Coeliac disease: diagnostics and therapies beyond the gluten-free diet

Coeliac disease, once seen as a condition primarily affecting children, is now recognised as a complex systemic disorder with diverse symptoms across all ages. The gluten-free diet remains the cornerstone of treatment; however, with a modern understanding of its pathophysiology, emerging therapies hold the promise of alleviating the burdens of strict dietary adherence and improving patient quality of life.
- Coeliac disease is a systemic immune disorder with a wide range of extraintestinal symptoms affecting around 1% of the global population.
- Current diagnostic tests, including serology and small bowel biopsy, need to be utilised correctly and early to capture underdiagnosed cases.
- The gluten-free diet remains the cornerstone of treatment; however, it carries a high treatment burden, with strict adherence difficult for many patients, and inconsistent mucosal healing.
- Novel therapies for coeliac disease are advancing rapidly, targeting various stages of the disease pathway, but none have yet completed a successful phase 3 trial.
- The future of coeliac disease management will likely involve a combination of expert dietary guidance and pharmacological strategies.
Gluten appeared in the human diet when agricultural practices enabled the cultivation of cereal grains around 10,000 years ago.1 Beginning in the ‘Fertile Crescent’ (located in the Middle East), this cultivation aided the congregation of people into stable communities.2 Residing adjacent to the Fertile Crescent in what was then Cappadocia, the Greek physician Aretaeus first described a condition he called koiliakos (of the abdomen) describing ‘undigested food and loose stools’ 2000 years ago.2 Aided by the ability to read ancient Greek, Dr Samuel Gee anglicised koiliakos to ‘coeliac’ in 1887 when he gave the first modern description of coeliac disease, noting that ‘if the patient can be cured at all, it must be by means of diet’.3
In 1924, without knowing the environmental driver of coeliac disease, a predominantly exclusive ‘banana diet’ was attributed to saving an untold number of lives of severely unwell children.4,5 However, it was not until the 1940s that Dr Willem Dicke established that the exclusion of wheat led to a clinical improvement.6 Associated histological small intestinal pathology was demonstrated in the mid-1950s and, with the advent of the flexible fibreoptic endoscope in 1958, the diagnosis and management of coeliac disease fell upon the modern gastroenterologist.7-10 More advanced diagnostics have seen the diagnosis of coeliac disease in people not necessarily presenting with bowel symptoms, underscoring coeliac disease to be a multisystem immune disorder, rather than an isolated intestinal disease.11
Despite affecting between 0.7% and 1.4% of the global population, and with an increasing incidence and prevalence, the only treatment remains a strict gluten-free diet (GFD), which is expensive, is socially isolating, carries a high treatment burden and may not, as once believed, restore the bowel to full health.12-17 With an advanced understanding of its immunopathogenesis, there are now multiple potential novel therapeutics in various phases of global clinical trial development in humans, many of which are also being conducted in Australia. This article provides an overview of coeliac disease, with a focus on the use of established and potentially new diagnostics, and emerging therapies beyond the GFD.
Pathophysiology of coeliac disease
Gluten is a mixture of hundreds of proteins, mainly composed of gliadin and glutenin proteins, so named because of their high concentration of the amino acids proline and glutamine.18 These amino acids render the gluten protein resistant to complete digestion in the human gut, with the resultant nondegraded peptides being seen as toxic in people with coeliac disease. Gliadin and glutenin proteins are collectively referred to as prolamins because of this abundance of proline and glutamine.18,19 Gluten or glu- (from the Latin glūten, which translates to ‘glue’ or ‘gluey substance’) collectively refers to these and other structurally related prolamins, which are also found in rye as secalins, in barley as hordeins and in oats as avenins.20 These nondigested smaller peptide chains of amino acids from gluten proteins are the drivers of coeliac disease and, consequently, any foods containing these are excluded in a strict GFD.21
