Advances in lipid management: current challenges and new horizons
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality worldwide, with elevated low-density lipoprotein cholesterol being a major modifiable risk factor. Effective lipid management is crucial for CVD prevention, yet a substantial proportion of patients do not achieve guideline-recommended lipid goals. This treatment gap underscores the need for implementation strategies to optimise the use of currently available therapies for lipid lowering. Furthermore, the landscape of lipid management is rapidly evolving, with several novel therapies showing promise in clinical trials.
- Treatment gaps exist between guideline recommendations for lipid goals and clinical practice.
- There has been a significant evolution of lipid-lowering therapies in the past decade.
- Several safe and effective therapies are available in clinical practice to achieve lipid goals.
- Implementation strategies are needed to optimise lipid management.
- Novel therapies in clinical trials could expand options for lipid-modifying treatment in the future.
Despite being preventable, cardiovascular disease (CVD) continues to be one of the leading causes of death in Australia.1 Although smoking and diabetes are often the focus of CVD prevention in clinical practice, high cholesterol remains an overlooked yet treatable condition. The INTERHEART study showed that elevated cholesterol and apolipoprotein B (apoB) levels are leading CVD risk factors, accounting for about 50% of the population-attributable risk for myocardial infarction worldwide.2 Long-term exposure to high plasma levels of apoB-containing lipoproteins, such as low-density lipoprotein (LDL), significantly increases the risk of CVD.3,4 Randomised controlled trials of LDL-cholesterol (LDL-C)-lowering therapies have shown that every 1 mmol/L reduction in LDL-C safely reduces CVD risk by 20 to 25%, with reductions in risk that accumulate over time.5 Intensive LDL-C lowering can also stabilise atherosclerotic plaque.6,7 Therefore, current guidelines recommend lipid goals or thresholds for lipid-lowering therapies that are more intensive than prior guidelines.8 Similar to the management of hypertension, combination therapies (including polypills for improving adherence to treatments) may be needed to achieve effective lipid lowering in patients at high and very high risk of CVD.9
This review discusses the current challenges, treatment options, implementation strategies and new horizons for lipid-focused CVD prevention in Australia.
The scope of the problem
Several studies have shown gaps between guideline recommendations for lipid lowering and clinical practice. Most patients at high risk of CVD in Australia are not prescribed cholesterol-lowering therapies.10 Moreover, almost two-thirds of statin-treated patients are not meeting LDL-C goals.11 In patients with acute coronary syndrome, almost half do not meet lipid goals within 12 months of the event, leaving them at increased residual risk.12 Despite ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors being safe and effective therapies for LDL-C lowering, their use remains low in patients with CVD, even in tertiary care.10 There is also scope to improve lipid management in older patients, in Indigenous Australians and in people living in rural or remote areas.13-15 Furthermore, patients with familial hypercholesterolaemia (FH), a genetic condition affecting about one in 250 people, remain largely undiagnosed and undertreated.16-18 Patients with FH have an estimated threefold increased risk of premature coronary artery disease.19 Yet, the roughly 100,000 Australians diagnosed with FH are thought to represent only 10% of the actual burden of this disease.16
This current state of undertreatment exists despite the increasing number of safe and effective lipid-lowering therapies. Several factors contribute to suboptimal LDL-C-goal attainment at the clinician, patient and health system level. At the clinician level, contributing factors may include clinician time pressure, competing priorities, therapeutic inertia and limited familiarity with new guidelines and therapies.20 At the patient level, misinformation about medications, cost, difficulty accessing or adhering to medication, and side effects are important factors.21,22 At the health system level, lack of multidisciplinary lipid clinics, lack of standardised procedures and health alerts, and delays in accessing healthcare are potential factors.22 There is also a need to improve access to effective lipid-lowering therapies through the PBS.21
The current state of play
The European Society of Cardiology (ESC) 2019 Guidelines recommend that patients at very high risk of CVD achieve at least a 50% reduction in LDL-C from baseline, with an LDL-C goal of less than 1.4 mmol/L.8 Patients at high risk should aim for at least a 50% reduction in LDL-C from baseline and an LDL-C goal of less than 1.8 mmol/L.8
The wider acceptance of nonfasting blood samples for lipid assessment may make lipid testing more acceptable to patients, leading to more routine testing to meet lipid goals.23 Although more therapies are now available for lipid lowering, lifestyle management with dietary modifications and increased physical activity remains the cornerstone of treatment.8 In addition, addressing secondary causes of dyslipidaemia like obesity, diabetes mellitus and alcohol excess is imperative.
