Obesity: a therapeutic target in people with cardiovascular disease
Obesity is a major public health problem that contributes indirectly and directly to cardiovascular disease and mortality. The management of obesity is rapidly evolving with new pharmacological treatments leading to weight loss similar to that achieved with weight loss surgery. Glucagon-like peptide-1 receptor agonists have also been shown to improve cardiovascular outcomes in patients with obesity.
- Obesity is a multifaceted disease that contributes directly and indirectly to atherosclerotic cardiovascular disease (CVD), heart failure, atrial fibrillation and multiple CVD risk factors, including dyslipidaemia, type 2 diabetes, hypertension and sleep disorders.
- Healthcare practitioners should be equipped with the skills to ask about weight and address weight loss in a nonjudgemental manner.
- Modest weight loss (5 to 10%) contributes to improvements in cardiovascular risk factors, with greater amounts of weight loss (>10 to 15%) contributing to reductions in major adverse cardiovascular outcomes.
- The use of glucagon-like peptide-1 receptor agonists has been shown to induce more than 10% weight loss with associated improvements in cardiovascular outcomes.
- The obesity treatment landscape is rapidly evolving with several cardiovascular outcome trials for antiobesity medications underway.
- A multidisciplinary team approach, including a cardiologist, endocrinologist and allied healthcare professionals, can assist GPs in developing individualised care plans for people with obesity to reduce its associated cardiovascular morbidity and mortality.
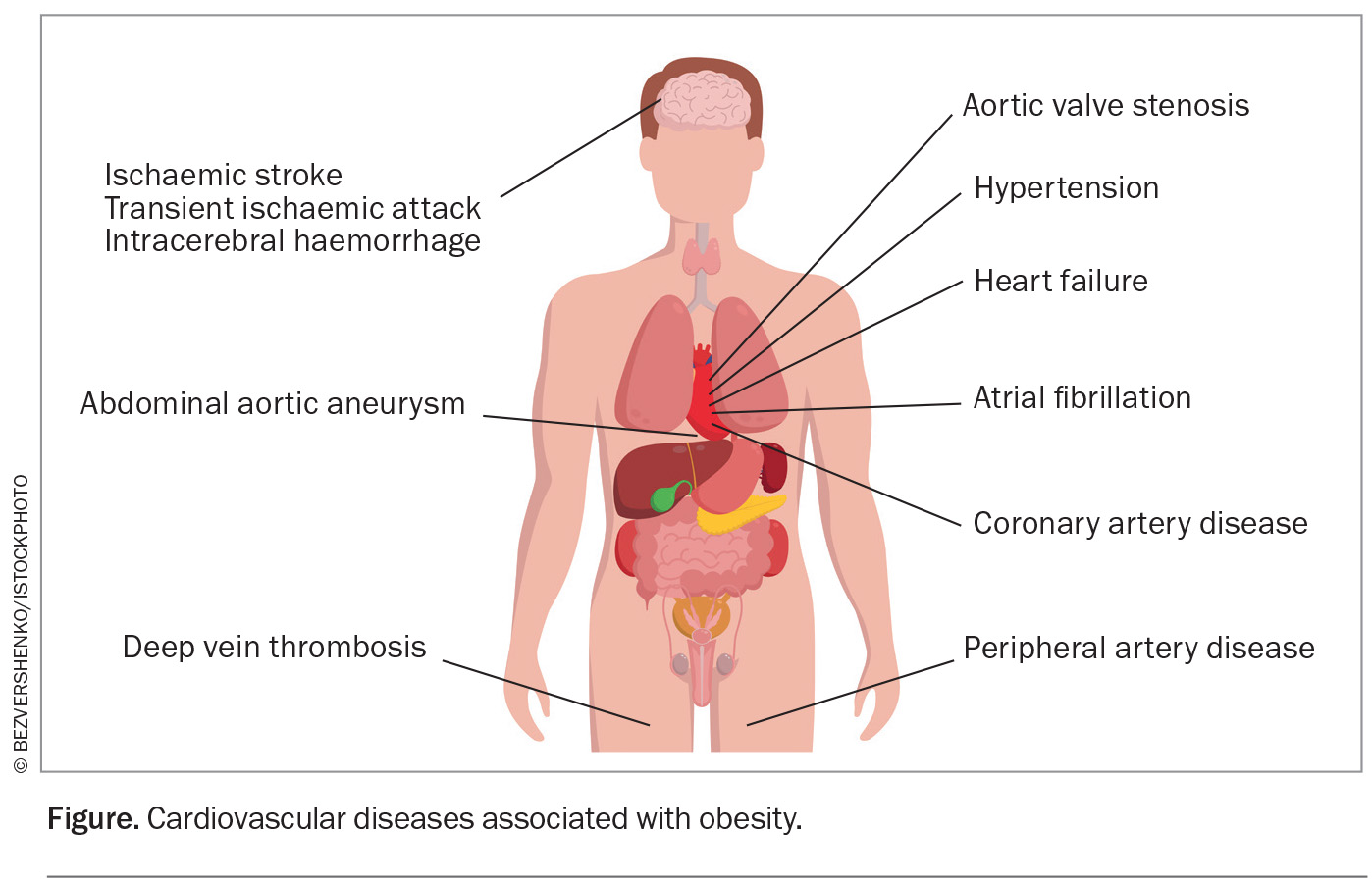
Obesity is a major public health problem worldwide, with at least half of the world’s population projected to be overweight or obese by 2035.1 Obesity is a multifaceted disease that contributes directly and indirectly to atherosclerotic cardiovascular disease (CVD), heart failure, atrial fibrillation (Figure) and multiple CVD risk factors, including dyslipidaemia, type 2 diabetes, hypertension and sleep disorders. It is also increasingly recognised that obesity leads to increased CVD mortality independent of cardiovascular risk factors.2-11 In 2015, obesity was estimated to have accounted for four million deaths globally, two-thirds of which were caused by CVD.12
Obesity definitions
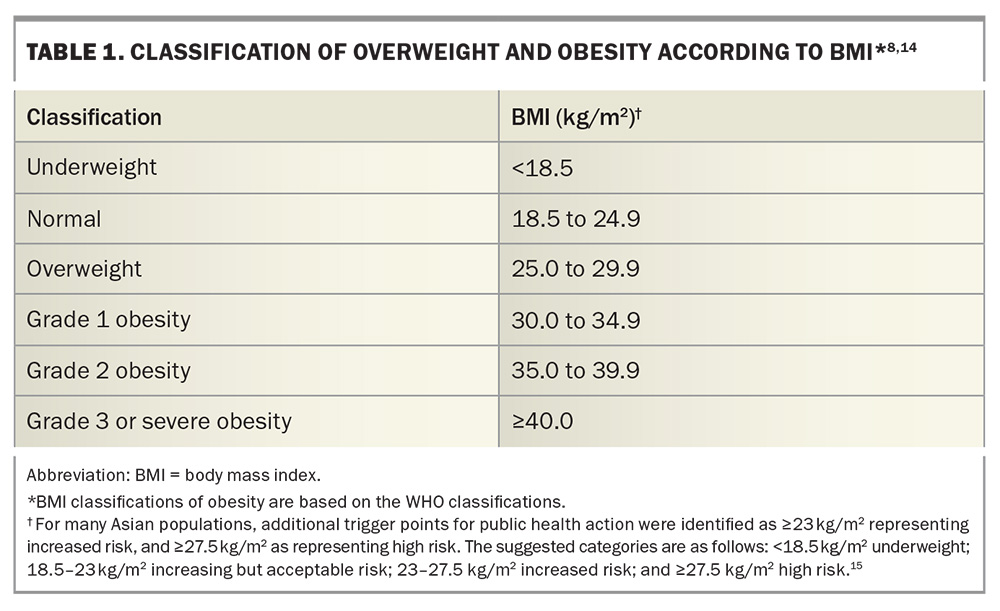
Despite its links with CVD, obesity is underdiagnosed.9 Obesity can be defined using several different parameters, including body mass index (BMI), measurements of central obesity (including waist circumference and waist-to-hip ratio) and body composition. BMI is considered the most practical way to evaluate the degree of obesity, albeit it does not distinguish between fat-free mass (a protective factor) and fat mass (which is associated with adverse outcomes). Central obesity is defined as a waist circumference of more than 102 cm for men and more than 88 cm for women, and a waist-to-hip ratio of 0.90 or more for men and 0.85 or more for women. The waist-to-hip ratio has been suggested to be superior to BMI in predicting cardiovascular risk.13 Nevertheless, BMI remains the most common obesity indicator for studies evaluating obesity interventions and for access to pharmacological and surgical therapies. The most widely accepted BMI classifications of obesity are those from the WHO (Table 1).14
Why is the treatment of obesity important?
