Obesity, atrial fibrillation and cardiovascular risk: a classic trifecta
Obesity is widely established to play a causal role in the development of atrial fibrillation (AF) through the augmentation of cardiovascular risk factors, such as hypertension, type 2 diabetes and obstructive sleep apnoea. The classic combination of obesity and AF is being increasingly recognised among patients and requires a patient-centred and evidence-based approach for management.
Obesity is a global epidemic with a prevalence that has been increasing progressively since 1980.1 According to the WHO, 39% of all adults were overweight and 13% had obesity in 2016.1 By 2025, the prevalence of obesity is projected to reach 18% among men and 21% among women.2-9 GPs and specialists may encounter patients with cardiovascular (CV) conditions, such as atrial fibrillation (AF), in the presence of obesity. Management in these cases requires a patient-tailored and evidence-based approach.10 This article describes a clinical case of newly diagnosed AF in a woman who copresents with severe obesity and hypertension. Management of her AF requires a careful consideration of her comorbidities and risk factors.
Case scenario
A 38-year-old woman attends your general practice clinic regularly. She has class 3 obesity, with a body mass index (BMI) of 44 kg/m2. Her comorbidities include hypertension, for which you have prescribed perindopril 5 mg daily, as well as newly diagnosed AF with rapid ventricular response, which is causing symptoms of palpitations and lightheadedness. Her CHA2DS2-VASc score is 1, and a decision was made to commence her on a non-vitamin K antagonist oral anticoagulant. You have prescribed metoprolol 25 mg twice daily. You are considering a referral to a cardiologist for AF ablation (pulmonary vein isolation).
What is the significance of the patient’s class 3 obesity in terms of elevating her CV risk?
The mortality associated with CV disease (CVD) may be reduced with primary prevention approaches to care, which particularly include the management of key risk factors such as obesity.3,11 Obesity accelerates the process of atherosclerosis via several mechanisms including insulin resistance and inflammation. It is often associated with other CV risk factors, such as hypertension, dyslipidaemia and elevated blood glucose levels, which collectively increase the risk of accelerated atherosclerosis and early onset of CVD.2-5
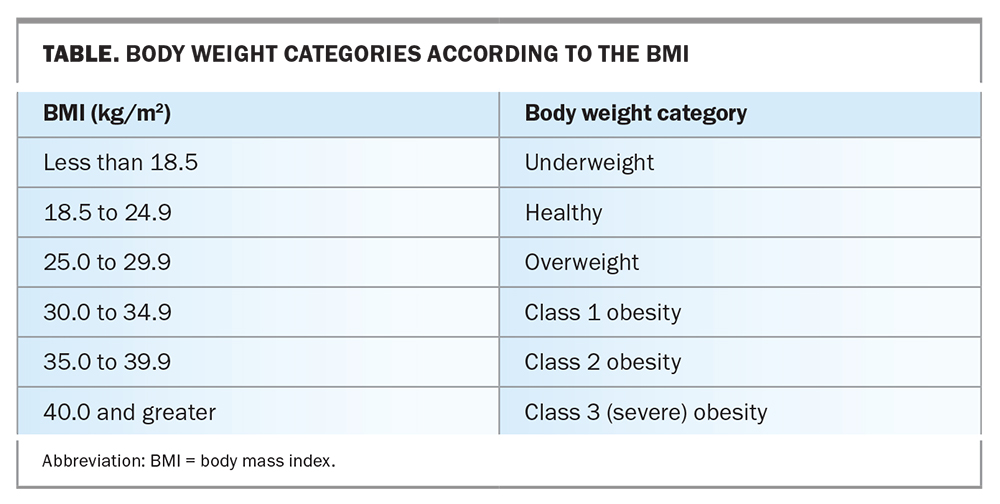
A large meta-analysis has shown that BMIs 25 kg/m2 and greater are strongly associated with an elevated CV risk.4-6 The BMI is the standard measure to quantify obesity (Table); however, the total cumulative exposure to excessive adiposity, as expressed in BMI-years or waist circumference-years, may be an even stronger predictor of CV risk.5-7 A relationship between the BMI and all-cause mortality has been consistently reported worldwide and is independent of sex.3 In particular, BMIs 40 kg/m2 or greater are associated with a reduction in life expectancy by about 10 years, and BMIs 30 to 34.9 kg/m2 are associated with a reduction in life expectancy by about three years compared with BMIs 18.5 to 24.9 kg/m2.7-11 BMIs greater than 25 kg/m2 are positively associated with an increased risk of mortality due to coronary artery disease and stroke.6 Evidence points to a direct causal link between obesity and CVD.12
What are the associations among the patient's class 3 obesity, hypertension and obstructive sleep apnoea?
