New pharmacological treatments for obesity: incretin analogues, their mechanism of action, efficacy and safety

Incretin analogues are very effective weight loss medications resulting in weight loss approaching that achieved with bariatric surgery. They also have cardiovascular benefits. However, these treatments are costly and their availability has been limited because of overdemand.
- Obesity increases the risk of disease, including serious cardiometabolic disease.
- Incretin analogue treatment results in weight loss with a substantial reduction in cardiovascular risk factors. The newer agents have been associated with weight loss of 15 to 20% bodyweight, which approaches that seen with bariatric surgery.
- For the first time, a pharmacological obesity treatment, semaglutide, has been shown to reduce major adverse cardiovascular events in people with obesity and established cardiovascular disease and there are ongoing cardiovascular outcome trials with other incretin therapies.
- Incretin analogues are an increasingly important class of drugs that provide substantial health benefits for people with obesity with adverse effects limited primarily to gastrointestinal disturbances. Equity of access is a problem because these medications are costly and there have been global shortages because of overdemand.
Obesity and its related medical complications are a major problem in Australia and worldwide. In 2017 to 2018, 25% of children and adolescents aged 2 to 17 years and 67% of adults (aged ≥18 years) in Australia had overweight or obesity. Obesity increases the risk of disease, including serious cardiometabolic disease. Prevention of obesity is key because once a person has developed obesity, long-term weight loss is rare. Until recently, the most durable weight-loss intervention was bariatric surgery, which is also associated with excellent health outcomes. However, with the advent of incretin treatment, there are now pharmacological options that result in weight loss of 15 to 20% bodyweight, which approaches that achieved with bariatric surgery, and substantial cardiovascular benefit. The weight loss is sustained while patients remain on incretin treatment. However, these treatments are costly and their availability has been limited because of their popularity, which has been problematic and stressful for people with obesity.
What are incretins and how do they result in weight loss?
Incretins are hormones produced in the gastrointestinal tract that are rapidly secreted in response to a meal. They communicate nutrient intake to systems that regulate postprandial homeostasis, including the stimulation of pancreatic insulin release in a glucose-dependent manner, the so-called incretin effect, which occurs after the oral but not intravenous administration of glucose. In addition, they regulate gastric motility, nutrient absorption, blood