Although the management of traditional CVD risk factors applies to patients with and without obesity, a systematic evaluation involving 97 prospective cohorts and 1.8 million participants estimated that blood pressure-, cholesterol- and glucose-lowering interventions would address only half of the excess risk of coronary heart disease and three-quarters of the excess risk of stroke associated with increased BMI.10 Furthermore, modest reductions in weight (5 to 10%) can induce clinically significant improvements in these CVD risk factors, and the degree of benefit in terms of a reduction in CVD risk factors and clinical outcomes have been shown to increase with higher percentages of weight loss.9,16-19 There are also benefits on non-cardiovascular outcomes such as joint pain, obstructive sleep apnoea and quality of life.20,21
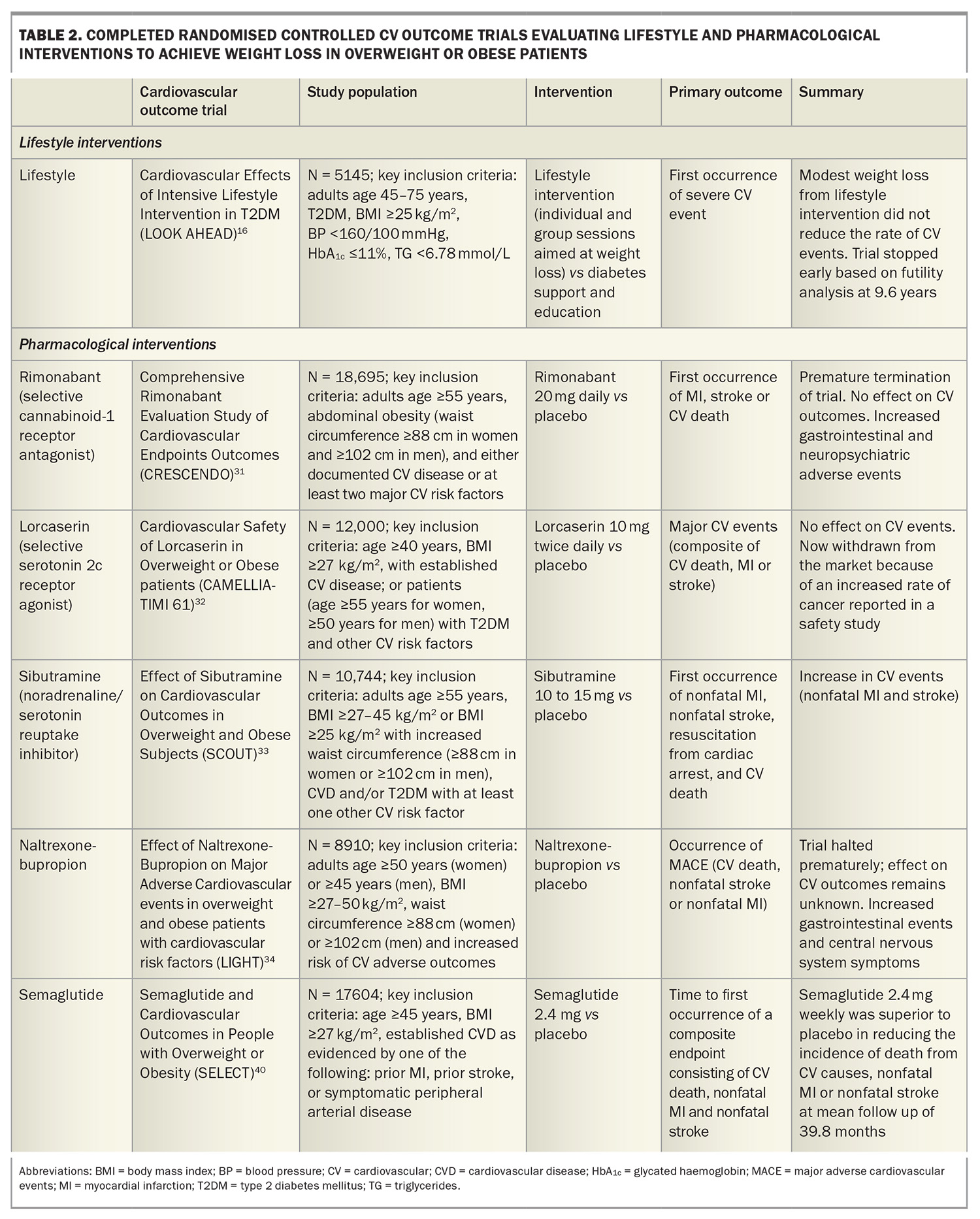
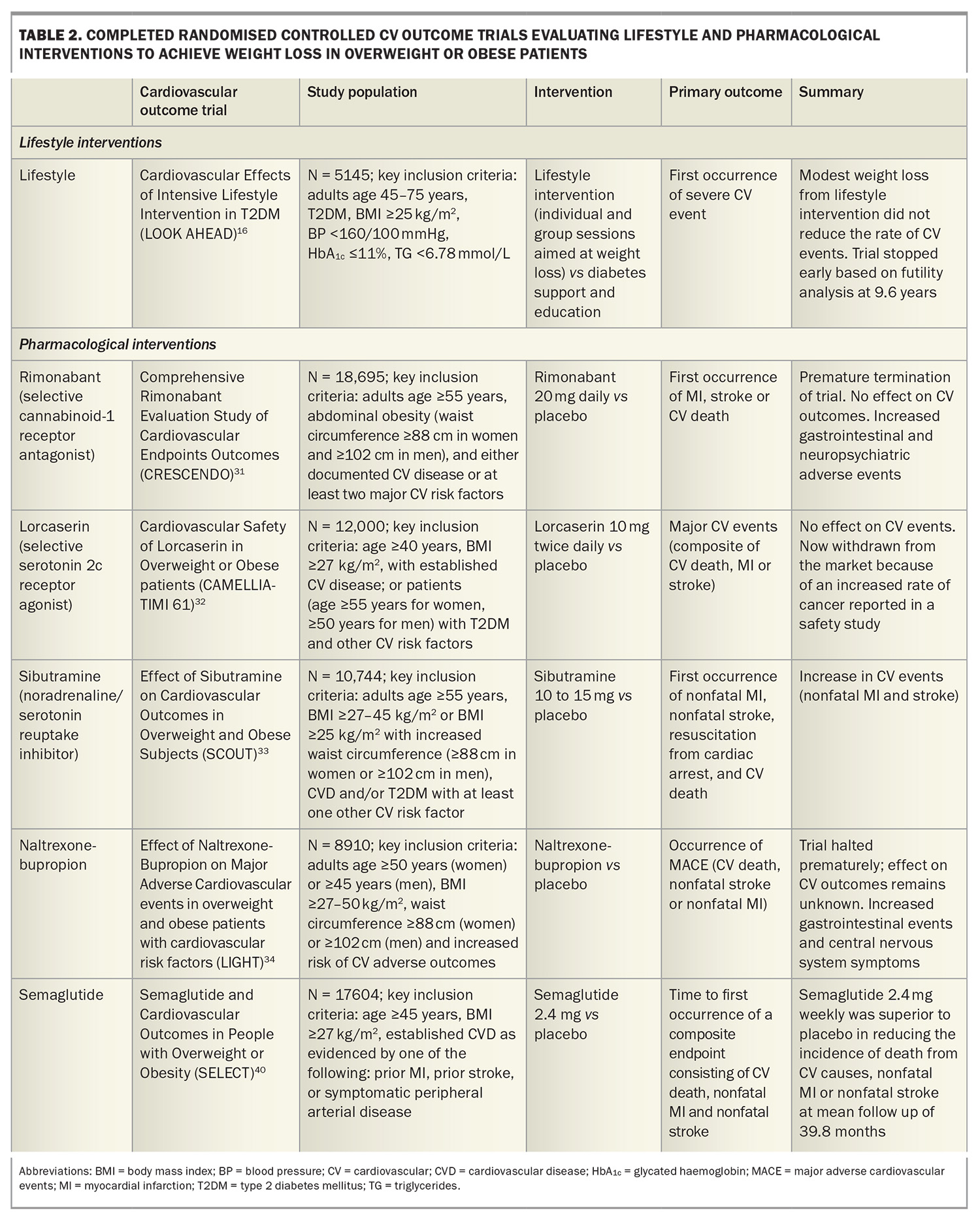
A systematic review and meta-analysis of randomised controlled trials evaluating weight-reduction diets with or without exercise reported an 18% reduction in premature mortality over a median trial duration of two years in adults with obesity.7 About 55% of the weighting in this meta-analysis was contributed by the Action for Health in Diabetes (Look AHEAD) trial, which examined if intensive lifestyle interventions for weight loss would reduce cardiovascular morbidity and mortality in people with overweight or obesity and type 2 diabetes. The intensive lifestyle intervention group achieved greater weight loss in the intervention group compared with the control group (6.0% vs 3.5%). The intensive lifestyle group also produced greater reductions in glycated haemoglobin (HbA1c) levels and improvements in all traditional CVD risk factors (except for LDL-cholesterol levels).16 However, despite these improvements in CVD risk factors and modest weight loss, reductions in cardiovascular outcomes were not demonstrated, so the trial was discontinued at a median follow up of 9.6 years following a futility analysis.11,16 These findings suggest that although lifestyle-based, weight loss interventions are beneficial in the short term, it is difficult for most patients to maintain sufficient weight loss to achieve longer-term reductions in cardiovascular events. Furthermore, weight maintenance remains a challenge, with 80% of weight loss expected to be regained over the following five years.22
Recent developments add new impetus to the notion that larger degrees of weight loss (10 to 20% of body weight) may indeed improve important cardiovascular outcomes.23,24 A post hoc analysis of the Look AHEAD trial reported that at least 10% weight loss in the first year was associated with a 21% lower risk of major adverse cardiac events.23 Similar findings have been reported in analyses of people who have undergone metabolic surgery.11,25-29 