Similar to obesity, hypertension has a profound impact on global health. The excessive accumulation of adipose tissue initiates a cascade of events that give rise to elevated blood pressure; as such, obesity-induced hypertension is commonly recognised. Increased sympathetic activity, elevated adipokines and insulin levels in individuals with obesity are proposed to cause new-onset hypertension or may accelerate pre-existing hypertension. Renal function, sodium excretion, salt sensitivity and renin–angiotensin–aldosterone system activity are also significantly altered in the presence of obesity, which promotes the development of hypertension.13 Long-term follow-up data indicate that CVD-related mortality is exponentially increased in the presence of both hypertension and obesity.14 The prevalence of obstructive sleep apnoea (OSA) also increases significantly in patients with an elevated BMI and has also been shown to predict hypertension and adverse CV outcomes, such as the development of AF.15
Can weight loss reverse the patient's CV risk profile and alleviate her current comorbidities?
Weight management is of central importance in managing CV risk in patients with obesity. Weight loss beneficially affects traditional CV risk factors, such as hypertension, type 2 diabetes and dyslipidaemia, and may reverse OSA. In a randomised controlled trial of patients with type 2 diabetes, lifestyle interventions that led to at least a 10% reduction in body weight in the first year of the study resulted in a 21% lower risk of CVD related mortality.16 This study suggests that achieving a threshold of weight loss may be necessary before a mortality benefit is gained.
What options are available to help with weight loss?
Recommended weight loss interventions include lifestyle, behavioural, pharmacological and surgical options. Dietary modifications (e.g. consuming a Mediterranean diet) have been shown to be beneficial in the primary prevention of CVD, which is predominantly related to the consequent weight loss. Consuming a ketogenic diet might lead to greater weight loss and a positive effect on blood sugars and dyslipidaemia.17
Traditional pharmacological treatments for weight reduction, such as phentermine and bupropion with naltrexone, have varying degrees of efficacy and are restricted by cost and safety concerns. Until recently, they have not been widely adopted.18-21 Phase 3 studies of the glucagon-like peptide 1 receptor agonist drug semaglutide (STEP trials 1 to 4) showed an average weight loss of about 15% together with improvements in CV risk factors.22 Ongoing CV outcome studies are evaluating oral semaglutide in patients with obesity and established CVD. Trials in patients with AF are planned.
Bariatric surgical procedures, such as gastric sleeve or bypass surgery, have been shown to result in notable weight loss in patients with class 3 obesity. In observational studies, this weight reduction has resulted in reduced incidence rates of type 2 diabetes, CVD related death, myocardial infarction and stroke over a 15-year follow-up period.23 Although surgery is highly effective for achieving significant weight loss, the proportion of people able to access bariatric surgery is low compared with the number of people living with obesity; this discrepancy is attributed to limited availability with prolonged waiting times, cost and surgical morbidity. Bariatric surgery will continue to be an important option for selected patients with severe obesity but is not broadly applicable among patients with obesity.
What is the association between the patient's class 3 obesity and AF?
A wide array of epidemiological data and randomised studies have confirmed the strong causative relationship between obesity and incident AF. Obesity has been suggested as one of the major factors responsible for the rising epidemic of AF.10,24-26 The mechanisms by which obesity results in AF are multifactorial and include the promotion of hypertension, type 2 diabetes and OSA. In addition, the accumulation of excess epicardial adiposity has both direct and indirect effects on the atrial myocardium.
Both pharmacological and interventional catheter ablation treatment options have been shown to be significantly less effective in patients with obesity than in patients who do not have obesity. In particular, late recurrence after an apparently effective ablation procedure is a well-recognised complication in patients with obesity. The complication rates of catheter ablation procedures may also be higher in patients who have obesity. Randomised controlled trials have shown that comprehensive risk factor management centred around weight loss and increasing physical exercise, either as a primary strategy or in association with catheter ablation, can significantly reduce the AF burden and symptom recurrence.27,28 Optimal effects are seen in patients who achieve a greater than 10% reduction in body weight, but benefits have also been reported in those with a 3% to 9% weight reduction.27 The REVERSE-AF trial showed that weight reduction achieved through physician-led risk factor management frequently resulted in a significant reduction in AF burden and reversal of persistent AF to paroxysmal AF.27 Patients achieving 10% or greater reductions in body weight showed a high prevalence of AF freedom.28 Bariatric surgery in patients with severe obesity and AF has also been associated with significant reduction in AF burden and reduced incidence of new AF.29
What are some considerations before planning AF ablation in this patient?