flow and food intake.1 The two dominant incretins are glucagon-like peptide-1 (GLP-1), secreted by enteroendocrine L cells (located in the ileum and colon), and glucose-dependent insulinotropic peptide (GIP), secreted by K cells (located mainly in the duodenum). These hormones are rapidly degraded by dipeptidyl peptidase-4 and neutral endopeptidase 24.11 with renal clearance resulting in a short half-life (two minutes).2 GLP-1 is also produced in cerebral preproglucagon neurons where it is released locally and acts as a neurotransmitter. Peripheral and central GLP-1 systems appear to be separate and act independently.3
Of the two dominant incretins, GIP has been shown to have a greater effect on insulin release following oral glucose ingestion in healthy humans. At euglycaemia or hyperglycaemia, GLP-1 suppresses glucagon secretion and when blood glucose levels are 5 mmol/L or less, it either has no effect or may increase glucagon secretion, whereas GIP stimulates glucagon secretion, with enhanced activity at lower glycaemia.
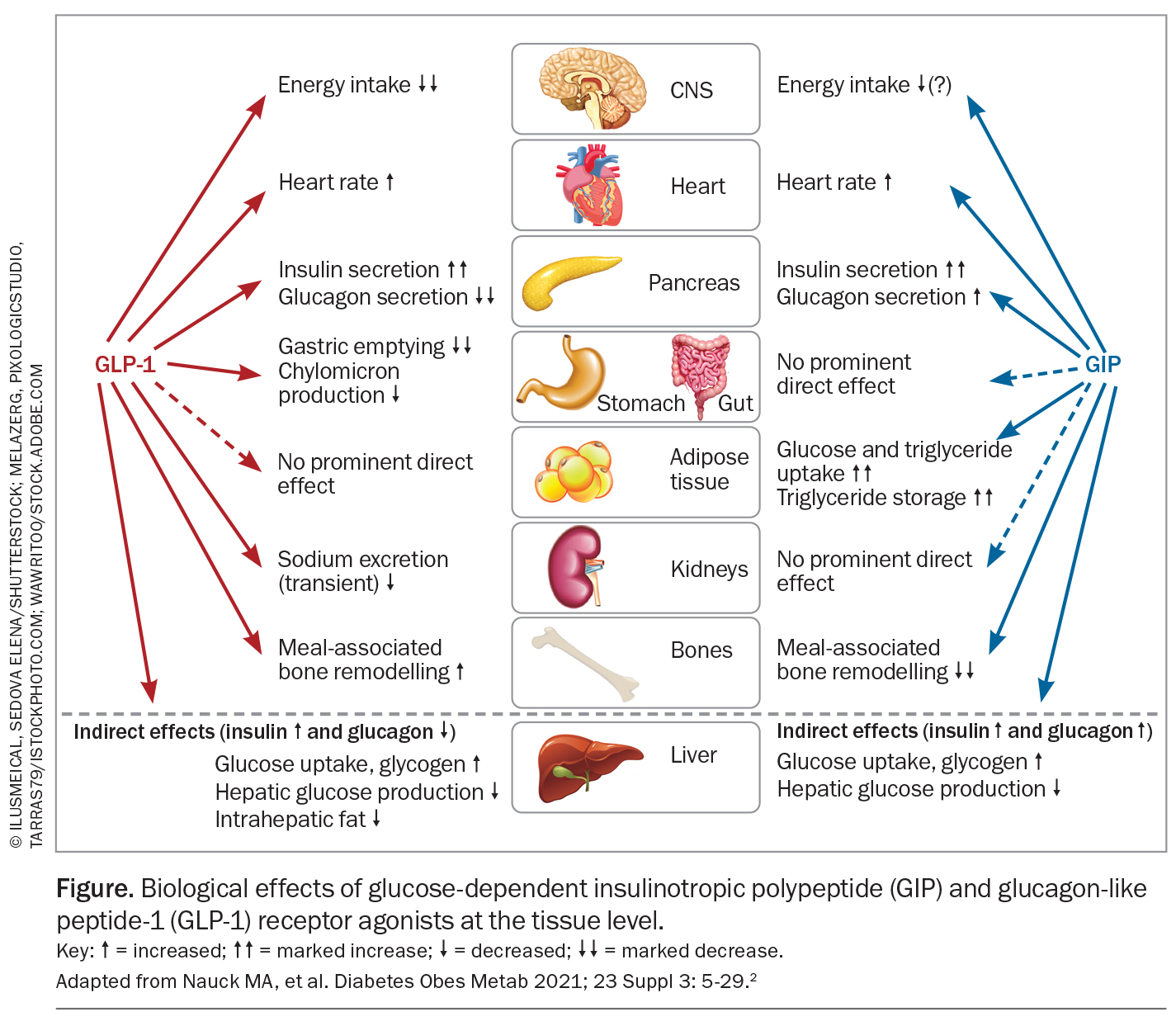
GLP-1 and GIP receptors are widespread in humans with abundant levels in pancreatic beta and, to a lesser degree, alpha cells. They have also been found in heart, subcutaneous and visceral adipose tissue, lung, kidney, blood vessels, bone and gastrointestinal tract. In rodents and nonhuman primates, receptors have also been found in brain tissue involved in appetite regulation, satiety, energy intake and expenditure (i.e. hypothalamus and brain stem nuclei), as well as regions involved in synaptic plasticity, memory, reward functions and emotional responses (Figure).2
Effects of incretins on the gastrointestinal tract
Gut-released GLP-1 acts as a hormone and binds primarily to receptors in the gastrointestinal system. It also acts on vagal afferent neurons that signal the brain on the gut nutrient status. Physiological and pharmacological doses of GLP-1 slow gastric emptying, but GIP has no such effect. Although tachyphylaxis to this effect may occur with long-acting GLP-1 receptor agonists, GLP-1-related slowing of gastric emptying does not appear to be responsible for the nausea and vomiting often reported with the use of GLP-1 receptor agonist treatment. High doses of GLP-1 receptor agonist resulting in complete cessation of gastric emptying are not usually associated with gastrointestinal symptoms, which appear to be centrally mediated.2
In slowing gastric emptying, gastric acid and pancreatic exocrine secretion are also reduced by GLP-1. GLP-1 and GLP-1 receptor agonists also reduce intestinal motility and this may affect nutrient absorption. These effects have been referred to as the ‘ileal brake’ that provides a signal to stop the eating, digestion and absorption of nutrients, and may contribute to the diarrhoea sometimes associated with GLP-1 receptor agonist treatment.
