fibrates or bempedoic acid (not yet available in Australia). Lipid-lowering monotherapy or specific combinations of lipid-lowering drugs can be used to achieve specific LDL-cholesterol targets (Table 3 and Table 4, respectively).16 Current targets for LDL-cholesterol depend on underlying CVD risk. Australian guidelines suggest targets of less than 2 mmol/L for primary prevention and less than 1.8 mmol/L for secondary prevention, consistent with PBS criteria for PCSK9 inhibitors (Table 2).9 Other guidelines, such as the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) guidelines have targets of less than 1.4 mmol/L for those with very high CVD risk (Figure 5).17 Randomised controlled CVD outcome trials using monoclonal antibody PCSK9 inhibitors have achieved mean LDLcholesterol levels of 0.8 to 0.9 mmol/L, with a significant proportion of patients with levels below 0.5mmol/L and approaching physiological LDL-cholesterol levels between 0.6 and 1.3 mmol/L.18-20
Advantages
Inclisiran is conjugated with N-acetylgalactosamine (GalNAc), a ligand that specifically binds to hepatic carbohydrate receptors. It is therefore specifically absorbed by the liver and its actions are liver-specific and do not directly affect other tissues.
Patient adherence is a key issue for all drugs. The prolonged activity of inclisiran requires a low frequency of administration compared with other PCSK9 inhibitor therapies and is likely to improve adherence. Inclisiran is given as an injection at six-monthly intervals, compared with monthly or fortnightly injections of monoclonal antibody PCSK9 inhibitors and oral medications. Additionally, it does not affect hepatic cytochromes and has a low potential for drug interactions.
The efficacy of inclisiran in lowering LDL-cholesterol levels is comparable with monoclonal antibody PCSK9 inhibitors. A recent meta-analysis, however, showed slightly lower LDL-cholesterol levels after 24 weeks with the addition of monoclonal antibodies to maximally tolerated statins (mean percentage reduction with evolocumab 61.8% vs 50.1% with inclisiran).21 ApoB reduction was also greater with evolocumab (58.4% vs 45.1%).21 Like monoclonal antibodies, inclisiran also reduces levels of non-HDL cholesterol by 40 to 50% and Lp(a) by 20 to 30% thereby contributing significantly to CVD risk reduction in the longer term.12,13,22 There are no clinically significant changes in HDL-cholesterol and triglyceride levels with inclisiran treatment.
Adverse events and contraindications
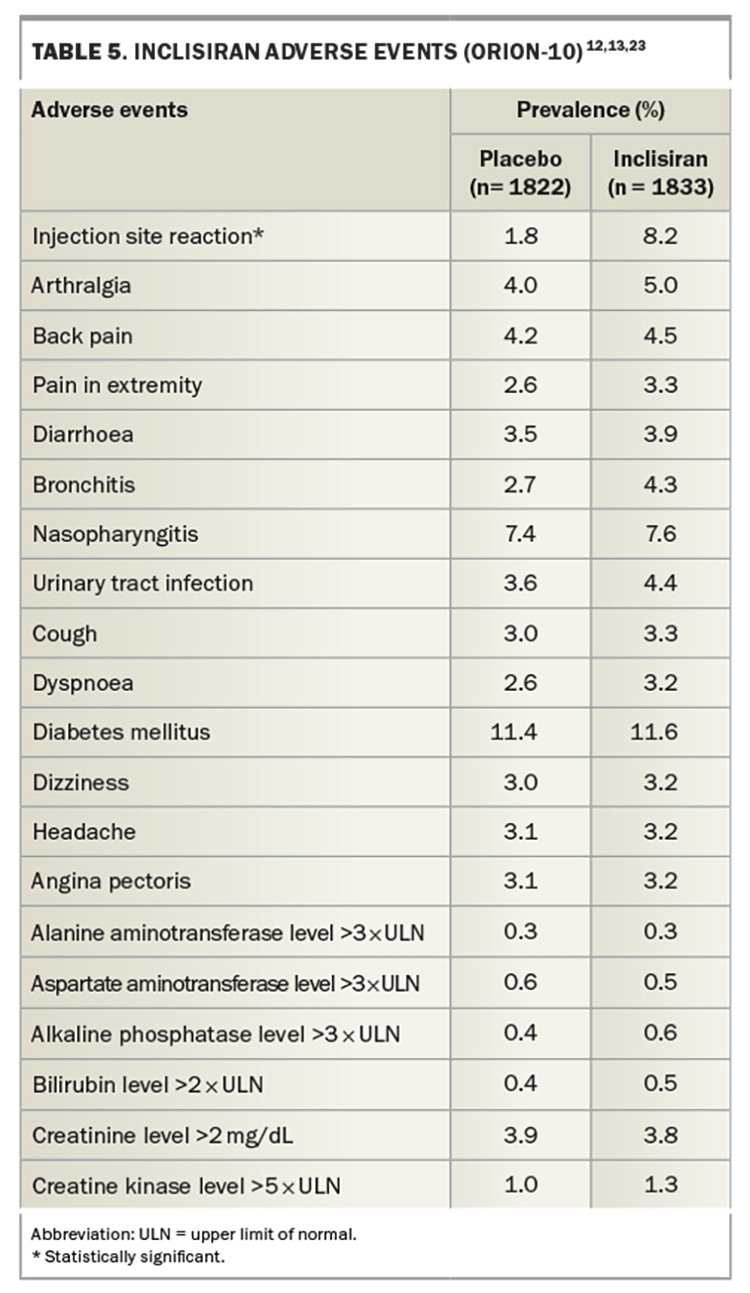
Adverse events in inclisiran-treated and placebo groups in the ORION-10 trial are summarised in Table 5.12 Adverse events were similar for both groups, with the exception of local skin reactions, which occurred more frequently with inclisiran, but were usually mild and reversible.12