In people with coeliac disease, the nondigested peptides cross the mucosal epithelium and, once within the lamina propria, are deamidated by the enzyme transglutaminase-2, which converts these peptides to being highly immunogenic.12,22-25 The peptides are presented on the surface of antigen-presenting cells by genetically defined human leucocyte antigen (HLA) heterodimers and recognised by gluten-specific CD4+ T cells.26,27 This interaction between antigen-presenting cells, HLA, gluten peptides and T cells lies at the centre of the coeliac disease pathogenesis.28 Given this, the clinically determinable absence of an at-risk HLA allele in an individual virtually excludes the diagnosis of coeliac disease.
Once activated, pathogenic gluten-specific CD4+ T cells secrete cytokines with a resultant inflammatory milieu. Gluten- specific CD4+ cells provide helper signals to B cells; as plasma cells, they then become capable of secreting antibodies against deamidated gliadin peptide (DGP) and transglutaminase-2, both of which are used clinically to screen for coeliac disease.29 Killer T lymphocytes degrade surface enterocytes and result in villous atrophy.20
Diagnosis of coeliac disease
Clinical manifestations
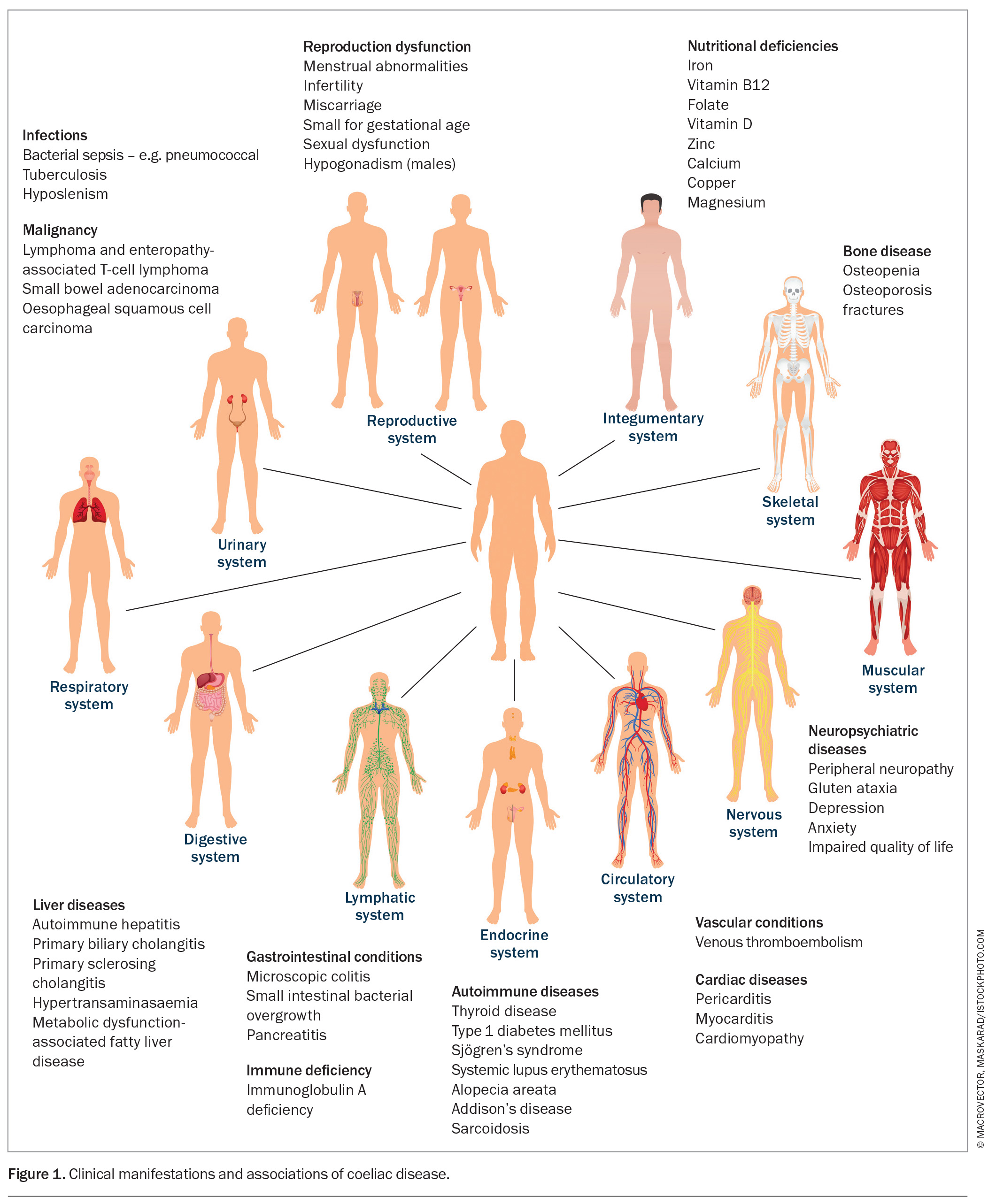
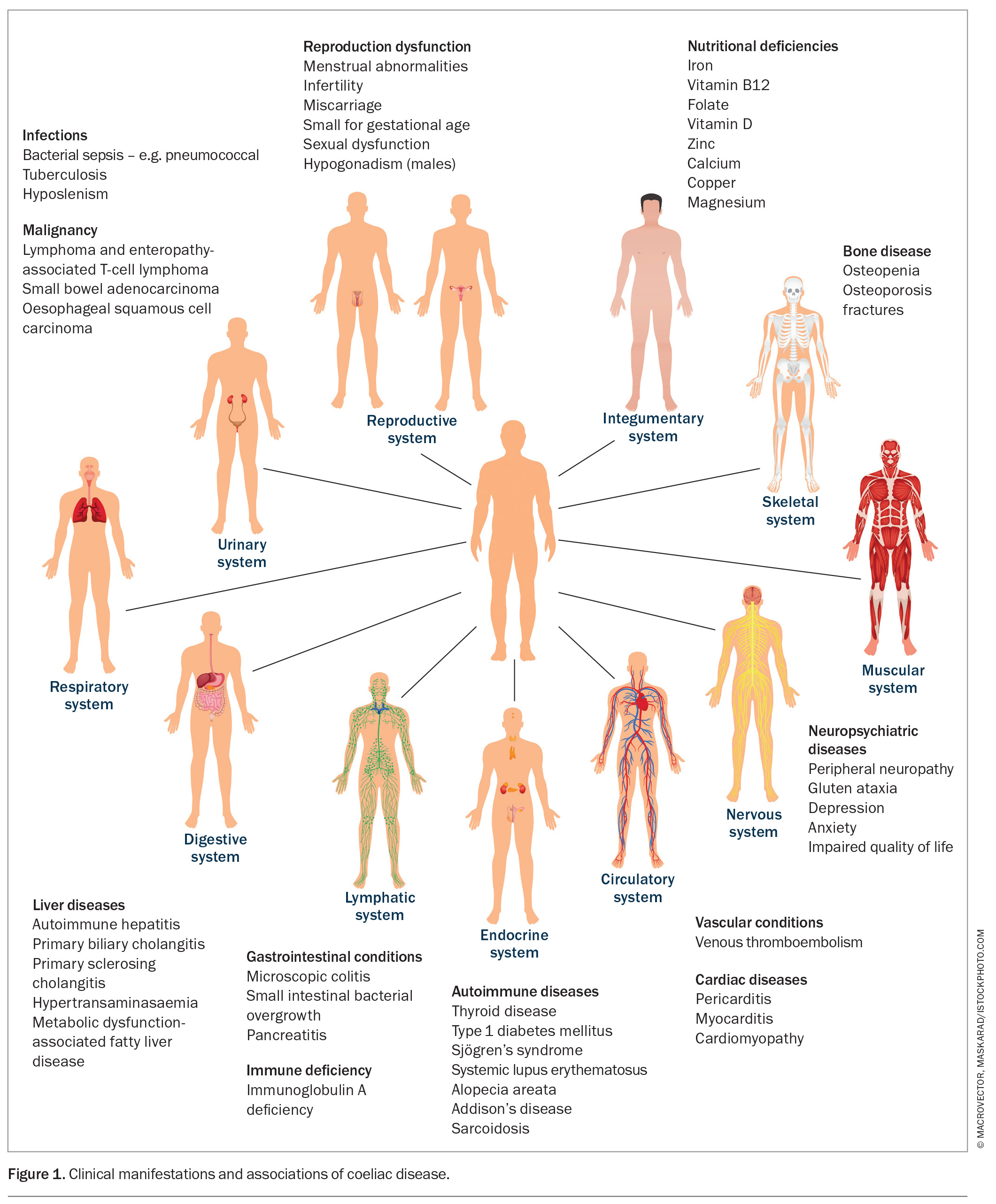
The recently held view, even up to the late 20th century, that coeliac disease occurs predominantly in Caucasians presenting with bloating and diarrhea is now believed to be more an exception than the rule.30 Coeliac disease presents with a broad spectrum of extraintestinal symptoms (Figure 1) in people of almost any ethnicity, including those from North Africa, the Middle East, Asia and northwestern India.12,13,30,31 This broad heterogeneity of its clinical presentation likely contributes to its global underdiagnosis.32
Although coeliac disease affects one in 70 people in Australia, only one in five receive a diagnosis.33,34 Delays of over 10 years can occur before a diagnosis is established, with a reduced quality of life for those remaining undiagnosed.35,36 Attempts to identify cases in high-risk groups, such as first-degree relatives (affecting about 10%), are recommended.11 A recent Australian study that screened over 200 first-degree relatives of people with diagnosed coeliac disease in an inner city metropolitan centre found that 14% of the genetically at-risk children had the disease but were undiagnosed; this is especially pertinent in the context of the reduced regional access to healthcare services.37
Coeliac disease can present with a chameleon-like cluster of symptoms, including faltering growth, dental enamel defects, delayed puberty, amenorrhoea, unexplained infertility, anaemia (both microcytic due to iron depletion or macro-cytic due to low vitamin B12 levels), low-impact fractures from osteoporosis or osteopenia, and unexplained neurological symptoms, including recurrent headaches.30,38,39 Low levels of vitamin B12, folate, calcium, potassium, magnesium, phosphate and vitamin D, as well as elevated liver enzymes, should also prompt a consideration of coeliac disease, as should unexplained thrombocytosis as a marker of functional hyposplenism.13 Dermatitis herpetiformis occurs in up to 10% of people with coeliac disease, and there is an increased prevalence of coeliac disease in those with Hashimoto’s thyroiditis, type 1 diabetes, Down’s syndrome and other autoimmune diseases (Figure 1).40,43
However, after following a strict GFD for one to two years, the symptoms of coeliac disease can change. Pronounced nausea and vomiting can subsequently follow a single oral gluten exposure one to two hours post-ingestion. Elevated levels of interleukin (IL)-2 have been shown to correlate with these symptoms, validating the long-held symptom reporting by patients.44 An Australian study presented as an abstract at the International Society for the Study of Coeliac Disease meeting in Sheffield, UK in 2024 pointed towards micro-amounts of gluten resulting in IL-2 elevation as a marker of immune activation in coeliac disease (ACTRN12621000781842).