Energy intake and expenditure
Physiological plasma levels of GLP-1 and GIP do not affect appetite. However, at pharmacological concentrations GLP-1, but not GIP, reduces appetite and prospective food intake and increases satiety. GLP-1 receptor agonists can stimulate GLP-1 receptors in the area postrema, a circumventricular organ in the lower brainstem that has fenestrated capillaries and can sense hormonal signals in the general circulation, and appetite centres in the hypothalamus, which has fenestrated capillaries in the median eminence. The area postrema is responsible for emesis and nausea and is likely the main contributor to these GLP-1 receptor agonist-related side effects. Peripherally administered liraglutide is taken up by the hypothalamus (arcuate and paraventricular nuclei) where it stimulates pro-opiomelanocortin and amphetamine-regulated transcript to reduce appetite and induce weight loss.4 However, GLP-1 receptor agonists and gut-derived GLP-1 do not cross the blood–brain barrier and cannot access GLP-1 receptors in the brain parenchyma, which can only be accessed by brain (pre-proglucagon neuron) derived GLP-1.3 Paradoxically, GIP-receptor knockout mice studies showed that when fed a high-fat diet the mice were resistant to weight gain. Based on animal studies, it has been suggested that antagonism of the GIP receptor may enhance GLP-1-receptor activity whereas chronic GIP-receptor agonism may desensitise GIP-receptor activity mimicking antagonism.1 Neither GIP or GLP-1 at physiological or pharmacological doses have been shown to affect energy expenditure.2
Incretin therapies currently available in Australia
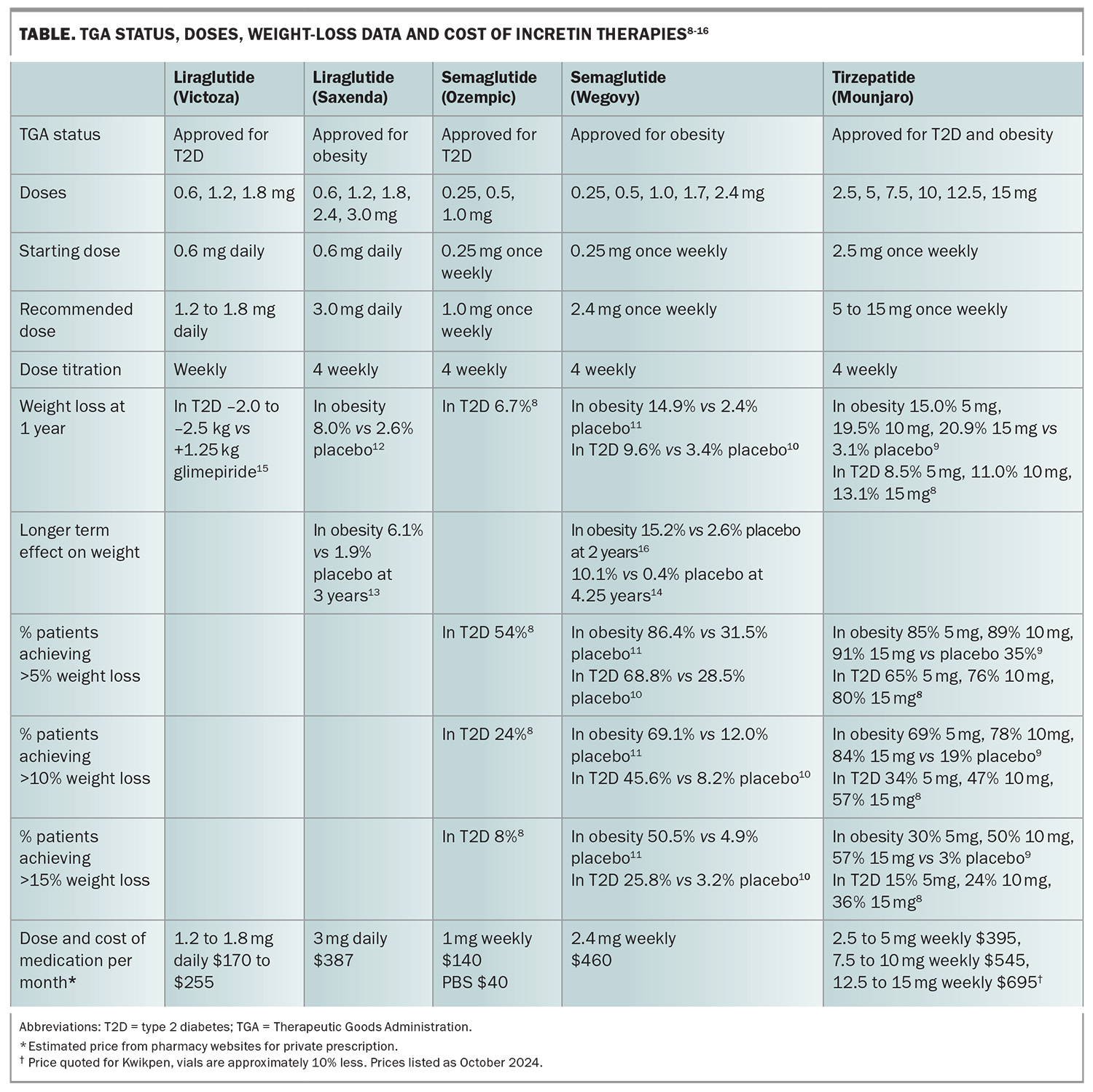
Dulaglutide, liraglutide and semaglutide, which are synthetic versions of human GLP-1, and tirzepatide, a single molecule dual GLP-1 and GIP receptor coagonist, are available in Australia. Liraglutide, semaglutide and tirzepatide have been trialled for the management of obesity. Although dulaglutide has not been studied as a treatment for obesity, dulaglutide treatment in people with type 2 diabetes results in modest weight loss, which was shown to be inferior to liraglutide 1.8 mg, semaglutide 1.0 mg and tirzepatide in head-to-head trials.5-7 In Australia, liraglutide, semaglutide and tirzepatide are TGA approved as a treatment for type 2 diabetes and obesity. All these medications are given by a subcutaneous injection in the abdomen, upper arms or thighs. The doses, weight-loss data and cost are summarised in the Table.8-16 As for all other weight-loss treatments, people with type 2 diabetes do not achieve as much weight loss as those without type 2 diabetes, and these agents appear to be equally effective in patients who have undergone bariatric surgery.8-11,17
Body composition changes
Incretin analogues produce substantial weight loss approaching that achieved with bariatric surgery. Whenever weight is reduced there is a loss of adipose tissue as well as lean tissue. However, with these agents, there is relatively greater fat loss, including visceral fat loss, such that the proportion of lean mass to total body mass is increased with their use.11,18
Efficacy in children and adolescents