Metabolic surgery resulting in weight loss of 20 to 35% of body weight was found to be associated with lower rates of all-cause mortality, cardiovascular mortality, incident heart failure, myocardial infarction and stroke (p<0.001 for all comparisons).29 It is noted, however, that these outcomes were derived from nonrandomised, cohort studies.11,25-29 Nonetheless, metabolic surgery should be considered an important adjunct to reduce CVD risk in patients with a BMI of 40 kg/m2 or more, or over 35 kg/m2 in the presence of obesity-associated comorbidities despite lifestyle interventions and pharmacological therapy.7,29 The three most commonly performed procedures in Australia are sleeve gastrectomy, laparoscopic adjustable gastric banding and Roux-en-Y gastric bypass.
Prioritising obesity treatment to improve cardiovascular outcomes
Reducing the risk of CVD by treating dyslipidaemia, hypertension and diabetes is considered standard evidence-based practice. However, the treatment of obesity requires further recognition as an important priority. In a study of pooled data on more than 10,000 patients with CVD, fewer than 20% of patients had a healthy BMI at the time of their CVD event, and more than a third of the patients with obesity said they had not received advice on physical activity or nutrition, with nearly one in five indicating they had not been informed that they were overweight.30 Perhaps what has contributed to the lack of momentum in the treatment of obesity has been the lack of high-level evidence that lifestyle or pharmacological interventions for overweight or obesity improves cardiovascular outcomes (Table 2).24 Indeed, several pharmacological weight loss agents have been withdrawn from the market because of increased cardiovascular events or adverse side effects,31-34 and until recently only a small number of agents were available and indicated to manage obesity. Currently, phentermine, orlistat, liraglutide, semaglutide and tirzepatide are approved by the TGA to manage obesity in Australia.
Furthermore, observational analyses of overweight and obese patients with CVD suggest an ‘obesity paradox’, whereby elevated BMI may be associated with lower mortality and cardiovascular events. However, this likely reflects reverse causality and the limitations of using BMI to identify visceral obesity, with other studies describing a U-shaped association with respect to weight, and severe obesity being associated with an increased risk of cardiovascular outcomes.6-8,24
Evidence for the new pharmacological therapies
Although lifestyle interventions represent a cornerstone of weight management, sustaining weight loss over the long term is challenging. The management of obesity is changing with the advent of pharmacological agents that lead to substantial weight loss approaching that seen with metabolic surgery.