Blood tests
When coeliac disease is suspected, anti-tissue transglutaminase (anti-tTG) immunoglobulin (Ig)A and DGP IgG antibody levels should be requested, given their high sensitivity (>85%) and specificity (>90%).45-49 An IgA level is often routinely supplied with the reporting of an anti-tTG result, with the aim of excluding false-negative anti-tTG IgA results based on low IgA levels. IgA deficiency occurs in up to 3% of patients with coeliac disease, representing an increase of 10- to 15-fold compared with the general population.50 Levels of IgA must be very low rather than slightly out of range for this to be a clinical concern. An initial request for levels of anti-tTG IgA, serum IgA and DGP IgG generally overcomes this issue, as an IgG-based test is included and avoids the need for repeated venesections. Antiendomysial antibody testing has a higher specificity of more than 99% but is generally only requested in cases of diagnostic uncertainty, given that the test is labour intensive and expensive.30
The accuracy of serological testing depends on an adequate intake of gluten before testing. As such, patients should not be told to start a GFD until their serology has been performed. A clinical response to a GFD test does not confer a diagnosis of coeliac disease, and symptoms can be inappropriately attributed to gluten when other components in the diet or diseases (such as irritable bowel syndrome or inflammatory bowel disease) may be the actual cause.20 An incomplete initial work-up may result in potential delays in arriving at the correct diagnosis.51 Wheat is not composed solely of gluten, and wheat-derived fructans can result in bowel symptoms in patients unaffected by coeliac disease.52,53 The only instance in which serological testing would not be required before starting a gluten- containing diet is if accurate genetic testing reveals an absence of an at-risk genotype (HLA-DQ2, -DQ8 and -DQ7 haplotypes), as this effectively excludes a diagnosis of coeliac disease.34
Gluten challenge
For undiagnosed people on a GFD, although there are data suggesting that a shorter gluten challenge could be sufficient, the generally accepted clinical approach is to consume the equivalent of four pieces of bread daily (equivalent to 10–12 g of gluten per day) for a minimum of six weeks prior to testing.54,55 For those not wishing to undertake a conventional dietary gluten challenge using bread or other foods (which can complicate the attribution of symptoms), vital wheat gluten, which is the material remaining after thoroughly washing and drying wheat flour, can be used as a substitute.56 An initial challenge of 1 to 2 g of vital wheat gluten daily can be uptitrated over the course of one to two weeks to 16 g daily, which equates to about 12 g of gluten protein. A gentle uptitration reduces the risk of severe vomiting or other potential symptoms in those with undiagnosed coeliac disease.57,58
An exciting development arising from the RESET Nexvax2 studies was the finding of elevated IL-2 levels in the blood of people with coeliac disease, but not in those without coeliac disease, with levels peaking two to four hours after a single oral dose of gluten.59 This has been recently adapted to a whole-blood assay system, where collected blood is combined with gluten peptides in a blood collection tube outside the body. It represents a potential new diagnostic test for coeliac disease in those already consuming a GFD, negating the need for an oral gluten challenge.60
Duodenal biopsies
Currently, a confirmatory duodenal biopsy ensures a correct diagnosis before subjecting patients to a lifelong GFD, as well as providing a baseline reference for a post-treatment histological response. Coeliac disease can affect the small bowel in a patchy manner and multiple biopsies are suggested; two from the first part of the duodenum and four from the second.11,61 Biopsies should be collected with a single pass of the biopsy forceps via endoscopy.62 In clinical practice, correct interpretation of the duodenal biopsy is highly dependent on both sufficient sampling and correct laboratory orientation, leaving the results prone to interobserver variability. High-quality, well-orientated and correctly cut samples reduces the risk of an erroneous assessment and diagnosis.63,64
No-biopsy approach in children
The European Society for Paediatric Gastroenterology, Hepatology and Nutrition released revised guidelines supporting a no-biopsy diagnosis for children in 2020. A biopsy can be omitted if, with the agreement of the family, any of the following are present:
- characteristic symptoms of coeliac disease
- a high anti-tTG IgA value 10 times or greater the upper limit of normal
- positivity for endomysial antibodies detected in a second serum sample.
If present, the child should be referred to a paediatric gastroenterologist for diagnosis.65 The absence of standardisation of anti-tTG assays and the real risk of misdiagnosis without following these strict criteria can limit this approach in clinical practice. It is also still recommended that a biopsy be performed in children if these criteria are not met, especially in those with a positive transglutaminase-IgA titer (<10 times the upper limit of normal).65
Genetic testing
The main susceptibility genetic haplotypes for coeliac disease are HLA-DQ2, -DQ8 and -DQ7, which are present in over 99% of patients with coeliac disease, although they are also present in 40 to 50% of the Australian population.66 Given this, a positive gene test result for coeliac disease does not confirm a diagnosis of coeliac disease, but its absence confers a negative predictive value of greater than 99%. This is useful to help exclude coeliac disease in patients already on a GFD before undergoing a gluten challenge and to exclude coeliac disease in first-degree relatives, negating the need for future screening. In children genetically at risk who return initially negative serology results, retesting (in the absence of intercurrent symptoms) before each anticipated growth opportunity (e.g. before puberty and adolescence) is clinically recommended.
Although genotyping has been available both publicly and privately in Australia and frequently requested for many years, two Australian papers raised concerns over reporting discrepancies in genetic testing and national laboratory performance in 2017 and 2018.67,68 Given these concerns, although likely to be valid, HLA typing should be carefully reviewed if being used to exclude coeliac disease in a patient on a GFD or to define future risk in a high-risk patient, such as a first-degree relative. The interpretation of HLA typing is complex, and it is important that laboratories ensure the results are accurate and able to be understood by practicing clinicians as per academically open-access and accepted Australian guidelines.27 If there are clinical concerns, it would be best to on-refer the patient for review by a clinician with expertise in coeliac disease.
Undiagnosing coeliac disease
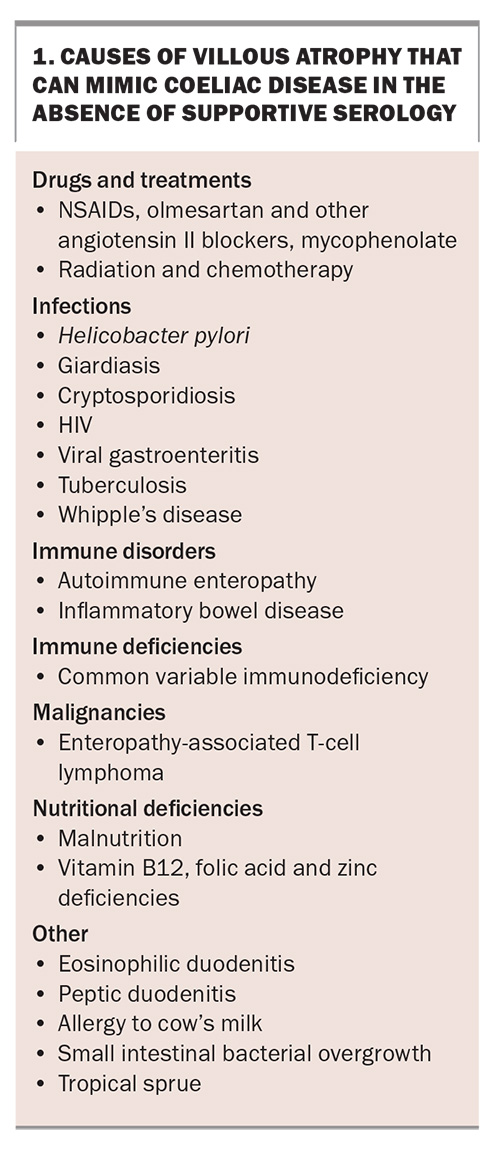
Up to 20% of people labelled with coeliac disease may not in fact have coeliac disease, with these patients unnecessarily following a GFD.69,70 Anti-tTG IgA and DGP IgG tests have been commercially available in Australia since the turn of the 21st century, although prior to this, less accurate tests for anti-gliadin antibodies with lower sensitivity and specificity were used.71 Consequently, many diagnosed with these serological tests (e.g. older patients who may have been diagnosed before the turn of this century) may have been diagnosed incorrectly, as there may have been another cause for the coexistent histological changes present at the time. Causes of villous atrophy other than coeliac disease are common and can often be misinterpreted as coeliac disease, particularly if the anti-tTG IgA and DGP IgG results were negative at the time of diagnosis (or an older, less specific though positive serological test prior to 2000 was used).
Common causes of villous atrophy, which can be mistaken for coeliac disease, include the use of NSAIDs and angiotensin II receptor antagonists (e.g. olmesartan), Helicobacter pylori infection, inflammatory bowel disease, enteric infections, tropical sprue and rarer diseases such as common variable immunodeficiency (Box 1). These should be considered if the serological test results are negative, or if the initial histological changes are not improving as expected. The diagnosis of coeliac disease based solely on histological results or when combined with older serological tests should be done so cautiously. The rare entity of seronegative coeliac disease (the finding of villous atrophy in the absence of positive serological tests) requires repeated gluten challenges for confirmation.13
Clinical clues to a misdiagnosis of coeliac disease include the finding of normal histology in a patient who is not strictly compliant with a GFD, an inability to locate initial serological results, an older historical diagnosis using outdated serological tests or a diagnosis of ‘seronegative’ coeliac disease.