The ARREST-AF cohort study showed significantly improved outcomes of catheter ablation in patients who lost weight with comprehensive risk factor management from a dedicated clinic.28 Long-term follow up of these patients indicated that persistent weight loss is associated with excellent long-term outcomes of catheter ablation. Similarly, observational studies of bariatric surgery in patients with severe obesity and AF have indicated that it is possible to achieve ablation outcomes comparable with those in patients who do not have obesity.30 In these studies, weight loss was accompanied by improvements in both blood pressure management and glycaemic control and reductions in sleep apnoea incidence and severity. There is a higher incidence of both minor and major complications in patients with obesity undergoing AF ablation, although this has not been universally reported and does not seem prohibitive.31,32 The National Health Service in the UK has proposed that ablation should not be offered to patients with BMIs greater than 40 kg/m2, and patients with BMIs between 35 and 40 kg/m2 will need to demonstrate weight reductions of at least 10% of their body weight to become eligible for this treatment.33 In Australia, a patient-centred approach has been adopted, wherein the timing of ablation is discussed in relation to lifestyle modifications and other weight loss strategies without specifying particular BMI cutoffs.
Although clinical trials have not yet evaluated the impact of glucagon-like peptide 1 receptor agonists on weight loss and AF outcomes in patients with AF, a number of trials are ongoing. These drugs have the potential to greatly improve AF outcomes in patients with obesity, but to date, definitive data are lacking.
Case management
Based on the patient's young age, you refer her to a specialist cardiologist or electrophysiologist. Her initial investigations should include routine blood tests (including thyroid function, renal function and fasting glucose level), 12-lead ECG (which may provide evidence of left ventricular hypertrophy or other structural abnormality) and an echocardiogram (to evaluate left ventricular function and left atrial size and to exclude valvular pathology). You also consider a simple home screening study for OSA.
At present, her CHA2DS2-VASc score is 1 (assuming normal left ventricular function and the absence of type 2 diabetes), and guidelines suggest that this is a grey zone for anticoagulation indications. However, you prescribe her a non-vitamin K antagonist oral anticoagulant. It is unclear how long she has had AF, and you attempt to restore her sinus rhythm. As the patient is young, an antiarrhythmic agent (e.g. sotalol) should be initiated rather than a simple rate control agent, along with a discussion of the potential side effects and risks. You then plan a cardioversion with a preceding transoesophageal echocardiogram to exclude left atrial appendage thrombus, as you are uncertain of her AF duration.
The long-term strategy is directed towards weight loss with appropriate referral to a specialist weight loss clinic where weight reduction options are considered. You also focus on achieving adequate blood pressure control.
Conclusion
Patients are increasingly presenting with concurrent obesity and AF. In young patients who have severe obesity and recurrent and difficult-to-control AF despite pharmacotherapy, GPs should discuss with them the advantages and disadvantages, and risks and benefits, of AF ablation as a treatment option. MT
COMPETING INTERESTS: Dr Barwad: None. Professor Kalman has received research and fellowship support from Medtronic Inc. and Biosense Webster Inc. and research support from Zoll Medical.
References
1. World Health Organization (WHO). Obesity and overweight. Geneva: WHO; 2021. Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed Jun 2023).
2. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016; 387: 1377-1396.
3. Aune D, Sen A, Prasad M, et al. BMI and all-cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016; 353: i2156.
4. Berenson GS, Srinivasan SR, Bao WH, Newman WP 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med 1998; 338: 1650-1656.
5. McGill HC Jr, McMahan CA, Malcom GT, et al. Relation of glycohemoglobin and adiposity to atherosclerosis in youth. Arterioscler Throm Vasc Biol 1995; 15: 431-440.
6. Bogers RP, Bemelmans WJ, Hoogenveen RT, et al.; BMI-CHD Collaboration Investigators. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med 2007; 167: 1720-1728.
7. Reis JP, Allen N, Gunderson EP, et al. Excess body mass index- and waist circumference-years and incident cardiovascular disease: the CARDIA study. Obesity (Silver Spring) 2015; 23: 879-885.
8. di Angelantonio E, Bhupathiraju S, Wormser D, et al.; Global BMI Mortality Collaboration. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016; 388: 776-786.