Glucagon-like peptide-1 agonists
Glucagon-like peptide-1 (GLP-1) is an endogenous incretin produced in the intestines after food intake that enhances insulin secretion and suppresses glucagon release. Cardiovascular outcome studies have evaluated the safety and efficacy of GLP-1 receptor agonists in patients with type 2 diabetes, with meta-analyses reporting significant reductions in major adverse cardiovascular events (hazard ratio [HR] 0.86; 95% confidence interval [CI] 0.80–0.93) with no increase in the risk of severe hypoglycaemia.35 Although these studies did not selectively include patients who were overweight or obese, the average BMI at baseline was over 30 kg/m2. These studies included the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) and Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes (SUSTAIN-6) trials.36,37 Both studies found that injectable GLP-1 receptor agonists (up to 1.8 mg once-daily liraglutide or up to 1.0 mg once-weekly semaglutide) reduced the risk of major adverse cardiovascular events in patients with type 2 diabetes who were at high cardiovascular risk.36,37
The Semaglutide Treatment Effect in People with obesity (STEP) trials evaluated high-dose semaglutide of 2.4 mg once weekly in people with obesity. The STEP-1 trial showed that from baseline to week 68, the mean change in body weight in people without diabetes was –14.9% in the semaglutide group compared with –2.4% in the placebo group (p<0.001).38 The STEP-5 trial reported sustained weight loss with semaglutide out to 104 weeks.39
The recent Semaglutide and Cardiovascular Outcomes in People with Overweight or Obesity (SELECT) trial was the first randomised, placebo-controlled, cardiovascular outcome trial evaluating semaglutide in patients without diabetes who were overweight or obese (Table 2).40 A total of 17,604 patients aged 45 years or older who had pre-existing CVD and a BMI of 27 kg/m2 or more without diabetes were randomly assigned to receive once-weekly subcutaneous semaglutide titrating up to a dose of 2.4 mg or placebo. Semaglutide reduced the incidence of death from cardiovascular causes, nonfatal myocardial infarction or nonfatal stroke at a mean follow up to 39.8 months.40 Patients randomised to semaglutide also showed greater improvements in a variety of secondary endpoints, including HbA1c levels, systolic and diastolic blood pressure and levels of high-sensitivity C-reactive protein and lipids.40