Postdiagnosis of coeliac disease
The only treatment for coeliac disease is a GFD, which poses considerable challenges. The treatment burden of a GFD is very high, likely because of the continuous lifelong and daily concern about food choices.72 The pronounced vomiting and nausea experienced when inadvertently exposed to gluten while following a strict GFD leads to patient anxiety about becoming unwell after accidental gluten exposure; this is an important consequence of a GFD.73-75 Gluten exposure when eating at restaurants or travelling is difficult to avoid, and the hypervigilance around gluten-free options is also associated with anxiety and depression, which may require early psychology input.76,77 A GFD is expensive, difficult for the financially vulnerable and, unlike other approved treatments, does not attract a financial rebate. Food consumption is central to human interaction and a GFD in adolescents can be socially isolating. Regional and remote patients have less access to gluten-free options, and those with a reading disability, who are visually impaired or who are intellectually challenged may find food labelling difficult to interpret. Consequently, in patients with coeliac disease, the strict following of a GFD ranges from 42 to 91%.72,78
A clinical improvement can occur within days to weeks of starting a GFD; however, the return to normal serological levels of anti-tTG IgA and DGP IgG usually occurs within six months and can take longer.20,79 Persistent antibody elevation is often an indicator of inadvertent exposure or nonadherence to a GFD. Dietitians experienced in coeliac disease can play an important role in identifying areas where inadvertent gluten exposure occurs, providing advice for travel and assisting when social isolation is evident.
Screening for hypothyroidism (three- to fourfold higher risk in people with coeliac disease) and type 1 diabetes (affecting 5% of people with coeliac disease) is important, and in those with abnormal liver enzymes, possible diagnoses include autoimmune hepatitis, primary biliary cirrhosis and primary sclerosing cholangitis.80 An initial baseline panel of bloods including the following tests provides a comprehensive initial data set:
- a full blood count
- electrolytes and liver function test
- calcium and phosphate test
- iron studies, including ferritin
- assessment of serum levels of folate and vitamins B12 and D
- assessment of copper, zinc and magnesium levels
- thyroid-stimulating hormone and thyroid autoantibody tests
- antinuclear antibody test
- extractable nuclear antigen test
- parathyroid hormone test.
An elevated level of C-reactive protein can point to another diagnosis, as it is usually normal in coeliac disease. A visit six months post-necessary supplementation to confirm normalisation of initially abnormal results is clinically appropriate.
Up to 75% of people with coeliac disease may have osteopenia or osteoporosis at the time of diagnosis, and a dual-energy x-ray absorptiometry scan should be considered within a year of diagnosis in adults and no later than 30 to 35 years of age.80-82 In coeliac disease, functional hyposplenism can predispose an individual to sepsis from encapsulated organisms, and vaccination against pneumococcus is recommended, as is an annual influenza vaccination.11,83-85 Coeliac Australia, a membership-based organisation, provides the latest in research updates (https://coeliac.org.au/).
Mucosal healing is observed in 95% of children within two years of starting a GFD, whereas demonstrable mucosal healing after two years of following a GFD varies from 12 to 79% in adults.86,87 The variability in histological improvement aligns with clinical concerns over histology reporting in biopsies collected from patients on a GFD. Technical challenges such as misorientation have led to discrepant reports of mucosal ‘healing’. However, as it remains standard clinical practice in adults to repeat small bowel biopsies post-initiation of a GFD to confirm healing, this should not be done before two years of following a GFD in the absence of new or concerning symptoms.
Nonresponsive histology
In people with a confirmed diagnosis of coeliac disease, a persistently positive serology is strongly associated with ongoing intestinal damage; however, conversely, negative serology does not predict small bowel mucosal healing.88 The rate of mucosal healing in adults on a GFD ranges from six months to 10 years.80 Refractory coeliac disease (RCD), although often considered a cause of nonhealing mucosa, is rarely the cause. A study including patients with coeliac disease on a strict GFD for an average of six years from Australia, New Zealand and the USA found that the small bowel histology had failed to completely normalise in more than 90%.89 In a Spanish study, over 50% of adults with coeliac disease had villous atrophy present two years after starting a strict GFD.90 It is becoming increasingly recognised that a GFD may be insufficient to induce complete mucosal remission in some individuals, but this knowledge has not yet translated into clinical management.80 This finding can cause patients considerable anxiety.89
Nonresponsive coeliac disease
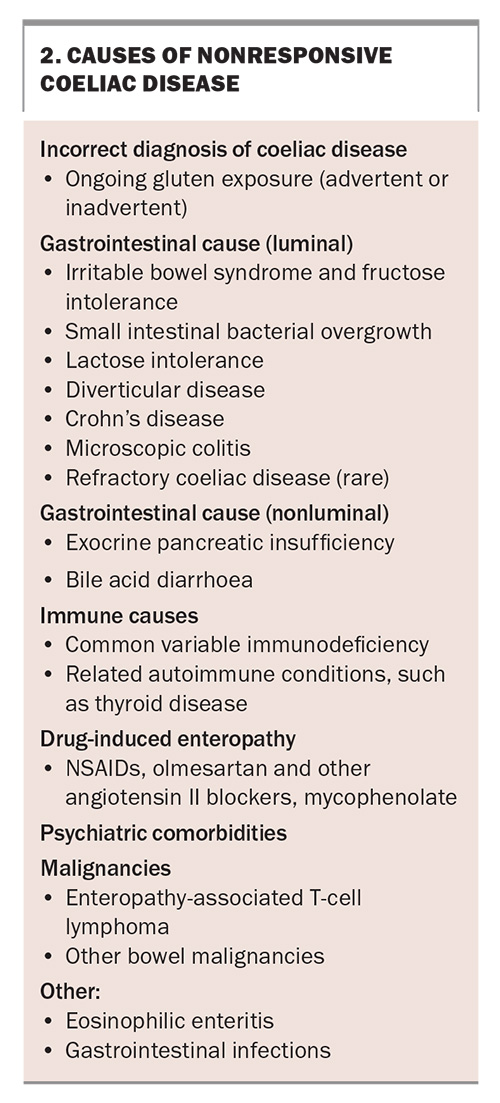
Nonresponsive coeliac disease (NRCD) is defined as a failure to respond symptomatically or to resolve biochemical abnormalities after at least six months of starting a GFD.91 However, this definition is currently under review. Histology results are not included in this definition and would likely be abnormal if tested. NRCD may be better defined including nonresponsive histology as well as persistent gastrointestinal symptoms.
When faced with a patient with NRCD, the first step should be to review the original diagnosis, including histology and serology, to ensure it was correct. The most common cause of NRCD is gluten exposure, and while understanding the patient’s concern about this suggestion, referral to an experienced dietitian is often useful.92 If the dietitian feels there is no potential for gluten exposure, the most likely cause is a slow response (e.g. in older individuals or in those homozygous for HLA-DQ2.5). Other conditions not necessarily related to coeliac disease, such as microscopic colitis, Crohn’s disease, irritable bowel syndrome, small intestinal bacterial overgrowth, Helicobacter pylori infection, lactose intolerance, diverticular disease, pancreatic exocrine insufficiency, gastroparesis and constipation, can also result in persistent small bowel histological changes and symptoms that do not seem to be clinically responding to a GFD (Box 2).93,94 RCD, although often thought of early, is rarely a cause of NRCD.95
Refractory coeliac disease
RCD is defined as persistent or recurrent malabsorptive symptoms and signs with villous atrophy, despite consuming a strict GFD for more than 12 months, in the absence of all other causes of NRCD and overt malignancy.96 The main risk group includes those aged older than 50 years who have never been diagnosed with coeliac disease or who have not followed a GFD.
There are two types of RCD: RCD-I and RCD-II. Although those who develop RCD-II are clinically unwell, it can be difficult to differentiate between RCD-I and inadvertent ongoing gluten exposure, both clinically and with advanced testing. Again, it is important to ensure the initial diagnosis was correct and that villous atrophy was not associated with other causes or that the patient was not a slow responder. RCD-II presents with significant weight loss, nutritional deficiencies and biochemical abnormalities, and confers a poor 5-year survival rate.97,98 RCD-II is generally considered a preneoplastic condition, with 50% of patients developing enteropathy-associated T-cell lymphoma within five to 10 years.99,100 RCD-II can be diagnosed clearly with the demonstration of clonal expansion of surface CD3– and intracytoplasmic CD3+ intraepithelial lymphocytes, along with monoclonal rearrangement of the gamma chain of the T-cell receptor.101 Involving a gastroenterologist specialising in coeliac disease is recommended for suspected cases of NRCD and RCD.