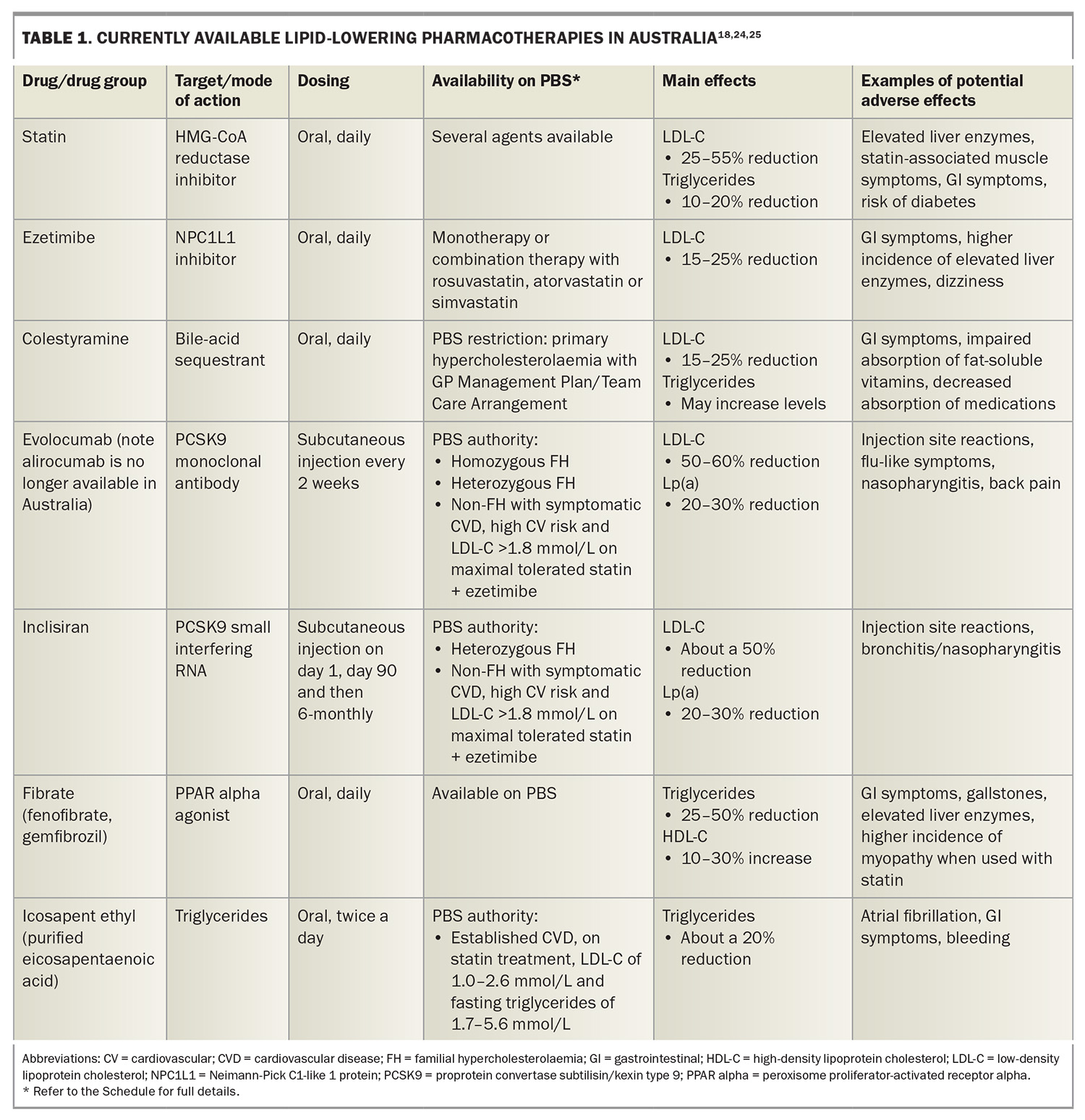
Statins remain the mainstay of lipid-lowering therapy, having been proven safe and effective in lowering LDL-C levels and reducing CVD events in primary and secondary prevention.8 However, there has been new emphasis on a high-intensity lipid-lowering therapy strategy, particularly in patients at high and very high risk of CVD.9 Available therapies in Australia that can be added to statins for LDL-C lowering include ezetimibe, bile-acid sequestrants and PCSK9-directed therapies (Table 1).18,24,25
Ezetimibe is well tolerated and has been shown to reduce CVD events.26 Upfront combination of moderate doses of statins plus ezetimibe in patients at high risk may improve outcomes and has fewer adverse effects compared with high-dose statin monotherapy.9,27 The PBS has recently removed the authority restrictions on prescribing ezetimibe, which allows for earlier use of combination therapy (https://www.pbs.gov.au/pbs/search?term=ezetimibe).