Although the mechanism of the reduction in cardiovascular events may be through weight loss and its impact on traditional CVD risk factors (e.g. lipid levels, glycaemia, blood pressure), the early separation in cardiovascular outcomes between the semaglutide and placebo groups after treatment initiation before substantial weight loss occurred suggests potential direct cardioprotective benefits beyond weight loss. This could include effects on inflammatory and prothrombotic factors or other pleiotropic effects, with further support for weight-loss independent benefits provided by a recent mediation analysis.41
Two recent studies have also found that treatment with semaglutide aiming for a target dose of 2.4 mg once weekly led to significant weight loss, improved quality of life and increased the six-minute walk distance in patients with obesity and heart failure with a preserved or mildly reduced ejection fraction with or without type 2 diabetes.42,43 Given that obesity is associated with lower levels of N-terminal proB-type natriuretic peptide (NT-proBNP), it is notable that the reduction in weight observed in both studies was associated with a reduction in NT-proBNP levels, suggesting a beneficial effect on myocardial remodelling.
Beyond its effects on cardiovascular outcomes, semaglutide aiming for a target dose of 1.0 mg weekly was also found to reduce the risk of clinically important kidney outcomes and death from cardiovascular causes in patients with type 2 diabetes and chronic kidney disease (defined by estimated glomerular filtration rate [eGFR] of 50 to 75 mL/min/1.73 m2 and urinary albumin to creatinine ratio of >300 and <5000 or eGFR 25 to <50 mL/min/1.73 m2 and urinary albumin to creatinine ratio of >100 and <5000).44 The primary outcomes were major kidney disease events, a composite of the onset of kidney failure (dialysis, transplantation or an eGFR of <15 mL/min/1.73 m2), at least 50% reduction in eGFR from baseline or death from kidney-related or cardiovascular causes. The risk of a primary outcome event was 24% lower in the semaglutide group than in the placebo group (HR 0.76; 95% CI 0.66–0.88; p = 0.0003) at a median follow up of 3.4 years. The results were similar for a composite of the kidney-specific components of the primary outcome (HR 0.79; 95% CI 0.66–0.94) and for death from cardiovascular causes (HR 0.71; 95% CI 0.56–0.89). The risk of major cardiovascular events was 18% lower in the semaglutide group than in the placebo group (HR 0.82; 95% CI 0.68–0.98; p = 0.029).44