Elevated alanine and aspartate transaminase levels (one to three times the upper limit of normal) seen in randomised controlled trials with inclisiran use were similar to those seen in the placebo group.12,13,22 According to a recent review of drug-induced liver injury, regular monitoring of routine liver tests is not recommended with inclisiran as elevated serum alanine transaminase levels were invariably transient, mild to moderate in severity, and without accompanying symptoms or jaundice.23 A study of patients with mild to moderate hepatic impairment showed clinically significant increases in liver enzymes both before and after inclisiran therapy, which is likely to be attributed to underlying liver disease.24 No enzyme elevation occurred in those with normal liver function.24
Inclisiran therapy is not recommended for patients with severe liver disease and end-stage renal disease (creatine clearance rate <15 mL/min) and should be used with caution in patient with severe renal impairment.25 It is approved for use with no dose adjustment in patients with mild or moderate liver disease, those with mild, moderate or severe renal failure or in older people.25
Long-term outcomes
Cost-efficacy data from long-term CVD outcomes studies are not yet available, as the first outcome data are expected in 2026 with the results of the ORION-4 randomised control trial in over 16,000 patients with prior CVD, treated over a period of 5 years.26 Other inclisiran trials in patients with and without prior CVD (VICTORION-2 PREVENT and VICTORION-1 PREVENT) are expected to report in 2027-29.27,28
An exploratory analysis of 3655 participants from the ORION-9, -10 and -11 trials, using nonadjudicated pooled patient-level data, showed a significant 26% reduction in prespecified CVD events, including cardiac death, cardiac arrest, nonfatal myocardial infarction and stroke (fatal and nonfatal), along with a 50.6% (1.37 mmol/L) reduction in LDL-cholesterol.13 Reductions in non-prespecified CVD events (20% reduction in total myocardial infarction and 14% reduction in total strokes) were not statistically significant. The ORION-9 to -11 meta-analyses were not powered for CVD outcomes, but these exploratory data are encouraging.
Conclusion
The post-statin era of genetic manipulation by targeting mRNA is now established, with effective therapies for certain lipid disorders and new therapies likely to become available for clinical practice in the near future. Control of LDL-cholesterol levels, however, remains the major focus of current lipid therapy. mRNA-based PCSK9 inhibition with inclisiran expands the selection of available drugs for LDL-cholesterol control and, when added to statins and ezetimibe, can achieve target LDL-cholesterol levels with a high degree of efficacy and safety in most patients. MT
COMPETING INTERESTS: Professor Hamilton-Craig is honorary Chairman of the SA Lipid Group, sponsored by Amgen (Australia).
This article is for general information purposes only, and the full Product Information should be consulted before prescribing any of the mentioned medications.
References
1. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391: 806-11.
2. Nobel Prize Committee Press Release 6 October 2006. Available online at: https://www.nobelprize.org/prizes/medicine/2006/press-release/ (accessed July 2024).