Bile-acid sequestrants are rarely used owing to their gastrointestinal side effects, impact on fat-soluble vitamin absorption and medication interactions.28
Statin intolerance is an important issue, with ezetimibe and PCSK9-directed therapies being alternative options. Nutraceutical regimens (such as plant sterols, red yeast rice and berberine) have a modest effect in lowering LDL-C levels and are another alternative for patients with statin intolerance.8
The PCSK9-directed therapies currently available in Australia are the monoclonal antibody evolocumab and the small interfering RNA (siRNA) inclisiran. Evolocumab is administered subcutaneously every two or four weeks by the patient and can reduce LDL-C by 50 to 60% when added to a statin.8 The triple combination of a high-intensity statin, ezetimibe and a PCSK9 monoclonal antibody can achieve, on average, an 85% reduction in LDL-C from baseline.8 Inclisiran can reduce LDL-C by 50% in patients on statin therapy and was listed on the PBS in April 2024.29 It reduces the hepatic production of PCSK9 at the RNA level and is highly specific to the hepatocyte, leading to few off-target effects. With its long duration of action, inclisiran is administered subcutaneously by a healthcare professional every six months after the day 1 and day 90 doses. Longer-term data for PCSK9-directed therapies have shown safety and efficacy.30,31
Fibrates have a role in reducing triglyceride levels, especially in preventing pancreatitis in patients with extreme hypertriglyceridaemia (>10 mmol/L).32 Fenofibrate can reduce the progression of microvascular disease in patients with diabetes.32 However, a recent CVD outcome trial of pemafibrate (not available in Australia) did not show a reduction in CVD events in patients with diabetes, hypertriglyceridaemia, low high-density lipoprotein-cholesterol (HDL-C) levels and well-controlled LDL-C.33 Niacin and mixed formulations of omega-3 fatty acids have also not been shown to reduce CVD events and are therefore not routinely recommended for CVD prevention.34-38