The semaglutide 2.4 mg weekly dose was TGA approved in August 2024 for chronic weight management. This includes adults with an initial BMI of 30 kg/m2 or more, or 27 to 30 kg/m2 with at least one weight-related comorbidity (such as hypertension, dyslipidaemia, obstructive sleep apnoea, CVD, pre-diabetes or type 2 diabetes). It is currently not subsidised under the PBS and is only available via private prescription.
Glucagon-like peptide-1/glucose-dependent insulinotropic polypeptide receptor agonist
Tirzepatide is a once-weekly subcutaneous injectable peptide with agonist activity at both the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors. GIP activation appears to act synergistically with GLP-1 receptor activation to allow greater weight reduction than that achieved with GLP-1 receptor monoagonism.45
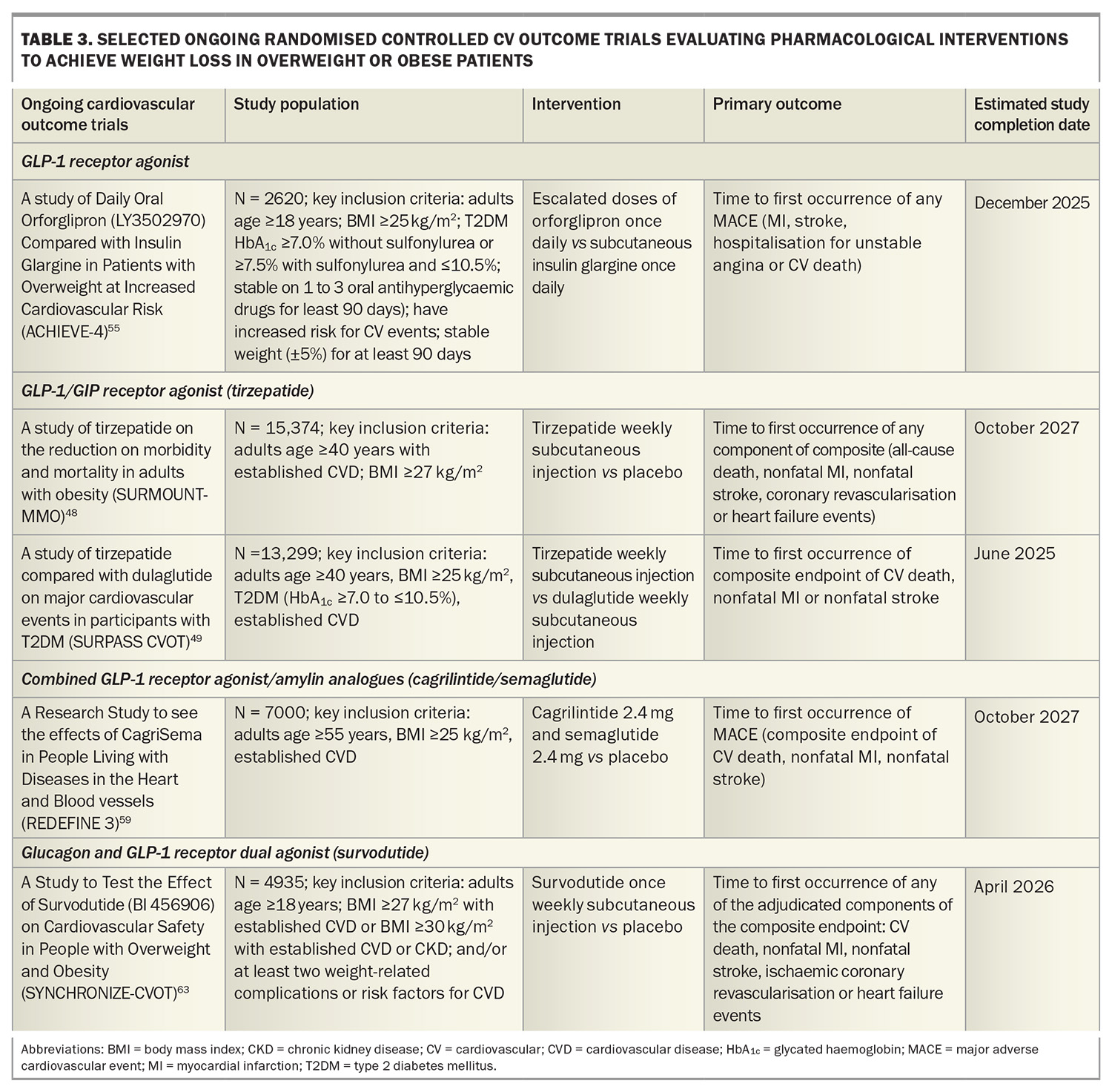
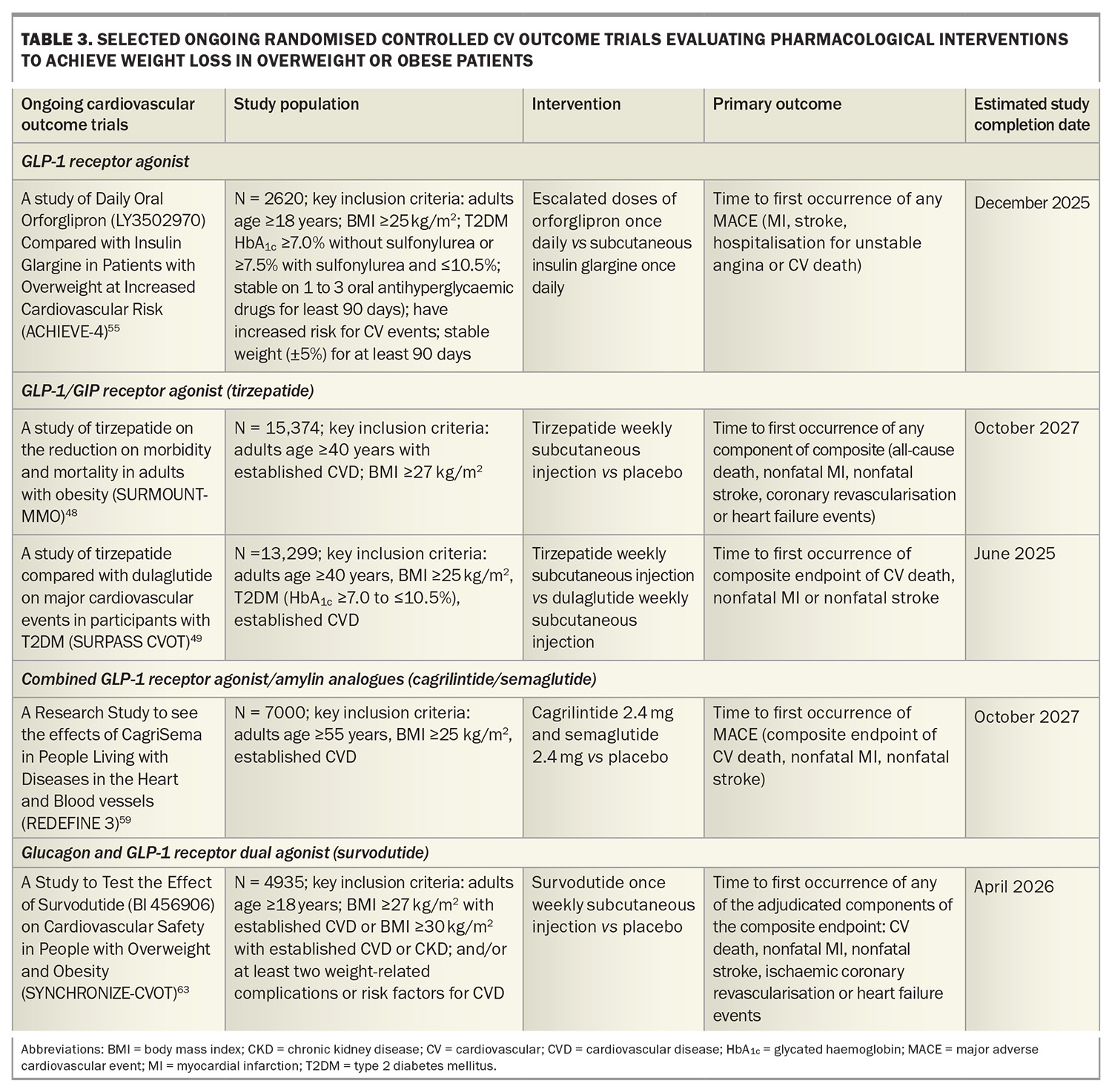
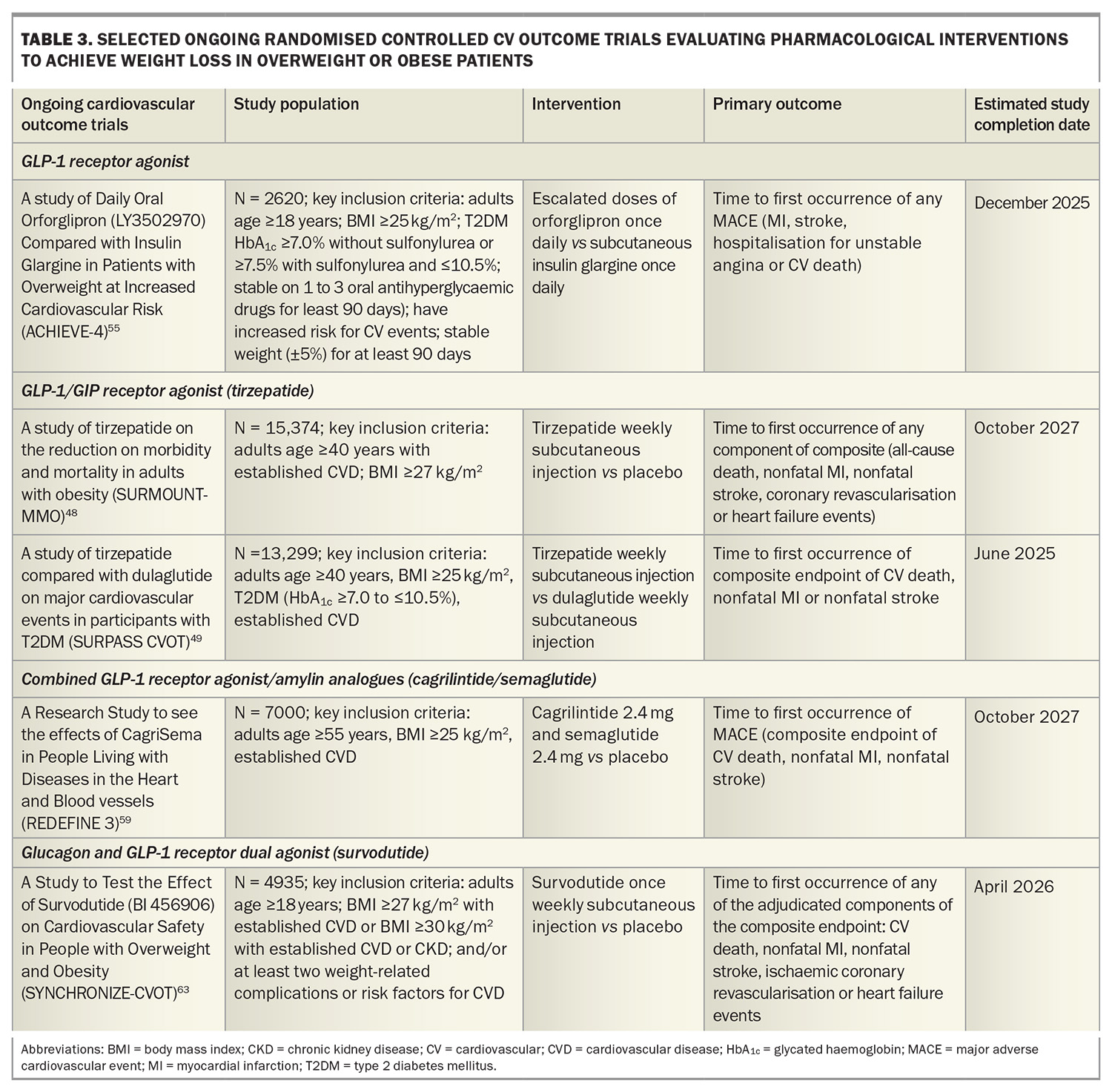
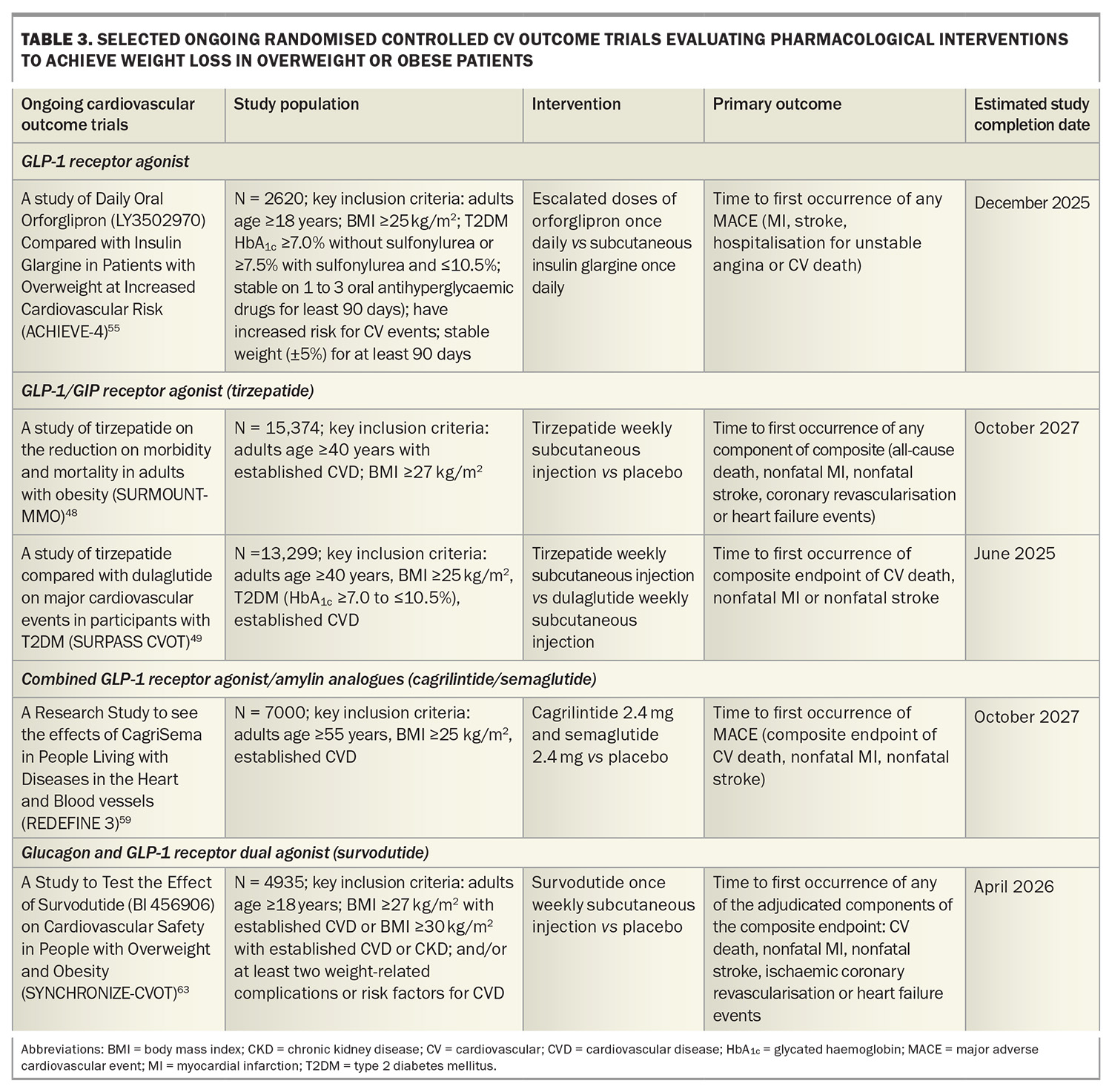
A Study of Tirzepatide Versus Semaglutide Once Weekly as Add-on Therapy to Metformin in Participants With Type 2 Diabetes (SURPASS-2) demonstrated noninferiority or superiority of tirzepatide at all doses (5 mg, 10 mg, 15 mg) compared with semaglutide 1 mg as an add-on therapy to metformin with respect to the mean change in HbA1c level and weight loss from baseline to 40 weeks. The estimated mean change from baseline in HbA1c with tirzepatide 15 mg was 2.3% and a weight reduction of 11 kg.46 The tirzepatide once weekly for the treatment of obesity (SURMOUNT-1) trial showed that tirzepatide 15 mg weekly led to mean reductions in body weight up to 20.9% in patients with overweight or obesity but without diabetes after 72 weeks of treatment compared with 3.1% with placebo.47 Cardiovascular outcome trials are underway in patients with and without diabetes (Table 3).48,49
A Study of Tirzepatide in Participants with Heart Failure with Preserved Ejection Fraction with Obesity (SUMMIT) showed that weekly tirzepatide for a median follow up of two years led to a lower risk of a composite of death from cardiovascular causes or worsening heart failure (HR 0.62; 95% CI 0.41–0.95; p = 0.026) and improved quality of life compared with placebo in patients with heart failure with preserved ejection fraction and obesity.50
Tirzepatide was approved by the TGA for the treatment of overweight and obesity via private prescription in September 2024. Eligible adults include those with an initial BMI of 30 kg/m2 or more, or 27 to 30 kg/m2 with at least one weight-related comorbidity.