Liraglutide 3 mg daily for one year in adolescents with obesity was well tolerated and resulted in a 4.6% reduction in body mass index (BMI) compared with placebo.19 Liraglutide 3 mg daily in children with nonsyndromic obesity aged 6 years to younger than 12 years for one year resulted in a 7.4% reduction in BMI compared with placebo.20 In adolescents with obesity, semaglutide 2.4 mg once weekly resulted in similar weight loss to that seen in adults (16.1% mean change in body mass index from baseline vs 0.6% with placebo) at 68 weeks with improvement in cardiometabolic risk factors (i.e. waist circumference and glycated haemoglobin [HbA1c], LDL-cholesterol, triglycerides and alanine transaminase levels).21
What dose should be used?
For glycaemic control in people with type 2 diabetes, doses of liraglutide above 1.8 mg once daily do not have a substantially greater effect. Similarly, doses of semaglutide above 1 mg once weekly have a negligible effect on glycaemic control in most people with type 2 diabetes. However, for tirzepatide higher doses do have a greater effect on glycaemia. With 5 mg tirzepatide once weekly, 27% of people with type 2 diabetes achieved an HbA1c level below 5.7%, whereas 46% did so on 15 mg once weekly.8 Although there is greater weight loss at higher doses, it progressively wanes with doses exceeding 10 mg once weekly.
The dose of liraglutide recommended for the management of type 2 diabetes is 1.2 to 1.8 mg daily, whereas the dose for weight loss is 3 mg daily. Similarly, the dose of semaglutide recommended for people with type 2 diabetes is 1 mg once weekly, whereas for weight loss it is 2.4 mg once weekly. For tirzepatide, a maximum weekly dose of 15 mg is recommended for both type 2 diabetes and obesity. However, clinically it is apparent that many patients do not require the maximum recommended dose to achieve weight loss. Our recommendation is to increase the dose to that required for adequate appetite control and weight loss. Doses should not be increased until the current dose has been well tolerated. If a patient is experiencing side effects, dose titration should occur more slowly and if side effects are severe, the dose should be reduced back to the dose that was tolerated. The patient should maintain that dose for about a month before an attempt is made to increase the dose again.
How long should these medications be used?
Weight regain occurs when incretin analogues are ceased. This was well demonstrated in a trial in which people taking semaglutide 2.4 mg weekly for weight loss regained two-thirds of their prior weight loss within a year of ceasing semaglutide.22 This is not surprising as similar to nearly all medications, these medications are effective when used and stop working when ceased. Whether weight loss can be maintained with lower doses once patients have had a stable reduced weight for several years remains to be determined.
Benefits beyond weight loss
Liraglutide, semaglutide and tirzepatide all lower blood pressure, improve the lipid profile, reduce blood glucose levels, reduce C-reactive protein levels and improve physical functioning.9,11,12 These effects appear to be related to both weight loss and specific weight independent effects of GLP-1 receptor agonists.23 With these substantial beneficial effects on cardiometabolic risk factors, it is not surprising that liraglutide and semaglutide have also been shown to reduce cardiometabolic diseases.