Conversely, icosapent ethyl (IPE), a highly purified eicosapentaenoic acid, has been shown to reduce CVD events in patients with established CVD or diabetes and additional risk factors and with residual hypertriglyceridaemia despite statin therapy.24 It has been approved by the Therapeutic Goods Administration and was listed on the PBS in October 2024 (https://www.pbs.gov.au/pbs/search? term=icosapent+ethyl). It is cost-effective and recommended by international guidelines for hypertriglyceridaemia.25,32,39 Although IPE can increase the risk of atrial fibrillation and bleeding, these potential adverse events are outweighed by the benefits of treatment in reducing total CVD events.40 Notably, IPE also has antithrombotic and anti-inflammatory effects and its CVD benefits are independent of triglyceride lowering.24,41
The promise of novel lipid-lowering therapies
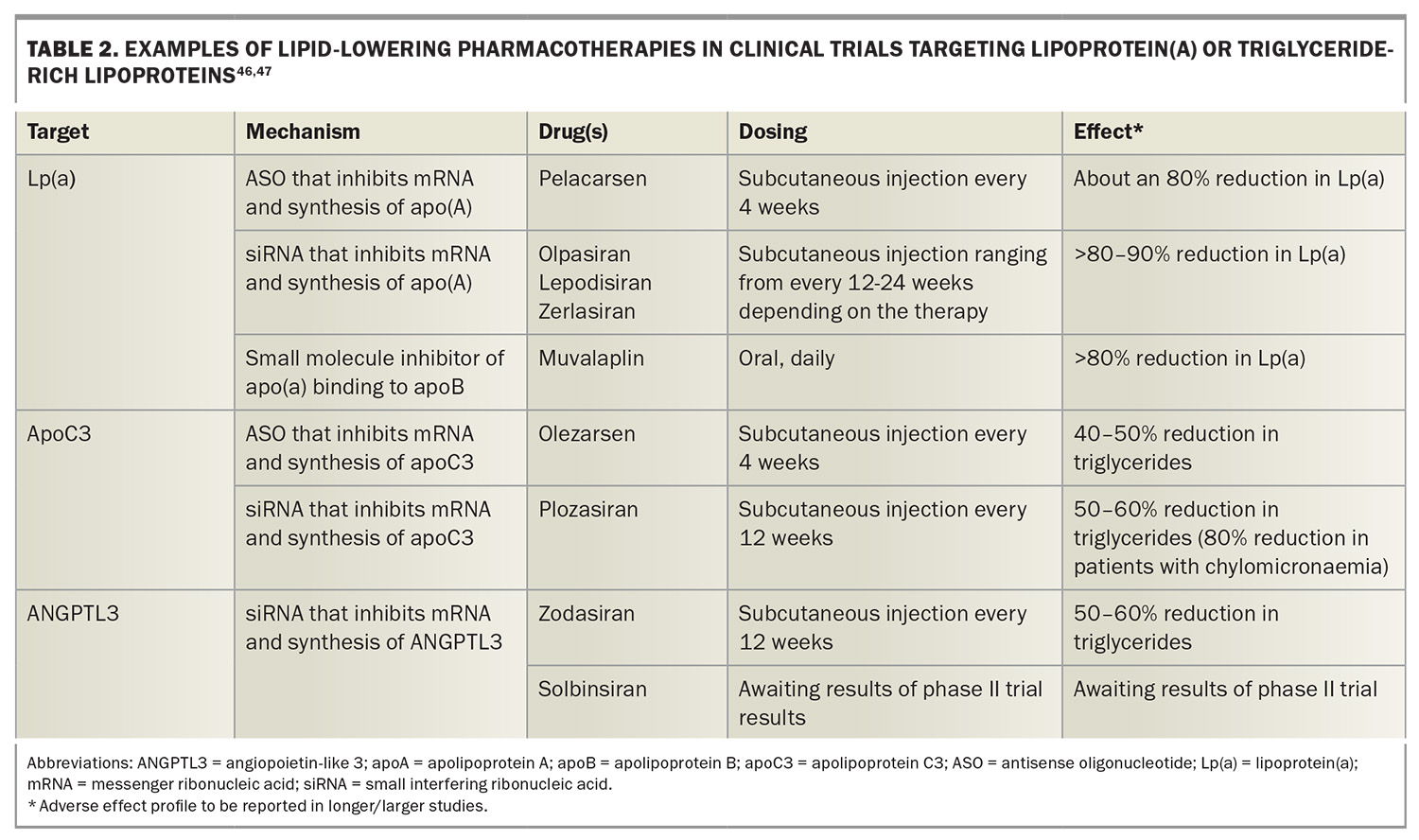
LDL-C currently remains the primary target in clinical practice. However, there are other apoB-containing lipoproteins, such as triglyceride-rich lipoproteins and lipoprotein(a) [Lp(a)], that are causally associated with CVD.42-45 Genetic studies and advances in drug development have led to several novel lipid-lowering therapies that target these atherogenic apoB-containing lipoproteins and are in clinical trials (Table 2).46,47 CVD outcome trials, as well as safety, acceptability and cost-effectiveness data, are crucial to enable such therapies to enter clinical practice. The long duration of action, especially with siRNA therapies, allows less frequent dosing regimens and potentially greater patient convenience.
In terms of targeting LDL-C, bempedoic acid has been shown to reduce CVD events compared with placebo in patients with statin intolerance.48 It is another alternative for patients with statin intolerance. It is available in the USA and Europe but currently not available in Australia. The future of LDL-C lowering also includes oral PCSK9 inhibitors.49 Other highly targeted therapies against PCSK9, such as vaccines and gene editing using CRISPR-Cas9, are in development.50,51 Further research is needed to resolve questions on bioethical issues, safety and costs, especially for gene editing therapies.