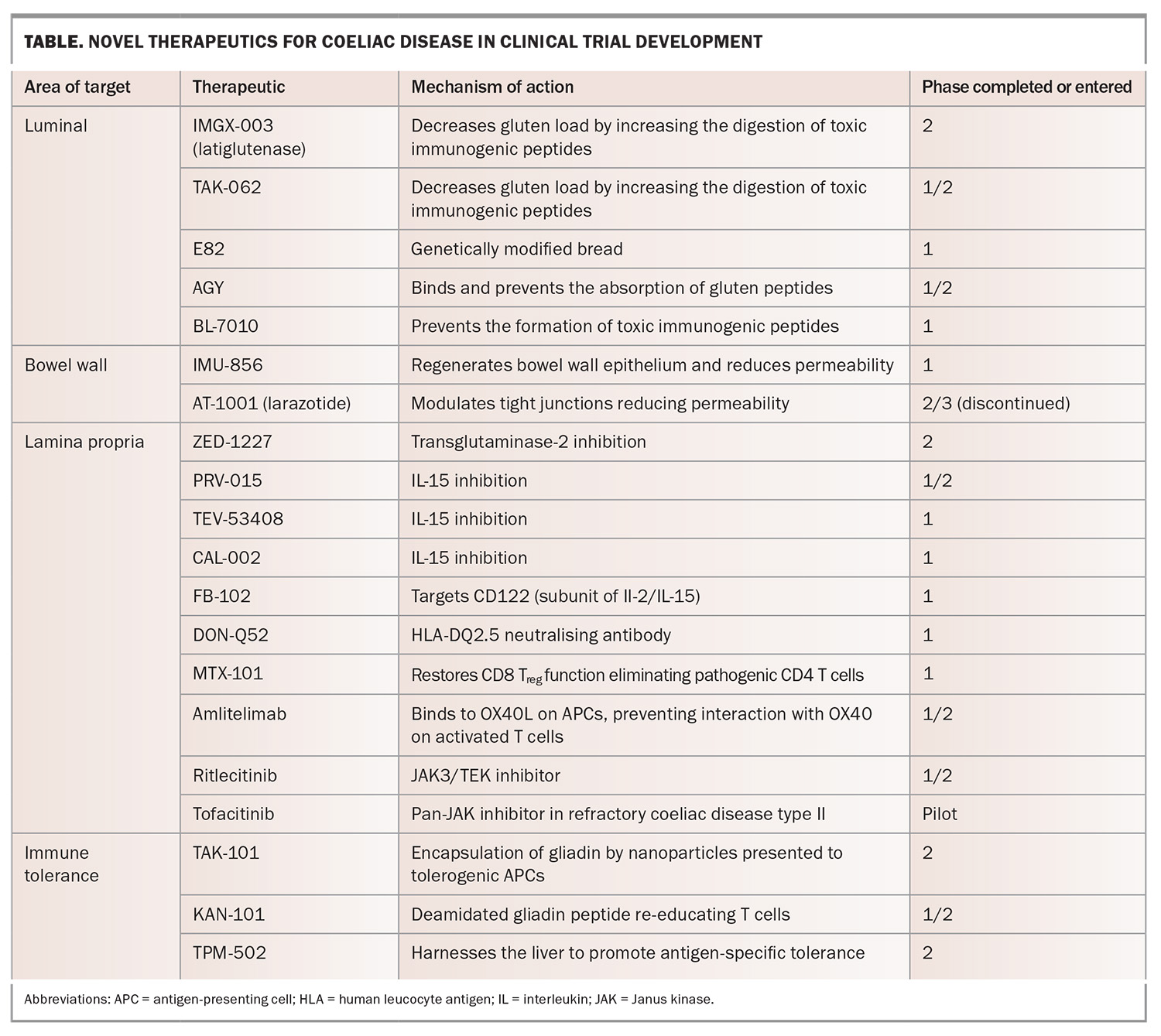
Novel therapies
Given that a GFD poses a substantial burden for patients with coeliac disease, it is not surprising that patients with coeliac disease are interested in alternative nondietary therapies.102,103 The field is moving rapidly, and at the time of writing this article, there are around 20 novel therapeutics at various stages of clinical studies, many of which are or have been trialled in Australia (Table). Understanding how they will be incorporated into the treatment landscape in the future will be an exciting challenge for those involved in treating coeliac disease.
Several novel therapies have progressed to phase 2 studies; however, none have yet successfully completed a phase 3 study, which is required for a therapy to be commercially available. The potential therapeutic targets follow the pathogenesis of coeliac disease and can be broadly divided into therapies acting:
- within the gut lumen to prevent exposure to gluten or to reduce the gluten load
- to regenerate the bowel wall epithelium or reduce epithelial permeability
- within the lamina propria to alter the aberrant immune response
- to restore the body’s immune tolerance to gluten.
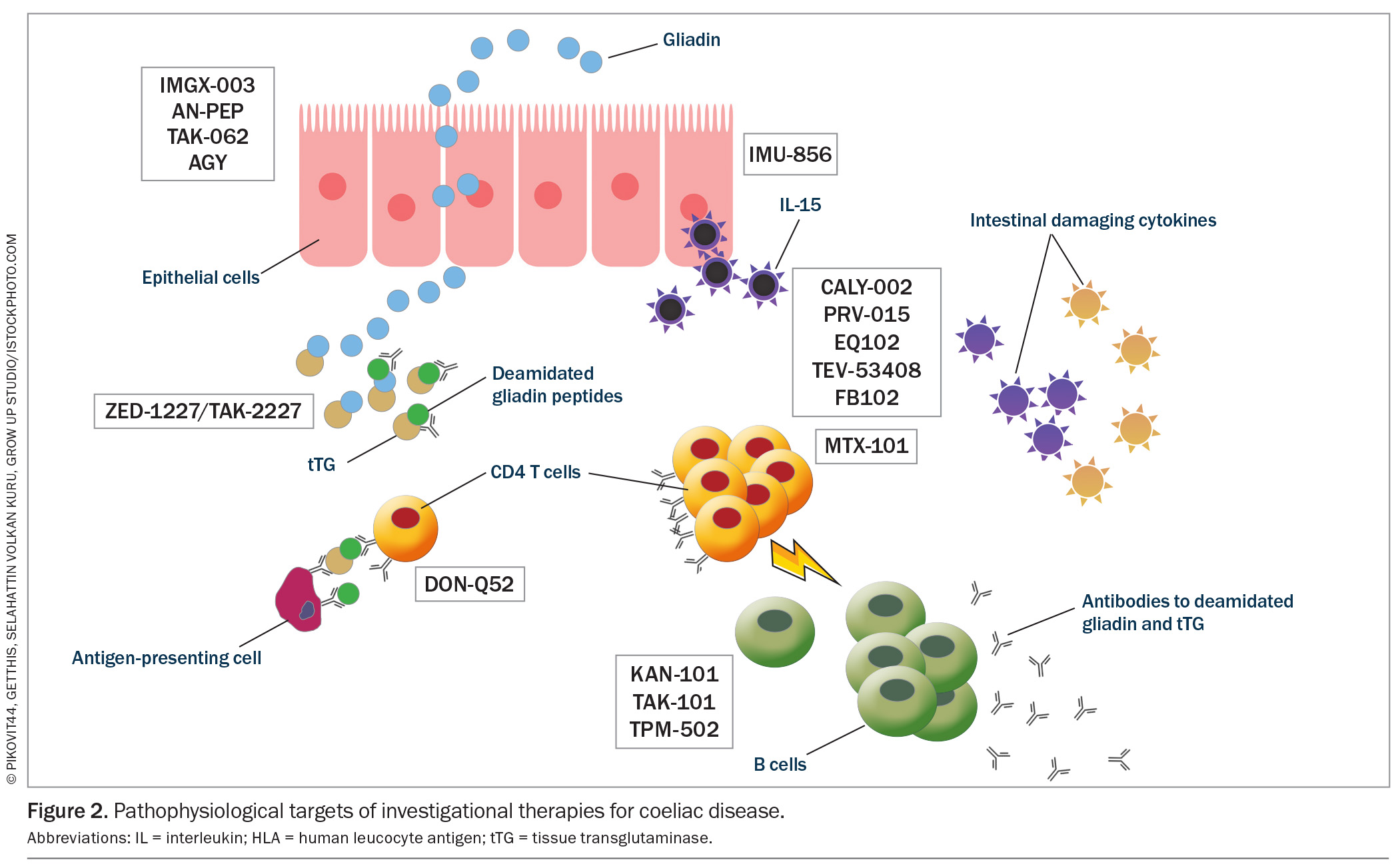
These current therapeutic targets are illustrated in the context of the pathophysiology of coeliac disease in Figure 2.