Prevention of type 2 diabetes
In adults with prediabetes and obesity liraglutide 3 mg daily for three years reduced the risk of progression to type 2 diabetes by 79% and a greater proportion reverted to normoglycaemia.13 Similarly, treatment with semaglutide 2.4 mg once weekly for one year in adults with prediabetes and obesity resulted in reversion to normoglycaemia in 81% compared with 14% on placebo.24 Recently released topline data from the SURMOUNT-3 trial showed that progression to type 2 diabetes was 94% lower among adults with prediabetes who received tirzepatide for an additional 104 weeks beyond the trial’s original 72 weeks compared with those who received placebo (p<0.0001).25
Cardiovascular effects
Incretin analogues increase heart rate by about two to three beats per minute via indirect effects on sinoatrial nodal tissue through increased sympathetic nervous system activation. Although this effect may have a detrimental effect on cardiac outcomes, GLP-1 receptor agonists have been shown to protect cardiac myocytes against ischaemia in animal and human studies. Interestingly, GLP-1 receptor agonists oppose sympathetic effects on cardiac ventricular excitability, reducing ventricular arrhythmic risk through stimulation of cardiac parasympathetic neurons. This, along with their beneficial effect on lipids, inflammation and glucose uptake, likely contributes to their beneficial cardiac effects.23,26 After a median exposure of 3.8 years of liraglutide 1.8 mg daily in patients with type 2 diabetes, the primary composite outcome of death from cardiovascular causes, nonfatal myocardial infarction and nonfatal stroke was reduced by 13%. Death from cardiovascular causes was reduced by 22%.27 After two years of treatment of low dose semaglutide (0.5 or 1 mg) in people with type 2 diabetes and established cardiac disease there was a 26% reduction in risk of a composite outcome of cardiovascular death, nonfatal myocardial infarction or nonfatal stroke that was primarily driven by stroke reduction.28 In people without diabetes but with obesity and pre-existing cardiovascular disease, 2.4 mg semaglutide once weekly was shown to reduce the risk of a composite of death from cardiovascular causes, nonfatal myocardial infarction or nonfatal stroke by 20% over a median of 3.25 years.14 Semaglutide has also been shown to result in greater improvements in exercise function and greater reduction in symptoms related to heart failure in people with heart failure with preserved ejection fraction.29
Renal effects
In people with type 2 diabetes at high risk of cardiovascular disease, liraglutide 1.8 mg daily over a median follow up of 3.8 years resulted in a 22% reduction in renal outcomes compared with placebo. This result was driven mainly by a lower incidence of macroalbuminuria in the liraglutide group than in the placebo group.30 Similarly, semaglutide (0.5 or 1 mg once weekly) for two years in people with type 2 diabetes resulted in a 36% reduction in progression to worsening of nephropathy compared with placebo.28 However, these studies were not primarily designed to assess renal outcomes and the benefits seen were primarily driven by a reduction in albuminuria. The Evaluate Renal Function with Semaglutide Once Weekly (FLOW) study was designed to assess the effect of semaglutide on kidney outcomes in patients with chronic kidney disease and type 2 diabetes. The median follow up was 3.4 years after early trial cessation was recommended at a prespecified interim analysis. Semaglutide treatment resulted in a 24% reduction in major kidney disease, a composite of kidney failure, at least a 50% reduction in estimated glomerular filtration rate from baseline or death from kidney-related or cardiovascular causes.31
Liver disease
In a 72-week phase 2 study of patients (n = 320) with nonalcoholic steatohepatitis (NASH) treated with semaglutide 0.1, 0.2 or 0.4 mg daily there was a reduction in steatosis but not fibrosis.32 In an open-label, substudy of patients with type 2 diabetes and NASH (n = 502), tirzepatide treatment resulted in a significant reduction in liver fat compared with insulin degludec after 52 weeks.33 In a 48-week smaller trial (n = 71) of semaglutide 2.4 mg once weekly in people with biopsy-confirmed NASH and compensated cirrhosis there was no significant improvement in fibrosis or resolution of NASH compared with placebo.34 It could be that longer duration of treatment is necessary to see any improvement and this is supported by a Swedish observational trial in people with chronic liver disease and type 2 diabetes. Patients who were treated with GLP-1 receptor agonists had a 49% lower risk of major adverse liver outcomes over 10 years compared with those not on GLP-1 receptor agonists.35
Obstructive sleep apnoea
In a one-year study of adults with moderate to severe obstructive sleep apnoea and obesity in whom about a half were treated with positive airway pressure at baseline and the remainder were untreated, treatment with tirzepatide 10 or 15 mg once weekly resulted in a clinically meaningful reduction in the apnoea–hypopnoea index in both groups compared with placebo. Tirzepatide also resulted in a marked reduction in the sleep apnoea-specific hypoxic burden, which comprises frequency, duration and depth of oxygen desaturation related to sleep events, and is considered to better reflect the risk of cardiovascular complications and death from sleep apnoea. Body weight, high sensitivity C-reactive protein level, systolic blood pressure and sleep-related patient-reported outcomes also improved.36