Lp(a) is an LDL-like particle that is genetically determined and its levels can be modestly lowered by PCSK9-directed therapies but are not lowered by statins.43 In the absence of specific Lp(a)-lowering therapies, current guidelines recommend intensive CVD risk-factor management in patients with elevated Lp(a) levels.43,52 RNA therapies that reduce the hepatic production of apolipoprotein(A) and which reduce Lp(a) level by more than 80 to 90% are currently in clinical trials. These therapies include the antisense oligonucleotide (ASO) therapy, pelacarsen, and siRNA therapies, such as olpasiran, zerlasiran and lepodisiran.53-56 CVD outcome trials of pelacarsen, olpasiran and lepodisiran are ongoing and will answer whether Lp(a) lowering reduces CVD events. Oral Lp(a) inhibitors such as muvalaplin are also in clinical trials.57
The future of triglyceride lowering includes agents that target apolipoprotein C3 (apoC3) and angiopoietin-like protein 3 (ANGPTL3), as these proteins regulate triglyceride-rich lipoprotein metabolism. Agents in clinical trials targeting apoC3 include the ASO therapy, olezarsen, and the siRNA therapy, plozarisan, both of which can reduce triglyceride levels by more than 50 to 60%.58,59 The monoclonal antibody against ANGPTL3, evinacumab, is approved overseas for homozygous FH, but is not available in Australia, while siRNA therapies against ANGPTL3, zodasiran and solbinsiran are in clinical trials.60-62
Familial chylomicronaemia syndrome, another under-recognised inherited lipid disorder affecting about one to 10 in one million people and causing severe hypertriglyceridaemia, increases the risk of pancreatitis and is inadequately treated by current lipid-lowering therapies.63 Agents targeting apoC3 can significantly reduce triglycerides and the risk of pancreatitis in patients with chylomicronaemia.64,65
HDL-C, the so-called ‘good cholesterol’ because of its inverse association with CVD, remains another potential therapeutic target. However, trials evaluating therapies that increase HDL-C levels have not shown reductions in CVD events.66 In addition, a recent trial evaluating CSL112, a therapy that increases cholesterol efflux, found that it did not significantly improve CVD outcomes when administered after acute myocardial infarction.67 Obicetrapib, an oral inhibitor of cholesteryl ester transfer protein (CETP), which increases HDL-C levels, is currently being studied in a CVD outcomes trial in patients at high risk of CVD.66,68 However, the potential impact of CETP inhibitors on CVD events may be mediated by the associated reductions in LDL-C.66,68 The care of people with genetically very low HDL-C levels, who are at increased risk of CVD, remains an unmet need and requires further clinical trials of HDL-targeted therapies.66
What needs to change
Updated guidelines on lipid management are overdue given that the last Australian guideline on overall care of patients with lipid disorders was published in 2005.69 The recently published 2025 Heart Foundation and Cardiac Society of Australia and New Zealand (CSANZ) guidelines on managing acute coronary syndrome provides more guidance on lipid lowering for secondary prevention.70 The FH Australasia Network published a guideline on enhancing the care of FH in 2020 and the Australian Atherosclerosis Society published a position statement on elevated Lp(a) in 2023.16,52 Furthermore, the International Atherosclerosis Society consensus statement on triglyceride management was published in 2024.25 As we are now in 2025, broader national guidelines for lipid management that reflect contemporary evidence are needed. Provision of short focused ‘how to’ articles for practical lipid management in the Australian context specifically for primary care is also important. The impact of the new Heart Foundation’s 2023 CVD risk calculator could be enhanced with updated guidelines for lipid management as well as updated guidelines for hypertension.71
The growing availability of lipid-lowering therapies offers clinicians and patients more options and flexibility, enabling personalised care plans. However, in Australia many of these new therapies are not accessible for routine clinical use (e.g. bempedoic acid and evinacumab) or are accessible only through clinical trials. Nonetheless, it is crucial to make full use of currently available therapies and ensure better adherence to both lifestyle changes and medications, as many patients who could benefit from available treatments are still not receiving them. To address these gaps, implementation strategies with a comprehensive, multifaceted and interdisciplinary approach is needed.21,22 In 2022, the Global Heart World Federation Cholesterol Roadmap laid out priorities for lipid management in CVD prevention, with the aim of lowering lifetime exposure to LDL-C at a population level.72,73 Strategies include:
- screening for FH
- recognising at-risk individuals with CVD risk scoring
- prioritising LDL-C lowering with lifestyle and pharmacotherapy
- improving access to therapies
- educating patients and clinicians
- promoting public policy.72,73
The currently available lipid-lowering therapies can potentially enable the attainment of guideline-recommended lipid goals. However, a clear action plan for the care of patients with lipid disorders in Australia needs to be developed and implemented nationally with key policy makers to further reduce the burden of CVD.73 The action plan requires further investment and collaboration at the patient, GP, specialist, allied health, health system and policy levels.