Therapies targeting within the lumen
Luminal strategies encompass four main mechanisms to reduce exposure to gluten peptides:
- genetically modifying wheat to remove toxic gluten peptides
- breaking down immunogenic gluten peptides in the gut before they are absorbed
- binding and sequestering gluten peptides out of the body before
- they can be absorbed
- reducing intestinal permeability to prevent absorption.
Latiglutenase and TAK-062 are designed to increase the intraluminal digestion of toxic gliadin peptides, rendering them nonimmunogenic when absorbed. Latiglutenase is the most progressed investigational therapy with several phase 1 and 2 studies published. It has been shown to be safe and able to reduce mucosal damage.104-108 TAK-062 has completed a phase 1 study showing that it was well tolerated with a median gluten degradation of more than 97% and is currently being trialled in a phase 2 study.107
E82 is a genetically modified wheat line that has been shown to reduce immune responsiveness, although a symptom improvement has not been shown.109 AGY is an egg yolk-derived antigliadin antibody that has been shown to bind to and prevent the absorption of gliadin in the intestine. It was deemed safe in a phase 1 study with a small number of participants.110 BL-7010 binds to gliadin and protects it from digestive enzymes, preventing their formation of immunogenic peptides. It has been shown to reduce immune reactivity with a decrease in tumour necrosis factor-α in patients with coeliac disease.111,112
Therapies targeting the bowel wall epithelium
Acting as a regulator of intestinal barrier function and regenerator of the bowel wall epithelium, IMU-856 is an orally available and systemically acting small-molecule modulator that targets sirtuin-6. IMU-856 has been shown in a phase 1 study to be well tolerated, improve measures of nutrient absorption (zinc, vitamin B12), reduce gluten-induced symptoms and attenuate mucosal damage.113
Larazotide (AT-1001) modulates tight junctions aiming to prevent the passage of gliadin peptides through the epithelial barrier. Despite being well tolerated and improving symptoms in patients with coeliac disease, a phase 3 study was halted when an interim analysis with disappointing results led to its discontinuation.114,115
Therapies targeting the lamina propria
ZED-1227 (a covalent and irreversible inhibitor of transglutaminase-2) was shown to attenuate mucosal damage and symptom scores when compared with placebo in a phase 2 study.29,116 PRV-015 (previously AMG-714) was the first anti-IL-15 evaluated in coeliac disease and, in a phase 2 study, was shown to be well tolerated with a symptom benefit, although it has not been shown to prevent mucosal injury.117,118 TEV-53408 was also designed to block IL-15 and is currently being trialled in a phase 1 study.119 CALY-002 similarly aims to block IL-15 and has completed a phase 1 study (NCT04593251). FB102 targets CD122, a subunit of IL-2/IL-15 receptors, which are key regulators of natural killer cells and certain T-cell subsets and is currently in a phase 1 study (ACTRN12624000974505). DON-Q52, a multispecific gluten peptide targeting the HLA-DQ2.5 neutralizing antibody preventing T-cell activation, is currently in a phase 1 study.120
MTX-101 is a bispecific autoimmune checkpoint inhibitor designed to restore CD8 T reg functionality and the cytolytic elimination of pathogenic CD4 T cells. Its dual configuration binds the CD8 T reg receptors, killer immunoglobulin-like receptors and CD8 and is currently in a phase 1 study (NCT06324604). Amlitelimab is an anti-OX40L monoclonal antibody that binds to OX40L on antigen-presenting cells, preventing interaction with OX40 on activated T cells, and is currently in a phase 2 study enrolling patients with NRCD (NCT06557772). Ritlecitinib is a selective and orally available inhibitor of Janus kinase-3 and the tyrosine kinase expressed in hepatocellular carcinoma family currently being tested in a phase 2 clinical study (NCT05636293).
Tofacitinib is a pan-Janus kinase inhibitor approved for the treatment of rheumatic conditions, such as rheumatoid arthritis and ankylosing spondylitis, as well as ulcerative colitis. In an open-label prospective pilot study (Eudra clinical trial number: 2018-001678-10) of six patients with RCD-II, 12 weeks of treatment with tofacitinib led to a clinical resolution. Mucosal improvement was observed in four of six patients. Rapid recurrence of symptoms including weight loss was observed in all patients after discontinuation of treatment, but restitution of therapy again led to a rapid and complete response.121,122
Therapies to restore immune tolerance to gluten
Several therapies have aimed to restore immune tolerance to gluten by rendering the pathogenic T cell unresponsive. Nexvax2 passed through multiple phase 1 studies before a phase 2 study was halted midway because of a lack of statistically significant protection from gluten-induced symptoms.123 Studies using the human hookworm Necator americanus to increase the regulatory T-cell population began around the same time as studies of Nexvax2.124 A more recent larger study showed that although gluten-associated symptoms improved after the intermittent consumption of low gluten doses, quality-of-life symptom scores were better in those without a hookworm infection and immune tolerance was not restored.125
More recently, several other compounds have been trialled to restore immune tolerance. TAK-101, similar to Nexvax2 in its aim to restore immune tolerance to gluten, differs in that it is gliadin encapsulated in a nanoparticle and delivered intravenously to tolerogenic antigen-presenting cells. A phase 1 study showed that it was well tolerated with no profound adverse effects, with the first of two phase 2 studies showing a decreased cytokine response to a gluten challenge, as well as protection from mucosal injury.126,127 A further phase 2 study is ongoing (NCT04530123).
KAN-101 is a liver-targeting glycosylation signature conjugated to a deamidated gliadin peptide, aiming to re-educate T cells so they do not respond to gluten antigens, and is currently in phase 1 and 2 studies (NCT05574010). TPM-502 is also designed to harness the liver to promote antigen-specific immune tolerance without broadly suppressing the immune response, with a phase 2 study having recently been completed (NCT05660109).
Conclusion
Initially a gut disease thought to predominantly affect children, coeliac disease is now recognised as a systemic immune disorder with a diverse range of extraintestinal symptoms and a global prevalence of around 1%. The approach of suspecting coeliac disease in patients with gastrointestinal symptoms, followed by a request for serology and, if the results are positive, small bowel biopsies for a diagnosis, although correct, may not hold true when looking to identify an underdiagnosed condition accurately.
Appreciating the chameleon-like systemic nature of the symptoms of coeliac disease, the historical inaccuracy of early serological tests, the requirement for accurate HLA typing and correct collection of duodenal biopsies post-maintenance of sufficient gluten intake, as well as an appreciation of the limitations of biopsies as the gold-standard test at the time of diagnosis and follow-up, will lead to greater rates of diagnostic accuracy. The potential incorporation of new diagnostics using IL-2 may eventually augment this diagnostic armamentarium. The early finding of micro-amounts of gluten being required to illicit an immune response and understanding the challenges of a strict GFD underscore the need to include expert dietitians in the management of coeliac disease.
Finally, although the GFD has been the only treatment since the end of World War II, multiple candidates are emerging as potential novel therapies. The GFD seems likely to remain an important part of clinical management, like dietary therapies for other diseases such as diabetes. However, given the limitations of the GFD, new therapies will hopefully lessen the burden of strict adherence in those not able, augment mucosal healing in those for whom such is not possible and reduce the debilitating symptoms experienced when inadvertently exposed to gluten while following a strict GFD. Australia has been and remains an important and active participant in the clinical trial landscape searching for a treatment other than the GFD for people with coeliac disease, which may finally be on the cusp of change. MT
COMPETING INTERESTS: Dr Daveson has received support from the University of Queensland for journal database access; is the Founder, Owner and Director of the Coral Sea Clinical Research Institute, which has participated in clinical trials focusing on new therapies for inflammatory bowel disease and coeliac disease including ImmusanT, Celgene, Gossamer Bio, Gilead, Arena, Janssen, Takeda, Abbvie, Eli Lilly, Sanofi, Regeneron, Lumen Bioscience, Roche, Abivax and Dr Falk Pharma; is a contracted consultant for the Wesley Research Institute; receives fees as a principal investigator; received an honorarium from Abbvie to present at Australian Gastroenterology week; and is the Director of the Coeliac and Immune Health Research Program at the Wesley Research Institute.