Novel agents
Looking to the next generation of obesity treatments, oral GLP-1 receptor agonists and combinations of GLP-1 receptor agonists with other enteropancreatic hormones (such as GIP agonists, glucagon receptor agonists and amylin analogues) are under investigation to enhance the weight loss and cardiovascular benefits of GLP-1 agonists.51
Oral GLP-1 receptor agonists
Oral GLP-1 receptor agonists are under development with data from phase 3 trials showing similar weight loss efficacy between high-dose oral semaglutide 50 mg (–17.4% weight loss at week 68) and subcutaneous semaglutide.52,53 Orforglipron, an oral nonpeptide GLP-1 receptor agonist, was also observed to demonstrate weight loss results similar to those observed with injectable GLP-1 receptor agonists in a phase 2 trial, with ongoing studies evaluating the efficacy of orforglipron on weight loss and cardiovascular outcomes (Table 3).54,55
GLP-1 agonist, GIP agonist and glucagon receptor agonist
Retatrutide, an agonist of the GIP, GLP-1 and glucagon receptors, has shown promise in clinical trials for significant weight loss. In a phase 2 trial including people with obesity, 12 mg once-weekly retatrutide treatment resulted in a mean weight reduction of 24.2% after 48 weeks.56 A phase 3 randomised controlled trial to Investigate the Efficacy and Safety of Retatrutide Once Weekly Compared to Placebo in Participants with Severe Obesity and Established Cardiovascular Disease (TRIUMPH-3) seeks to determine the percentage change from baseline in body weight in patients with a BMI of 35 kg/m2 or more and established CVD.57
GLP-1 receptor agonist and amylin analogue
Amylin is co-secreted with insulin from the pancreas and plays a key role in postprandial satiety regulation and improvement of glucose metabolism by delaying gastric emptying and inhibiting glucagon secretion. A phase 2 trial in adults with type 2 diabetes and a BMI of 27 kg/m2 or more showed that combining the GLP-1 receptor agonist semaglutide with the long-acting analogue cagrilintide resulted in significantly greater weight loss (–15.6%) compared with semaglutide alone (–5.1%) and cagrilintide alone (–8.1%) at 32 weeks.58 A Research Study to See the Effects of CagriSema in People Living With Diseases in the Heart and Blood Vessels (REDEFINE-3) phase 3 study will assess the effect of combined semaglutide and cagrilintide on major adverse cardiovascular events in people with obesity (with and without type 2 diabetes) and established CVD (Table 3).59
Glucagon and GLP-1 receptor dual agonist
In a phase 2 randomised controlled trial including patients without diabetes and a BMI of 27 kg/m2 or more, once-weekly subcutaneous survodutide (a glucagon receptor and GLP-1 receptor dual agonist) at doses of 0.6 mg, 2.4 mg, 3.6 mg and 4.8 mg was found to reduce body weight compared with placebo over 46 weeks, with a 14.9% reduction seen with the 4.8 mg dose. There were also reductions in blood pressure compared with placebo.60 Two ongoing phase 3 studies including A Study to Test Whether Survodutide Helps People with Overweight or Obesity Who do Not Have (SYNCHRONISE-1) or Have Diabetes (SYNCHRONISE-2) to Lose Weight will use a higher maintenance treatment dose (3.6 mg or 6 mg compared with placebo) over a longer treatment period (76 weeks).61,62 SYNCHRONISE- Cardiovascular Outcomes Trial is a phase 3, randomised, double-blind trial investigating the cardiovascular safety of survodutide in people with overweight and obesity with CVD, chronic kidney disease or risk factors for CVD (Table 3).63
Considerations for healthcare practitioners
Although addressing obesity can be challenging, all healthcare professionals should ask permission to discuss a patient’s weight in a sensitive, nonjudgemental manner (Box). Obesity should now be considered a CVD risk factor, in addition to the traditional risk factors, with an evolving body of evidence that weight reduction leads to improvements in health outcomes over and above its effect on intermediary CVD risk factors. Lifestyle interventions should form the basis of treating obesity, which usually involves a multidisciplinary collaboration including GPs, cardiologists, endocrinologists and allied healthcare professionals. However, if lifestyle approaches are insufficient, healthcare practitioners should discuss initiating pharmacotherapy, such as GLP-1 receptor agonists, while recognising the limitations of reimbursement and supply. An alternative approach for patients who have not achieved sufficient weight loss with lifestyle and pharmacotherapy is referral to appropriate specialists for consideration of metabolic surgery.
Conclusion
Major international guidelines now emphasise the role of obesity management as an important priority to reduce the burden of incident and recurrent cardiovascular events. Lifestyle management remains a cornerstone of obesity management with diverse health benefits; however, weight maintenance remains a challenge. The obesity treatment landscape is rapidly evolving with recent trials demonstrating the benefit of GLP-1 receptor agonists in inducing weight loss and reducing cardiovascular events. Cardiovascular outcome trials evaluating novel agents that target multiple pathways, including combinations of GLP-1 agonism, GIP agonism, glucagon receptor agonism and amylin analogues, are eagerly awaited. MT
COMPETING INTERESTS: Dr Sun: None. Professor Atherton has received travel sponsorship and/or research funding from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Novo Nordisk. He has given lectures and/or attended advisory boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Novo Nordisk with payments made to his employer.
References
1. World Obesity Federation. World Obesity Atlas 2023. Available online at: https://data.worldobesity.org/publications/?cat=19 (accessed December 2024).
2. Bogers RP, Bemelmans WJ, Hoogenveen RT, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med 2007; 167: 1720-1728.
3. Bianchettin RG, Lavie CJ, Lopez-Jimenez F. Challenges in Cardiovascular Evaluation and Management of Obese Patients: JACC State-of-the-Art Review. J Am Coll Cardiol 2023; 81: 490-504.
4. Hritani R, Al Rifai M, Mehta A, German C. Obesity management for cardiovascular disease prevention. Obes Pillars 2023;7:100069. doi:10.1016/j.obpill.2023.100069
5. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983;67: 968-977.
6. Lavie CJ, Arena R, Alpert MA, Milani RV, Ventura HO. Management of cardiovascular diseases in patients with obesity. Nat Rev Cardiol 2018; 15: 45-56.
7. Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ 2017; 359: 4849.
8. Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2021; 143: 984-1010.
9. Ryan DH, Deanfield JE, Jacob S. Prioritizing obesity treatment: expanding the role of cardiologists to improve cardiovascular health and outcomes. Cardiovasc Endocrinol Metab 2023; 12: 0279.
10. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects), Lu Y, Hajifathalian K, Ezzati M, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014; 383: 970-983.
11. Abdul Wahab R, le Roux CW. A review of the evidence on cardiovascular outcomes from obesity treatment. Obes Pillars 2023; 7: 100071.
12. GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 2017; 377: 13-27.
13. World Health Organisation. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8-11 December 2008. Available online at: https://www.who.int/publications/i/item/9789241501491 (accessed December 2024).
14. WHO Consultation on Obesity (1997: Geneva, Switzerland), World Health Organization. Division of Noncommunicable Diseases & World Health Organization. Programme of Nutrition, Family and Reproductive Health. (1998). Obesity: preventing and managing the global epidemic: report of a WHO Consultation on Obesity, Geneva, 3-5 June 1997. World Health Organization. Available online at: https://iris.who.int/handle/10665/63854 (accessed December 2024).
15. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies Lancet 2004; 363: 157-163.
16. Look AHEAD Research Group, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369: 145-154.
17. Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001; 134: 1-11.
18. Morris E, Jebb SA, Oke J, et al. Effect of weight loss on cardiometabolic risk: observational analysis of two randomised controlled trials of community weight-loss programmes. Br J Gen Pract 2021; 71: 312-319.
19. Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343-1350.