Conclusion
Despite the crucial role of lipid lowering in CVD prevention, many patients are not achieving guideline-recommended lipid goals, highlighting the need for a clear action plan on management of lipid disorders. The current armamentarium of lipid-lowering therapies is underutilised, and further strategies are required at every level of healthcare to optimise their use. Looking ahead, the addition of novel lipid-modifying therapies against a broad range of targets heralds a new era of precision preventive medicine. MT
COMPETING INTERESTS: Dr Chetty: None. Dr Lan has received research funding from Sanofi as part of a Clinical Fellowship in Endocrinology and Diabetes; education support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, CSL Seqirus, Eli Lilly, Novartis and Pfizer; speaker honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Menarini, Novartis and Sanofi; and has participated in advisory boards for Eli Lilly. Professor Watts has received honoraria for advisory boards and research grants from Amgen, Arrowhead, Esperion, Gemphire, Kowa, Novartis, Pfizer, Sanofi, Novo Nordisk and Regeneron.
References
1. Australian Institute of Health and Welfare. Deaths in Australia [Web report]. Canberra: Australian Government, 2025 (last updated 9 April 2025. Available online at: https://www.aihw.gov.au/reports/life-expectancy-deaths/deaths-in-australia/notes (accessed April 2025).
2. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364: 937-952.
3. Domanski MJ, Tian X, Wu CO, et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J Am Coll Cardiol 2020; 76: 1507-1516.
4. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017; 38: 2459-2472.
5. Cholesterol Treatment Trialists Collaborators; Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380: 581-590.
6. Räber L, Ueki Y, Otsuka T, et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. JAMA 2022; 327: 1771-1781.
7. Nicholls SJ, Kataoka Y, Nissen SE, et al. Effect of evolocumab on coronary plaque phenotype and burden in statin-treated patients following myocardial infarction. JACC Cardiovasc Imaging 2022; 15: 1308-1321.
8. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J 2019; 41: 111-188.
9. Ray KK, Reeskamp LF, Laufs U, et al. Combination lipid-lowering therapy as first-line strategy in very high-risk patients. Eur Heart J 2021; 43: 830-833.
10. Banks E, Crouch SR, Korda RJ, et al. Absolute risk of cardiovascular disease events, and blood pressure- and lipid-lowering therapy in Australia. Med J Aust 2016; 204: 320.
11. Talic S, Marquina C, Zomer E, et al. Attainment of low-density lipoprotein cholesterol goals in statin treated patients: real-world evidence from Australia. Curr Probl Cardiol 2022; 47: 101068.
12. Alsadat N, Hyun K, Boroumand F, Juergens C, Kritharides L, Brieger DB. Achieving lipid targets within 12 months of an acute coronary syndrome: an observational analysis. Med J Aust 2022; 216: 463-468.
13. Hamilton-Craig I, Colquhoun D, Kostner K, Woodhouse S, d’Emden M. Lipid-modifying therapy in the elderly. Vasc Health Risk Manag 2015; 11: 251-263.
14. Calabria B, Korda RJ, Lovett RW, et al. Absolute cardiovascular disease risk and lipid-lowering therapy among Aboriginal and Torres Strait Islander Australians. Med J Aust 2018; 209: 35-41.
15. Janus ED, Tideman PA, Dunbar JA, et al. Dyslipidaemia in rural Australia: prevalence, awareness, and adherence to treatment guidelines in the Greater Green Triangle Risk Factor Study. Med J Aust 2010; 192: 127-132.
16. Watts GF, Sullivan DR, Hare DL, et al. Integrated guidance for enhancing the care of familial hypercholesterolaemia in Australia. Heart Lung Circ 2021; 30: 324-349.
17. Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013; 34: 3478-3490.
18. Lan NSR, Bajaj A, Watts GF, Cuchel M. Recent advances in the management and implementation of care for familial hypercholesterolaemia. Pharmacol Res 2023; 194: 106857.
19. Clarke SL, Tcheandjieu C, Hilliard AT, et al. Coronary artery disease risk of familial hypercholesterolemia genetic variants independent of clinically observed longitudinal cholesterol exposure. Circ Genom Precis Med 2022; 15: e003501.
20. Nguy J, Hitchen SA, Lan NSR, et al. Barriers to prescribing proprotein convertase subtilisin-kexin type 9 inhibitors after coronary revascularisation. Intern Med J 2023; 53: 994-1001.
21. Talic S, Marquina C, Lybrand S, Liew D, Ademi Z. Calling for urgent actions to improve lipid management in Australia – low medication adherence and poor therapeutic goal attainment lead to poor outcomes and wasted resources. Curr Probl Cardiol 2023; 48: 102005.
22. Lan NSR, Chen RT, Dwivedi G, Watts GF, Nicholls SJ, Nelson AJ. Learnings from implementation strategies to improve lipid management. Curr Cardiol Rep 2025; 27: 9.