References
1. Parada A, Araya M. El gluten: su historia y efectos en la enfermedad celíaca. Revista médica de Chile 2010; 138: 1319-1325.
2. Losowsky MS. A history of coeliac disease. Dig Dis 2008; 26: 112-120.
3. Tommasini A, Not T, Ventura A. Ages of celiac disease: from changing environment to improved diagnostics. World J Gastroenterol 2011; 17: 3665-3671.
4. Haas SV. The value of the banana in the treatment of celiac disease. Am J Dis Child 1924; 28: 421-437.
5. The New York Times. Dr. Sidney Valentine Haas dies. New York, NY, USA; The New York Times Archive: 1964. Available online at: https://www.nytimes.com/1964/12/01/dr-sidney-valentine-haas-dies.html (accessed June 2025).
6. Dicke WK, Weijers HA, van de Kamer JH. Coeliac disease. II. The presence in wheat of a factor having a deleterious effect in cases of coeliac disease. Acta Paediatr (Stockh) 1953; 42: 34-42.
7. Paulley JW. Observation on the aetiology of idiopathic steatorrhoea; jejunal and lymph-node biopsies. Br Med J 1954; 2: 1318-1321.
8. Royer M., Croxatto O. Biopsia duodenal por aspiración bajo control radioscópico. Prensa Med Argent 1955; 42: 2515-2519.
9. Shiner M. Duodenal biopsy. Lancet 1956; 1: 17-19.
10. Hirschowitz BI, Curtiss LE, Peters CW, Pollard HM. Demonstration of a new gastroscope, the fiberscope. Gastroenterology 1958; 35: 50-53.
11. Rubio-Tapia A, Hill ID, Semrad C, et al. American College of Gastroenterology Guidelines update: diagnosis and management of celiac disease. Am J Gastroenterol 2023; 118: 59-76.
12. Lindfors K, Ciacci C, Kurppa K, et al. Coeliac disease. Nat Rev Dis Primers 2019; 5: 1-18.
13. Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med 2019; 17: 142.
14. Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology 2009; 137: 88-93.
15. Lohi S, Mustalahti K, Kaukinen K, et al., Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther 2007; 26: 1217-1225.
16. Catassi C, Kryszak D, Bhatti B, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med 2010; 42: 530-538.
17. Singh P, Arora A, Strand TA, et al. Global prevalence of celiac disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 16: 823-836.e2.
18. Biesiekierski JR. What is gluten? J Gastroenterol Hepatol 2017; 32: 78-81.
19. Besser HA, Khosla C. Celiac disease: mechanisms and emerging therapeutics. Trends Pharmacol Sci 2023; 44: 949-962.
20. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet 2018; 391: 70-81.
21. Hausch F, Shan L, Santiago NA, Gray GM, Khosla C. Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol 2002; 283: G996-G1003.
22. Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 2008; 135: 194-204.
23. Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med 2008; 205: 143-154.
24. Lebreton C, Ménard S, Abed J, et al. Interactions among secretory immunoglobulin A, CD71, and transglutaminase-2 affect permeability of intestinal epithelial cells to gliadin peptides. Gastroenterology 2012; 143: 698-707.
25. Rauhavirta T, Qiao S-W, Jiang Z, et al. Epithelial transport and deamidation of gliadin peptides: a role for coeliac disease patient immunoglobulin A. Clin Exp Immunol 2011; 164: 127-136.
26. Molberg Ø, Mcadam SN, Körner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med 1998; 4: 713-717.
27. Tye-Din JA, Cameron DJ, Daveson AJ, et al. Appropriate clinical use of human leukocyte antigen typing for coeliac disease: an Australasian perspective. Intern Med J 2015; 45: 441-450.
28. Daveson AJM, Tye-Din JA, Anderson RP. Editorial: inaccuracies in attribution of symptoms due to gluten-not just in those with self-reported noncoeliac gluten sensitivity. Authors’ reply. Aliment Pharmacol Ther 2020; 51: 403-404.
29. Stamnaes J, Sollid LM. Celiac disease: autoimmunity in response to food antigen. Semin Immunol 2015; 27: 343-352.
30. Elli L, Ferretti F, Orlando S, et al. Management of celiac disease in daily clinical practice. Eur J Int Med 2019; 61: 15-24.
31. Barada K, Bitar A, Mokadem MA, Hashash JG, Green P. Celiac disease in Middle Eastern and North African countries: a new burden? World J Gastroenterol 2010; 16: 1449-1457.
32. Singh P, Arora A, Strand TA, et al. Global prevalence of celiac disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 16: 823-836.e2.
33. Anderson RP, Henry MJ, Taylor R, et al. A novel serogenetic approach determines the community prevalence of celiac disease and informs improved diagnostic pathways. BMC Med 2013; 11: 188.
34. Anderson RP. Coeliac disease is on the rise. Med J Aust 2011; 194: 278-279.
35. Downey L, Houten R, Murch S, Longson D; Guideline Development Group. Recognition, assessment, and management of coeliac disease: summary of updated NICE guidance. BMJ 2015; 351: h4513.
36. Gray AM, Papanicolas IN. Impact of symptoms on quality of life before and after diagnosis of coeliac disease: results from a UK population survey. BMC Health Serv Res 2010; 10: 105.
37. Muir R, Sehgal A, Tye-Din JA, Daveson AJM. Undiagnosed coeliac disease identified by active case finding in first degree relatives of people with coeliac disease in Australia: a prospective observational study. Med J Aust 2023; 219: 371-373.
38. Olmos M, Antelo M, Vazquez H, Smecuol E, Mauriño E, Bai JC. Systematic review and meta-analysis of observational studies on the prevalence of fractures in coeliac disease. Dig Liver Dis 2008; 40: 46-53.
39. Ludvigsson JF, Michaelsson K, Ekbom A, Montgomery SM. Coeliac disease and the risk of fractures - a general population-based cohort study. Aliment Pharmacol Ther 2007; 25: 273-285.
40. Collin P, Salmi TT, Hervonen K, Kaukinen K, Reunala T. Dermatitis herpetiformis: a cutaneous manifestation of coeliac disease. Ann Med 2017; 49: 23-31.
41. Smyth DJ, Plagnol V, Walker NM, et al. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med 2008; 359: 2767-2777.
42. Carnicer J, Farré C, Varea V, Vilar P, Moreno J, Artigas J. Prevalence of coeliac disease in Down’s syndrome. Eur J Gastroenterol Hepatol 2001; 13: 263-267.
43. Elli L, Bonura A, Garavaglia D, et al. Immunological comorbity in coeliac disease: associations, risk factors and clinical implications. J Clin Immunol 2012; 32: 984-990.
44. Tye-Din JA, Daveson AJM, Ee HC, et al. Elevated serum interleukin-2 after gluten correlates with symptoms and is a potential biomarker for coeliac disease. Aliment Pharmacol Ther 2019; 50: 901-910.
45. Schwertz E, Kahlenberg F, Sack U, et al. Serologic assay based on gliadin-related nonapeptides as a highly sensitive and specific diagnostic aid in celiac disease. Clin Chem 2004; 50: 2370-2375.
46. Sugai E, Vázquez H, Nachman F, et al. Accuracy of testing for antibodies to synthetic gliadin-related peptides in celiac disease. Clin Gastroenterol Hepatol 2006; 4: 1112-1117.
47. Tonutti E, Visentini D, Picierno A, et al. Diagnostic efficacy of the ELISA test for the detection of deamidated anti-gliadin peptide antibodies in the diagnosis and monitoring of celiac disease. J Clin Lab Anal 2009; 23: 165-171.
48. van der Windt DA, Jellema P, Mulder CJ, Kneepkens CM, van der Horst HE. Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review. JAMA 2010; 303: 1738-1746.
49. Reeves GE, Squance ML, Duggan AE, et al. Diagnostic accuracy of coeliac serological tests: a prospective study. Eur J Gastroenterol Hepatol 2006; 18: 493-501.
50. Kumar V, Jarzabek-Chorzelska M, Sulej J, Karnewska K, Farrell T, Jablonska S. Celiac disease and immunoglobulin A deficiency: how effective are the serological methods of diagnosis? Clin Diagn Lab Immunol 2002; 9: 1295-1300.