9. Peto R, Whitlock G, Jha P. Effects of obesity and smoking on U.S. life expectancy. N Engl J Med 2010; 362: 855-857.
10. Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol 2017; 70: 2022-2035.
11. Grover SA, Kaouache M, Rempel P, et al. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: a modelling study. Lancet Diabetes Endocrinol 2015; 3: 114-122.
12. The Emerging Risk Factors Collaboration. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 2011; 377: 1085-1095.
13. Leggio M, Lombardi M, Caldarone E, et al. The relationship between obesity and hypertension: an updated comprehensive overview on vicious twins. Hypertens Res 2017; 40: 947-963.
14. Stamler J, Dyer AR, Shekelle RB, Neaton J, Stamler R. Relationship of baseline major risk factors to coronary and all-cause mortality, and to longevity: findings from long-term follow-up of Chicago cohorts. Cardiology 1993; 82: 191-222.
15. Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation 2017; 136: 583-596.
16. Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, Clark JM, Coday M, Curtis JM, Egan C, Evans M, Foreyt J, Foster G, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jeffery RW, Johnson KC, Kitabchi AE, Knowler WC, Kriska A, Lang W, Lewis CE, Montez MG, Nathan DM, Neiberg RH, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Redmon B, Regensteiner J, Rejeski J, Ribisl PM, Safford M, Stewart K, Trence D, Wadden TA, Wing RR, Yanovski SZ; Look AHEAD Research Group. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016; 4: 913-921.
17. Zhou C, Wang M, Liang J, He G, Chen N. Ketogenic diet benefits to weight loss, glycemic control, and lipid profiles in overweight patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trails. Int J Environ Res Public Health 2022; 19: 10429.
18. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ ACC/ TOS guideline for the management of overweight and obesity in adults. J Am Coll Cardiol 2014; 63: 2985-3023.
19. Bessesen DH, van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol 2018; 6: 237-248.
20. Electronic Medicines Compendium (EMC). Saxenda 6 mg/mL solution for injection in pre-filled pen. Sussex: Novo Nordisk Limited; 2022. Available online at: https://www.medicines.org.uk/emc/product/2313/smpc (accessed Jun 2023).
21. Markovic TP, Proietto J, Dixon JB, et al. The Australian Obesity Management Algorithm: a simple tool to guide the management of obesity in primary care. Obes Res Clin Pract 2022; 16: 353-363.
22. Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021; 397: 971-984.
23. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ ACC/TOS guideline for the management of overweight and obesity in adults. J Am Coll Cardiol 2014; 63: 2985-3023.
24. Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation 2017; 136: 583-596.
25. Mahajan R, Lau DH, Brooks AG, et al. Atrial fibrillation and obesity: reverse remodeling of atrial substrate with weight reduction. JACC Clin Electrophysiol 2021; 7: 630-641.
26. Javed S, Gupta D, Lip GYH. Obesity and atrial fibrillation: making inroads through fat. Eur Heart J Cardiovasc Pharmacother 2021; 7: 59-67.
27. Middeldorp ME, Pathak RK, Meredith M, et al. PREVEntion and regReSsive effect of weight-loss and risk factor modification on atrial fibrillation: the REVERSE-AF study. EP Europace 2018; 20: 1929-1935.
28. Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013; 310: 2050-2060.
29. Jamaly S, Carlsson L, Peltonen M, Jacobson P, Sjöström L, Karason K. Bariatric surgery and the risk of new-onset atrial fibrillation in Swedish obese subjects. J Am Coll Cardiol 2016; 68: 2497-2504.
30. Donnellan E, Wazni O, Kanj M, et al. Outcomes of atrial fibrillation ablation in morbidly obese patients following bariatric surgery compared with a nonobese cohort. Circ Arrhythm Electrophysiol 2019; 12: e007598. Erratum in: Circ Arrhythm Electrophysiol 2020; 13: e000047.
31. Javed S, Gupta D, Lip GYH. Atrial fibrillation in obesity: weighing up the evidence for catheter ablation. Clin Cardiol 2020; 43: 1064-1066.
32. Winkle RA, Mead RH, Engel G, et al. Impact of obesity on atrial fibrillation ablation: patient characteristics, long-term outcomes, and complications. Heart Rhythm 2017; 14: 819-827.
33. National Health Service (NHS) England. Clinical Commissioning Policy: Catheter ablation for paroxysmal and persistent atrial fibrillation 1903. Redditch: NHS England; 2020. Available online at: https://www.engage.england.nhs.uk/consultation/catheter-ablation/ (accessed Jun 2023).