Neurodegenerative disease
Animal data show that GLP-1 analogues exert beneficial effects on cognitive function (learning and memory) in models of Alzheimer’s disease, reduce amyloid plaques, reduce tau phosphorylation, attenuate cortical and hippocampal neuronal loss, increase neurogenesis, improve synapsis number, plasticity, reduce brain oxidative stress, exert cerebral anti-inflammatory activity and improve brain insulin receptor localisation and signalling.37,38 GLP-1 receptor agonists have also been shown to have beneficial effects in animal models of Parkinson’s disease with greater effects seen with GLP-1/GIP dual receptor agonists.37 A pooled randomised controlled trial analysis of 15,820 patients with type 2 diabetes treated with a GLP-1 receptor agonist or placebo has also suggested that GLP-1 receptor agonists may reduce dementia. Over a median follow up of 3.6 years, there was a 50% reduction in progression to dementia (placebo vs GLP-1 receptor agonist: 15 vs 32 patients).38 In a nationwide cohort study of 120,054 patients with type 2 diabetes with a median follow up of 7.4 years, there was an 11% reduction in the rate of dementia when GLP-1 receptor agonists were used as a second-line treatment compared with other glucose-lowering therapies and the risk reduced with increasing duration of GLP-1 receptor agonist exposure (liraglutide in 95% of patients).38 However, these findings need to be taken with caution as dementia was not their primary outcome.37,38 Small clinical trials of exenatide and liraglutide have shown improvements in motor symptoms, emotional wellbeing, activities of daily living and quality of life in people with Parkinson’s disease.37 These results have led to larger clinical trials that are ongoing in which the effects of incretin analogues on neurodegenerative diseases are being assessed.
Adverse effects
Gastrointestinal effects
The predominant adverse events associated with GLP-1 receptor agonist treatment are gastrointestinal, particularly nausea and to lesser degrees diarrhoea, vomiting or constipation, although these symptoms subside over time. Titrating the dose slowly reduces the risk of these side effects. However, 4.5% of semaglutide trial participants and 6.2% of people on the highest tirzepatide dose (15 mg weekly) ultimately stopped the drug because of these side effects.9,11 Early reports of pancreatitis in animal studies with the use of sitagliptin, clinical reports of humans treated with exenatide and demonstrations of preneoplastic pancreatic ductal lesions in animals treated with GLP-1 receptor agonists led to concerns about the pancreatic safety of GLP-1 receptor agonists. Levels of pancreatic enzymes, lipase and amylase often rise to a small degree (15 to 37%, respectively, in one study32) with use of GLP-1 receptor agonists, but this is rarely of any clinical significance. A comprehensive review of preclinical toxicology in normal and diabetic animals by European and US regulatory authorities did not find any evidence of incretin-related pancreatic toxicity. There has been no evidence of increased pancreatic disease in the large cardiovascular outcome trials studying the safety of GLP-1 receptor agonists.39 However, in a Canadian study using data from a large health claims database, comparing use of a GLP-1 receptor agonist with naltrexone/bupropion to treat obesity, there was an increased risk of pancreatitis. Although the event rate was low (4.6/1000 person-years for semaglutide, 7.9 for liraglutide and 1.0 for bupropion/naltrexone), there was a ninefold increased risk with GLP-1 receptor agonist overall.40 Some but not all studies have reported an increased risk of biliary disease, including cholelithiasis and acute cholecystitis, with the use of GLP-1 receptor agonists. However, animal studies do not support these agents having a direct effect on the biliary system. Weight loss increases the lithogenicity of bile and how much of the increased risk of biliary disease seen with GLP-1 receptor agonists is related to weight loss versus direct effects is uncertain.39
Anaesthesia
Several studies, including a retrospective audit and a matched pair case-control study of 205 pairs of patients undergoing gastroscopy, have shown that patients taking GLP-1 receptor agonists have higher rates of retained solid gastric contents.41 There have also been case reports of pulmonary aspiration in patients taking GLP-1 receptor agonists who have undergone anaesthesia.41 As the available GLP-1 receptor agonists have a long half-life there is no point stopping them before surgery and with emergency procedures this is not possible. The American Society of Anesthesiologists recommends treating patients who are taking GLP-1 receptor agonists as if they have a full stomach with appropriate airway protection and rapid induction of anaesthesia.41 Similar recommendations have been made by Australian experts. This Australian guideline also contains detailed advice on patient preparation for elective upper endoscopy.42
Medullary thyroid cancer