23. Nordestgaard BG, Langsted A, Mora S, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J 2016; 37: 1944-1958.
24. Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019; 380: 11-22.
25. Aggarwal R, Bursill C, Figtree GA, et al. Triglycerides revisited: a contemporary perspective on the assessment and management of cardiovascular risk due to elevated triglycerides. A consensus statement of the International Atherosclerosis Society. International Atherosclerosis Society; July 2024.
26. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372: 2387-2397.
27. Ya’Qoub L, Mansoor H, Elgendy IY. Upfront combination of statin and ezetimibe for patients with acute coronary syndrome: time for a new approach? J Am Heart Assoc 2023; 12: e031615.
28. Ross S, D’Mello M, Anand SS, Eikelboom J, et al. Effect of bile acid sequestrants on the risk of cardiovascular events: a mendelian randomization analysis. Circ Cardiovasc Genet 2015; 8: 618-627.
29. Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020; 382: 1507-1519.
30. O’Donoghue ML, Giugliano RP, Wiviott SD, et al. Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation 2022; 146: 1109-1119.
31. Wright RS, Raal FJ, Koenig W, et al. Inclisiran administration potently and durably lowers LDL-C over an extended-term follow-up: the ORION-8 trial. Cardiovasc Res 2024; 120: 1400-1410.
32. Virani SS, Morris PB, Agarwala A, et al. 2021 ACC Expert consensus decision pathway on the management of ASCVD risk reduction in patients with persistent hypertriglyceridemia: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021; 78: 960-993.
33. Das Pradhan A, Glynn RJ, Fruchart JC, et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med 2022; 387: 1923-1934.
34. AIM-HIGH Investigators, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365: 2255-2267.
35. HPS2-THRIVE Collaborative Group; Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014; 371: 203-212.
36. ASCEND Study Collaborative Group; Bowman L, Mafham M, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018; 379: 1540-1550.
37. Manson JE, Bassuk SS, Buring ; VITAL Research Group. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J Steroid Biochem Mol Biol 2020; 198: 105522.
38. Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA 2020; 324: 2268-2280.
39. Toth PP, Ferrières J, Waters M, Mortensen MB, Lan NSR, Wong ND. Global eligibility and cost effectiveness of icosapent ethyl in primary and secondary cardiovascular prevention. Front Cardiovasc Med 2023; 10: 1220017.
40. Bhatt DL, Steg PG, Miller M, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol 2019; 73: 2791-2802.
41. Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol 2020; 40: 1135-1147.
42. Reyes-Soffer G, Ginsberg HN, Berglund L, et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 2022; 42: e48-e60.
43. Kronenberg F, Mora S, Stroes ESG, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J 2022; 43: 3925-3946.
44. Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease. Circ Res 2016; 118: 547-563.
45. Ginsberg HN, Packard CJ, Chapman MJ, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies – a consensus statement from the European Atherosclerosis Society. Eur Heart J 2021; 42: 4791-4836.
46. Nordestgaard BG, Langsted A. Lipoprotein(a) and cardiovascular disease. Lancet 2024; 404: 1255-1264.
47. Zimodro JM, Rizzo M, Gouni-Berthold I. Current and emerging treatment options for hypertriglyceridemia: state-of-the-art review. Pharmaceuticals (Basel) 2025; 18: 147.
48. Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med 2023; 388: 1353-1364.
49. Koren MJ, Descamps O, Hata Y, et al. PCSK9 inhibition with orally administered NNC0385-0434 in hypercholesterolaemia: a randomised, double-blind, placebo-controlled and active-controlled phase 2 trial. Lancet Diabetes Endocrinol 2024; 12: 174-183.
50. Kim K, Choi SH. A New modality in dyslipidemia treatment: antisense oligonucleotide therapy. J Lipid Atheroscler 2022; 11: 250-261.
51. Walker HE, Rizzo M, Fras Z, Jug B, Banach M, Penson PE. CRISPR gene editing in lipid disorders and atherosclerosis: mechanisms and opportunities. Metabolites 2021; 11: 857.
52. Ward NC, Watts GF, Bishop W, et al. Australian Atherosclerosis Society position statement on lipoprotein(a): clinical and implementation recommendations. Heart Lung Circ 2023; 32: 287-296.
53. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med 2020; 382: 244-255.
54. O’Donoghue ML, Rosenson RS, Gencer B, et al. Small interfering RNA to reduce lipoprotein(a) in cardiovascular disease. N Engl J Med 2022; 387: 1855-1864.