3. Chebli J, Larouche M, Gaudet D. APOC3 siRNA and ASO therapy for dyslipidemia. Curr Opin Endocrinol Diabetes Obes 2024; 31: 70-77.
4. Fu Q, Hu L, Shen T, Yang R, Jiang L. Recent advances in gene therapy for familial hypercholesterolemia: an update review. J Clin Med 2022; 11: 6773.
5. Brandts J, Ray KK. Novel and future lipid-modulating therapies for the prevention of cardiovascular disease. Nat Rev Cardiol 2023; 20: 600-616.
6. Wilson FP. Commentary: The future of medicine is RNA. Medscape, November 2023. Available online at: https://www.medscape.com/viewarticle/998363?form=fpf (accessed July 2024).
7. Watts GF, Chan DC. RNA interference therapy for targeting ANGPTL3 and atherogenic lipoproteins: Findings and implications of a recent phase I study. Clin Transl Med 2023; 13: e1484.
8. Gennemark P, Walter K, Clemmensen N, et al. An oral antisense oligonucleotide for PCSK9 inhibition. Sci Transl Med 2021; 13: eabe9117.
9. Kosmas CE, Muñoz Estrella A, Sourlas A, et al. Inclisiran: a new promising agent in the management of hypercholesterolemia. Diseases 2018; 6: 63.
10. Friedrich M, Aigner A. Therapeutic siRNA: state-of-the-art and future perspectives. BioDrugs 2022; 36: 549-571.
11. Desai AS, Webb DJ, Taubel J. Zilebesiran, an RNA interference therapeutic agent for hypertension. N Engl J Med 2023; 389: 228-238.
12. Ray KK, Wright RS, Kallend D, et al; ORION-10 and ORION-11 Investigators. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020; 382: 1507-1519.
13. Ray KK, Raal FJ, Kallend DG, et al; ORION Phase III investigators. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J 2023; 44: 129-138.
14. Stoekenbroek RM, Kallend D, Wijngaard PL, Kastelein JJ. Inclisiran for the treatment of cardiovascular disease: the ORION clinical development program. Future Cardiol 2018; 14: 433-442.
15. Wright RS, Koenig W, Landmesser U, et al. Safety and tolerability of inclisiran for treatment of hypercholesterolemia in 7 clinical trials. J Am Coll Cardiol 2023; 82: 2251-2261.
16. Hamilton-Craig IR, Hamilton-Craig CR. Lipid lowering therapy for older people. Update on prescribing. Medic Today 2024; 25: 49-58.
17. Mach F, Baigent C, Catapano, et al; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111-188.
18. Morales-Villegas EC, Ray KK. Physiological level of LDL cholesterol: the master key a nobel dream comes true. Cardiovasc Pharm Open access 2017; 6: 223.
19. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713-1722.
20. Schwartz GG, Steg PG, Szarek Ml, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018; 379: 2097-2107.
21. Toth PP, Bray S, Villa G, et al. Network meta-analysis of randomized trials evaluating the comparative efficacy of lipid-lowering therapies added to maximally tolerated statins for the reduction of low-density lipoprotein cholesterol. J Am Heart Assoc 2022; 11: e025551.
22. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med 2017; 376: 1430-1440.
23. NCBI Bookshelf. Inclisiran. In: Liver Toxicity Clinical and Research Information on Drug-Induced Liver Injury. Updated 6 Jan 2023. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK588654/ (accessed July 2024).
24. Kallend D, Stoekenbroek R, He Y, Smith PF, Wijngaard P. Pharmacokinetics and pharmacodynamics of inclisiran, a small interfering RNA therapy, in patients with hepatic impairment. J Clin Lipidol 2022; 16: 208-219.
25. Therapeutic Goods Administration. Australian product information - Leqvio® (inclisiran) solution for injection. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2021-PI-02129-1 (accessed July 2024).
26. A randomized trial assessing the effects of inclisiran on clinical outcomes among people with cardiovascular disease (ORION-4). ClinicalTrials.gov ID NCT03705234.
27. A randomised, double-blind, placebo-controlled, multicentre trial, assessing the impact of inclisiran on major adverse cardiovascular events in participants with established cardiovascular disease (VICTORION-2 PREVENT). ClinicalTrials.gov ID NCT05030428.
28. A randomised, double-blind, placebo-controlled, multicentre study to evaluate the effect of inclisiran on preventing major adverse cardiovascular events in high-risk primary prevention patients (VICTORION-1 PREVENT). ClinicalTrials.gov ID NCT05739383.