There is a theoretical increased risk of medullary thyroid cancer (MTC) as preclinical studies showed a link between GLP-1 receptor agonism to the development of C-cell hyperplasia and MTC in rats and mice. Although GLP-1 receptors are expressed in rodents and have a functional role in bone metabolism, the density of GLP-1 receptors in monkey and human thyroid cells is extremely low and there is no evidence that calcitonin levels (which have been measured in all clinical trials of GLP-1 receptor agonists) or MTC rates are increased by the use of GLP-1 receptor agonists. Nevertheless, some human MTCs may express GLP-1 receptors so it is recommended to avoid these agents in people with a family or personal history of MTC or multiple endocrine neoplasia type 2.39
Retinopathy
After two years of low-dose semaglutide in people with type 2 diabetes there was a 76% increased risk of worsening retinopathy.28 However, there are no GLP-1 receptors in the eye so it is most likely that any worsening of retinopathy is related to the rapid fall in glucose levels with these agents.2
Psychiatric disease
A reciprocal link between obesity and depression exists, such that obesity increases the risk of depression and depression is predictive of developing obesity.43 Therefore, it is not surprising that there have been case reports of suicide in people taking incretin treatment and this is being monitored by the European Medicines Agency and US Food and Drug Administration. However, in a retrospective cohort study of electronic health records of 240,618 patients with overweight or obesity who were prescribed semaglutide or non-GLP-1 receptor agonist antiobesity medication and 1,589,855 patients with type 2 diabetes prescribed semaglutide or non GLP-1 receptor agonist hypoglycaemic treatment, during six-month follow up after initiation of treatment, there was lower risk of suicidal ideation in patients prescribed semaglutide regardless of whether there was a history of previous suicidal ideation.44 Although people with significant psychiatric illness are excluded from clinical trials, there has been no signal of increased psychiatric disorders in these studies but rather an improvement in quality of life measures with incretin therapy.
The future of incretins, new agents
Incretin analogues have proven to be very popular and currently not enough of these agents can be manufactured to meet demand, which has been particularly problematic for patients. These medications must only be used by people with health problems and not by healthy people with minimal or no excess adiposity who are seeking weight loss for purely cosmetic reasons. However, as the production of approved medications is intensified and with the arrival of new products that are currently undergoing phase 2 and 3 studies, availability should be much better. Products in more advanced studies include CagriSema, a combination of cagrilintide, an amylin analogue, and semaglutide; retatrutide, a triple compound of GIP/GLP-1/glucagon coagonists; survodutide, a dual glucagon/GLP-1 receptor agonist; maridebart /cafraglutide, a dual GIP receptor antagonist and GLP-1 receptor agonist; and orforglipron, an oral nonpeptide GLP-1 receptor agonist.45
Conclusion
Incretin analogues are very effective weight-loss medications with cardiovascular, renal and respiratory benefits and the promise of neuroprotection as well. Adverse effects are limited to gastrointestinal disturbances that can be managed with dose reduction and caution with anaesthesia. Liraglutide and semaglutide have been shown to reduce important cardiovascular events and there are ongoing similar trials with tirzepatide and other investigational incretins. No medications for overweight and obesity are currently subsidised by the PBS. This is a major problem as obesity predominantly affects people of lower socioeconomic status. The Pharmaceutical Benefits Advisory Committee will need to consider the ready availability of incretin analogues for the management of obesity against the long-term health and other costs of obesity to the Australian community. MT
COMPETING INTERESTS: Associate Professor Markovic is an investigator on pharmaceutical trials of semaglutide and CagriSema (NovoNordisk), tirzepatide and retatrutide (Lilly), BI 456906 (Boehringer Ingelheim) and maridebart /cafraglutide (Amgen) and is on the Advisory Board for Nestlé Health Science, VLCD. Professor Hocking has received honoraria for lectures and/or manuscript writing from Lilly Australia, AstraZeneca, Amgen and Sanofi-Aventis, Nestlé Health Sciences, iNova and Servier; support for attending meetings from Lilly Australia, Novo Nordisk, Amgen and CSL Seqirus; participation on Advisory Boards from Lilly Australia, Novo Nordisk, Ethicon, AstraZeneca; is an investigator on pharmaceutical trials of semaglutide and CagriSema (NovoNordisk), tirzepatide and retatrutide (Lilly), BI 456906 (Boehringer Ingelheim) and maridebart/cafraglutide (Amgen) and she is President of the National Association of Clinical Obesity Services.
References
1. Campbell JE. Targeting the GIPR for obesity: to agonize or antagonize? Potential mechanisms. Mol Metab 2021; 46: 101139.
2. Nauck MA, Quast DR, Wefers J, Pfeiffer AFH. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes Metab 2021; 23 Suppl 3: 5-29.
3. Trapp S, Brierley DI. Brain GLP-1 and the regulation of food intake: GLP-1 action in the brain and its implications for GLP-1 receptor agonists in obesity treatment. Br J Pharmacol 2022; 179: 557-570.
4. Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. J Clin Invest 2014; 124: 4223-4226.
5. Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet 2014; 384: 1349-1357.
6. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol 2018; 6: 275-286.
7. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018; 392: 2180-2193.
8. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021; 385: 503-515.
9. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022; 387: 1434-1435.
10. Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021; 397: 971-984.
11. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021; 384: 989-1002.
12. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373: 11-22.
13. le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017; 389: 1399-409.
14. Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023; 389: 2221-2232.
15. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373: 473-481.
16. Garvey WT, Batterham RL, Bhatta M, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med 2022; 28: 2083-2091.
17. Suliman M, Buckley A, Al Tikriti A, et al. Routine clinical use of liraglutide 3 mg for the treatment of obesity: Outcomes in non-surgical and bariatric surgery patients. Diabetes Obes Metab 2019; 21: 1498-501.
18. Volpe S, Lisco G, Racaniello D, et al. Once-weekly semaglutide induces an early improvement in body composition in patients with type 2 diabetes: a 26-week prospective real-life study. Nutrients 2022; 14: 2414.
19. Kelly AS, Auerbach P, Barrientos-Perez M, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med 2020; 382: 2117-2128.
20. Flox CK, Barrientos-Perez M, Bomberg, EM, et al. Liraglutide for children 6 to <12 years of age with obesity — a randomized trial. N Engl J Med 2024 Sep 10. Doi: 10.1056/NEJMoa2407379. Online ahead of print.
21. Weghuber D, Barrett T, Barrientos-Pérez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med 2022; 387: 2245-2257.
22. Wilding JPH, Batterham RL, Davies M, et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obes Metab 2022; 24: 1553-1564.
23. Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab 2016; 24: 15-30.
24. McGowan BM, Bruun JM, Capehorn M. Efficacy and safety of once-weekly semaglutide 2·4 mg versus placebo in people with obesity and prediabetes (STEP 10): a randomised, double-blind, placebo-controlled, multicentre phase 3 trial. Lancet Diabetes Endocrinol 2024; 12: 631-642.
25. Lilly Investors. News Release. Tirzepatide reduced the risk of developing type 2 diabetes by 94% in adults with pre-diabetes and obesity or overweight. August 20 2024. © Eli Lilly and Company, 2024.
26. Ang R, Mastitskaya S, Hosford PS, et al. Modulation of cardiac ventricular excitability by GLP-1 (glucagon-like peptide-1). Circ Arrhythm Electrophysiol 2018; 11: e006740.
27. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311-322.
28. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834-1844.
29. Kosiborod MN, Abildstrom SZ, Borlaug BA, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med 2023; 389: 1069-1084.
30. Mann JFE, Orsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017; 377: 839-848.
31. Perkovic V, Tuttle KR, Rossing P, et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med 2024; 391: 109-121.
32. Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021; 384: 1113-1124.
33. Gastaldelli A, Cusi K, Fernández Landó L, Bray R, Brouwers B, Rodríguez Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol 2022; 10: 393-406.
34. Loomba R, Abdelmalek MF, Armstrong MJ, et al. Semaglutide 2.4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol 2023; 8: 511-522.
35 Wester A, Shang Y, Toresson Grip E, Matthews AA, Hagstrom H. Glucagon-like peptide-1 receptor agonists and risk of major adverse liver outcomes in patients with chronic liver disease and type 2 diabetes. Gut 2024; 73: 835-843.
36. Malhotra A, Grunstein RR, Fietze I, et al. Tirzepatide for the treatment of obstructive sleep apnea and obesity N Engl J Med 2024; 391: 1193-1205.
37. Ferrari F, Moretti A, Villa RF. Incretin-based drugs as potential therapy for neurodegenerative diseases: current status and perspectives. Pharmacol Ther 2022; 239: 108277.
38. Norgaard CH, Friedrich S, Hansen CT, et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement 2022; 8: e12268.
39. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 2018; 27: 740-756.
40. Sodhi M, Rezaeianzadeh R, Kezouh A, Etminan M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 2023; 3330: 1795-1797.
41. Raven LM, Brown C, Greenfield JR. Considerations of delayed gastric emptying with peri-operative use of glucagon-like peptide-1 receptor agonists. Med J Aust 2023; 220: 14-16.
42. Clinical practice recommendation on the periprocedural use of GLP-1/GIP receptor agonists. June 2024. Available online at: https://www.anzca.edu.au/resources/professional-documents/endorsed-guidelines/periprocedural-glp-1-use-consensus-clinical-guide.pdf (accessed October 2024).
43. Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010; 67: 220-229.
44. Wang W, Volkow ND, Berger NA, Davis PB, Kaelber DC, Xu R. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat Med 2024; 30: 168-176.
45. Melson E, Ashraf U, Papamargaritis D, Davies MJ. What is the pipeline for future medications for obesity. Int J Obes 2024. Online ahead of print. Doi: 10.1038/s41366-024-01473-y.