20. Bliddal H, Bays H, Czernichow S, et al. Once-weekly semaglutide in persons with obesity and knee osteoarthritis. N Engl J Med 2024; 391: 1573-1583.
21. Malhotra A, Bednarik J, Chakladar S, et al. Tirzepatide for the treatment of obstructive sleep apnea: Rationale, design, and sample baseline characteristics of the SURMOUNT-OSA phase 3 trial. Contemp Clin Trials 2024; 141: 107516.
22. Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 2001; 74: 579-584.
23. Look AHEAD Research Group, Gregg EW, Jakicic JM, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016; 4: 913-921.
24. Neeland IJ, McGuire DK, Sattar N. Cardiovascular outcomes trials for weight loss interventions: another tool for cardiovascular prevention? Circulation 2021; 144: 1359-1361.
25. Aminian A, Zajichek A, Arterburn DE, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 2019; 322: 1271-1282.
26. Doumouras AG, Wong JA, Paterson JM, et al. Bariatric surgery and cardiovascular outcomes in patients with obesity and cardiovascular disease: a population-based retrospective cohort study. Circulation 2021; 143: 1468-1480.
27. Fisher DP, Johnson E, Haneuse S, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA 2018; 320: 1570-1582.
28. Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014; 311: 2297-2304.
29. Van Veldhuisen SL, Gorter TM, van Woerden G, et al. Bariatric surgery and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J 2022; 43: 1955-1969.
30. De Bacquer D, Jennings CS, Mirrakhimov E, et al. Potential for optimizing management of obesity in the secondary prevention of coronary heart disease. Eur Heart J Qual Care Clin Outcomes 2022; 8: 568-576.
31. Topol EJ, Bousser MG, Fox KA, et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet 2010; 376: 517-523.
32. Bohula EA, Wiviott SD, McGuire DK, et al. Cardiovascular safety of lorcaserin in overweight or obese patients. N Engl J Med 2018; 379: 1107-1117.
33. James WP, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 2010; 363: 905-917.
34. Nissen SE, Wolski KE, Prcela L, et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA 2016; 315: 990-1004.
35. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021; 9: 653-662.
36. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311-322.
37. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834-1844.
38. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021; 384: 989-1002.
39. Garvey WT, Batterham RL, Bhatta M, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med 2022; 28: 2083-2091.
40. Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023; 389: 2221-2232.
41. Colhoun H, Lincoff AM, Linder M, et al. Exploratory mediation analysis of the effect of semaglutide on cardiovascular outcomes in people with overweight or obesity in the SELECT randomised trial. Eur Heart J 2024; 45 (supplement 1): ehae666.2792.
42. Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med 2023; 389: 1069-1084.
43. Kosiborod MN, Petrie MC, Borlaug BA, et al. Semaglutide in patients with obesity-related heart failure and type 2 diabetes. N Engl J Med 2024; 390: 1394-1407.
44. Perkovic V, Tuttle KR, Rossing P, et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med 2024; 391: 109-121.
45. Cho YK, La Lee Y, Jung CH. The cardiovasculareffect of tirzepatide: a glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide dual agonist. J Lipid Atheroscler 2023; 12: 213-222.
46. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021; 385: 503-515.
47. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022; 387: 205-216.
48. A Study of Tirzepatide (LY3298176) on the Reduction on Morbidity and Mortality in Adults with Obesity (SURMOUNT-MMO) (NCT05556512). Information from clinicaltrials.gov. Available online at: https://classic.clinicaltrials.gov/ct2/show/NCT05556512 (accessed December 2024).
49. Nicholls SJ, Bhatt DL, Buse JB, et al. Comparison of tirzepatide and dulaglutide on major adverse cardiovascular events in participants with type 2 diabetes and atherosclerotic cardiovascular disease: SURPASS-CVOT design and baseline characteristics. Am Heart J 2024; 267: 1-11.
50. Packer M, Zile MR, Kramer CM, et al. Tirzepatide for heart failure with preserved ejection fraction and obesity. N Engl J Med 2024. Publish Ahead of Print e-pub doi.
51. Melson E, Ashraf U, Papamargaritis D, Davies MJ. What is the pipeline for future medications for obesity? Int J Obes (Lond) 2024.
52. Knop FK, Aroda VR, do Vale RD, et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023; 402: 705-719.
53. Aroda VR, Aberle J, Bardtrum L, et al. Efficacy and safety of once-daily oral semaglutide 25 mg and 50 mg compared with 14 mg in adults with type 2 diabetes (PIONEER PLUS): a multicentre, randomised, phase 3b trial. Lancet 2023; 402: 693-704.
54. Wharton S, Blevins T, Connery L, et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N Engl J Med 2023; 389: 877-888.
55. A Study of Daily Oral Orforglipron (LY3502970) Compared with Insulin Glargine in Participants with Type 2 Diabetes and Overweight at Increased Cardiovascular Risk (ACHIEVE-4) (NCT05803421). Information from clinicaltrials.gov. Available online at: https://classic.clinicaltrials.gov/ct2/show/NCT05803421 (accessed December 2024).
56. Jastreboff AM, Kaplan LM, Frías JP, et al. Triple-Hormone-Receptor Agonist Retatrutide for Obesity - A Phase 2 Trial. N Engl J Med. 2023;389: 514-526.
57. A Study of Retratrutide (LY3437943) In Participants With Obesity and Cardiovascular Disease (TRIUMPH- 3) (NCT05882045). Information from clinicaltrials.gov. Available online at: https://classic.clinicaltrials.gov/ct2/show/NCT05882045 (accessed December 2024).
58. Frias JP, Deenadayalan S, Erichsen L, et al. Efficacy and safety of co-administered once-weekly cagrilintide 2·4 mg with once-weekly semaglutide 2.4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet 2023; 402: 720-730.
59. A Research Study to See the Effects of CagriSema in People Living With Diseases in the Heart and Blood Vessels (REDEFINE 3) (NCT05669755). Information from clinicaltrials.gov. Available online at: https://classic.clinicaltrials.gov/ct2/show/NCT05669755 (accessed December 2024).
60. Le Roux CW, Steen O, Lucas KJ, Startseva E, Unseld A, Hennige AM. Glucagon and GLP-1 receptor dual agonist survodutide for obesity: a randomised, double-blind, placebo-controlled, dose-finding phase 2 trial. Lancet Diabetes Endocrinol 2024; 12: 162-173.
61. A Study to Test Whether Survodutide (BI 456906) Helps People Living with Overweight Or Obesity Who do Not Have Diabetes to Lose Weight (SYNCHRONISE-1) (NCT06066515). Information from clinicaltrials.gov. Available online at: https://classic.clinicaltrials.gov/ct2/show/NCT06066515 (accessed December 2024).
62. A Study to Test Whether Survodutide (BI 456906) Helps People Living with Overweight Or Obesity Who Also Have Diabetes to Lose Weight (SYNCHRONISE-2) (NCT06066528). Information from clinicaltrials.gov. Available online at: https://classic.clinicaltrials.gov/ct2/show/NCT06066528 (accessed December 2024).
63. A Study to Test the Effect of Survodutide (BI 456906) on Cardiovascular Safety In People with Overweight or Obesity (SYNCHRONIZE- CVOT) (NCT06077864). Information from clinicaltrials.gov. Available online at: https://classic.clinicaltrials.gov/ct2/show/NCT06077864 (accessed December 2024).