55. Nissen SE, Wolski K, Watts GF, et al. Single ascending and multiple-dose trial of zerlasiran, a short interfering RNA targeting lipoprotein(a): a randomized clinical trial. JAMA 2024; 331: 1534-1543.
56. Nissen SE, Linnebjerg H, Shen X, et al. Lepodisiran, an extended-duration short interfering RNA targeting lipoprotein(a): a randomized dose-ascending clinical trial. JAMA 2023; 330: 2075-2083.
57. Nicholls SJ, Nissen SE, Fleming C, et al. Muvalaplin, an oral small molecule inhibitor of lipoprotein(a) formation: a randomized clinical trial. JAMA 2023; 330: 1042-1053.
58. Bergmark BA, Marston NA, Prohaska TA, et al. Olezarsen for hypertriglyceridemia in patients at high cardiovascular risk. N Engl J Med 2024; 390: 1770-1780.
59. Ballantyne CM, Vasas S, Azizad M, et al. Plozasiran, an RNA interference agent targeting APOC3, for mixed hyperlipidemia. N Engl J Med 2024; 391: 899-912.
60. Watts GF, Schwabe C, Scott R, et al. RNA interference targeting ANGPTL3 for triglyceride and cholesterol lowering: phase 1 basket trial cohorts. Nat Med 2023; 29: 2216-2223.
61. Rosenson RS, Gaudet D, Hegele RA, et al. Zodasiran, an RNAi therapeutic targeting ANGPTL3, for mixed hyperlipidemia. N Engl J Med 2024; 391: 913-925.
62. Ray KK, Ruotolo G, Michael L, et al. Solbinsiran, a GalNAc-conjugated siRNA targeting ANGPTL3, reduces atherogenic lipoproteins in individuals with mixed dyslipidaemia in a durable and dose-dependent manner. JACC 2024; 83(13 Suppl): 1673.
63. Spagnuolo CM, Hegele RA. Etiology and emerging treatments for familial chylomicronemia syndrome. Expert Rev Endocrinol Metab 2024; 19: 299-306.
64. Watts GF, Rosenson RS, Hegele RA, et al. Plozasiran for managing persistent chylomicronemia and pancreatitis risk. N Engl J Med 2025; 392: 127-137.
65. Stroes ESG, Alexander VJ, Karwatowska-Prokopczuk E, et al. Olezarsen, acute pancreatitis, and familial chylomicronemia syndrome. N Engl J Med 2024; 390: 1781-1792.
66. Lan NSR, Watts GF. New perspectives on the high-density lipoprotein system and its role in the prevention and treatment of atherosclerotic cardiovascular disease. Curr Opin Endocrinol Diabetes Obes 2025; 32: 66-74.
67. Gibson CM, Duffy D, Korjian S, et al. Apolipoprotein A1 infusions and cardiovascular outcomes after acute myocardial infarction. N Engl J Med 2024; 390: 1560-1571.
68. Deng S, Liu J, Niu C. HDL and cholesterol ester transfer protein (CETP). Adv Exp Med Biol 2022; 1377: 13-26.
69. Tonkin A, Barter P, Best J, et al; National Heart Foundation of Australia; Cardiac Society of Australia and New Zealand. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: position statement on lipid management – 2005. Heart Lung Circ 2005; 14: 275-291.
70. Brieger D, Cullen L, Briffa T, et al; other members of the Heart Foundation Working Group. National Heart Foundation of Australia & Cardiac Society of Australia and New Zealand: Comprehensive Australian Clinical Guideline for Diagnosing and Managing Acute Coronary Syndromes 2025. Heart Lung Circ 2025; 34: 309-397.
71. Nelson MR, Banks E, Brown A, et al. 2023 Australian guideline for assessing and managing cardiovascular disease risk. Med J Aust 2024; 220: 482-490.
72. Ray KK, Ference BA, Séverin T, et al. World Heart Federation cholesterol roadmap 2022. Glob Heart 2022; 17(1): 75.
73. National Heart Foundation of Australia. Cholesterol roadblocks and solutions ‒ a report from Australia’s National Roundtable on Cholesterol [Internet]. Canberra: National Heart Foundation of Australia; 2022 [updated 30 June 2022]. Available online at: https://assets.contentstack.io/v3/assets/blt8a393bb3b76c0ede/blt441267e48b235b23/65adae2302a66524a292d5bc/ 221012_National-Cholesterol-Report_LS_FINAL.pdf (accessed May 2025).