51. Gibson PR. Editorial: inaccuracies in attribution of symptoms due to gluten-not just in those with self-reported non-coeliac glutensensitvity. Aliment Pharmacol Ther 2020; 51: 401-402.
52. Wieser H. Chemistry of gluten proteins. Food Microbiol 2007; 24: 15-19.
53. Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014 146: 67-75.e5.
54. Leffler D, Schuppan D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut 2013; 62: 996-1004.
55. Raju SA, Mooney PD, Aziz I, Kurien M, Sanders DS. Letter: gluten challenge in the era of noncoeliac gluten sensitivity—a change in clinical practice? Aliment Pharmacol Ther 2016; 43: 656.
56. Kasarda DD. Can an increase in celiac disease be attributed to an increase in the gluten content of wheat as a consequence of wheat breeding? J Agric Food Chem 2013; 61: 1155-1159.
57. Tye-Din JA, Daveson AJM, Goldstein KE, et al.; RESET CeD Study Group. Patient factors influencing acute gluten reactions and cytokine release in treated coeliac disease. BMC Med 2020 18: 362.
58. Daveson AJM, Tye-Din JA, Goel G, et al. Masked bolus gluten challenge low in FODMAPs implicates nausea and vomiting as key symptoms associated with immune activation in treated coeliac disease. Aliment Pharmacol Ther 2020; 51: 244-252.
59. Goel G, Tye-Din JA, Qiao SW, et al. Cytokine release and gastrointestinal symptoms after gluten challenge in celiac disease. Sci Adv 2019; 5: eaaw7756.
60. Anderson RP, Goel G, Hardy MY, et al. Whole blood interleukin-2 release test to detect and characterize rare circulating gluten-specific T cell responses in coeliac disease. Clin Exp Immunol 2021; 204: 321-334.
61. Hopper AD, Cross SS, Sanders DS. Patchy villous atrophy in adult patients with suspected gluten-sensitive enteropathy: is a multiple duodenal biopsy strategy appropriate? Endoscopy 2008; 40: 219-224.
62. Latorre M, Lagana SM, Freedberg DE, et al. Endoscopic biopsy technique in the diagnosis of celiac disease: one bite or two? Gastrointest Endosc 2015; 81: 1228-1233.
63. Taavela J, Koskinen O, Huhtala H, et al. Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PLoS One 2013; 8: e76163.
64. Villanacci V, Lorenzi L, Donato F, et al. Histopathological evaluation of duodenal biopsy in the PreventCD project. An observational interobserver agreement study. Apmis 2018; 126: 208-214.
65. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr 2020; 70: 141-156.
66. Anderson RP, Henry MJ, Taylor R, et al. A novel serogenetic approach determines the community prevalence of celiac disease and informs improved diagnostic pathways. BMC Med 2013; 11: 188.
67. Daveson AJM, Varney M, Jackson KE, Tye-Din JA. Discrepancies in genetic tersting results for coeliac disease: call for standardised testing and reporting. Med J Aus 2017; 207: 178-179.
68. Horan MP, Chai SY, Munusamy N, et al. High rates of variation in HLA-DQ2/DQ8 testing for coeliac disease: results from an RCPAQAP pilot program. J Clin Pathol 2018; 71: 900-905.
69. Vader W, Stepniak D, Kooy Y, et al. The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proc Natl Acad Sci U S A 2003; 100: 12390-12395.
70. Ianiro G, Bibbò S, Bruno G, et al. Prior misdiagnosis of celiac disease is common among patients referred to a tertiary care center: a prospective cohort study. Clin Transl Gastroenterol 2016; 7: e139.
71. Caio G, Riegler G, Patturelli M, Facchiano A, DE Magistris L, Sapone A. Pathophysiology of non-celiac gluten sensitivity: where are we now? Minerva Gastroenterol Dietol 2017; 63: 16-21.
72. Shah S, Akbari M, Vanga R, et al. Patient perception of treatment burden is high in celiac disease compared with other common conditions. Am J Gastroenterol 2014; 109: 1304-1311.
73. Silvester JA, Comino I, Kelly CP, Sousa C, Duerksen DR; DOGGIE BAG Study Group. Most patients with celiac disease on gluten-free diets consume measurable amounts of gluten. Gastroenterology 2020; 158: 1497-1499.e1.
74. Silvester, J.A., et al. Exposure sources, amounts and time course of gluten ingestion and excretion in patients with coeliac disease on a gluten-free diet. Aliment Pharmacol Ther 2020; 52: 1469-1479.
75. Stefanolo JP, Tálamo M, Dodds S, et al. Real-world gluten exposure in patients with celiac disease on gluten-free diets, determined from gliadin immunogenic peptides in urine and fecal samples. Clin Gastroenterol Hepatol 2021; 19: 484-491.e1.
76. Ludvigsson JF, Lebwohl B, Chen Q, et al. Anxiety after coeliac disease diagnosis predicts mucosal healing: a population-based study. Aliment Pharmacol Ther 2018; 48: 1091-1098.
77. Halmos EP, Deng M, Knowles SR, Sainsbury K, Mullan B, Tye-Din JA. Food knowledge and psychological state predict adherence to a gluten-free diet in a survey of 5310 Australians and New Zealanders with coeliac disease. Aliment Pharmacol Ther 2018; 48: 78-86.
78. Hall N, Rubin G, Charnock A. Systematic review: adherence to a gluten-free diet in adult patients with coeliac disease. Aliment Pharmacol Ther 2009; 30: 315-330.
79. Sbravati F, Cosentino A, Lenzi J, et al. Antitissue transglutaminase antibodies’ normalization after starting a gluten-free diet in a large population of celiac children-a real-life experience. Dig Liver Dis 2022; 54: 336-342.
80. Tye-Din JA. Review article: Follow-up of coeliac disease. Aliment Pharmacol Ther 2022; 56 Suppl 1: S49-S63.
81. Galli G, Lahner E, Conti L, Esposito G, Sacchi MC, Annibale B. Risk factors associated with osteoporosis in a cohort of prospectively diagnosed adult coeliac patients. United European Gastroenterol J 2018; 6: 1161-1168.
82. Pritchard L, Wilson S, Griffin J, Pearce G, Murray IA, Lewis S. Prevalence of reduced bone mineral density in adults with coeliac disease - are we missing opportunities for detection in patients below 50 years of age? Scand J Gastroenterol 2018; 53: 1433-1436.
83. Corazza GR, Zoli G, Di Sabatino A, Ciccocioppo R, Gasbarrini G. A reassessment of splenic hypofunction in celiac disease. Am J Gastroenterol 1999; 94: 391-397.
84. Ludvigsson JF, Olén O, Bell M, Ekbom A, Montgomery SM. Coeliac disease and risk of sepsis. Gut 2008; 57: 1074.
85. Simons M, Scott-Sheldon LAJ, Risech-Neyman Y, Moss SF, Ludvigsson JF, Green PHR. Celiac disease and increased risk of pneumococcal infection: a systematic review and meta-analysis. Am J Med 2018; 131: 83-89.
86. Wahab PJ, Meijer JW, Mulder CJ. Histologic follow-up of people with celiac disease on a gluten-free diet: slow and incomplete recovery. Am J Clin Pathol 2002; 118: 459-463.
87. Kreutz JM, Adriaanse MPM, van der Ploeg EMC, Vreugdenhil ACE. Narrative review: nutrient deficiencies in adults and children with treated and untreated celiac disease. Nutrients 2020; 12: 500.
88. Ciacci C, Cirillo M, Cavallaro R, Mazzacca G. Long-term follow-up of celiac adults on gluten-free diet: prevalence and correlates of intestinal damage. Digestion 2002; 66: 178-185.
89. Daveson AJM, Popp A, Taavela J, et al.; the RESET CeD Study Group. Baseline quantitative histology in therapeutics trials reveals villus atrophy in most patients with coeliac disease who appear well controlled on gluten-free diet. GastroHep 2020; 2: 22-30.
90. Fernández-Bañares F, Beltrán B, Salas A, et al.; CADER study group. Persistent villous atrophy in de novo adult patients with celiac disease and strict control of gluten-free diet adherence: a multicenter prospective study (CADER Study). Am J Gastroenterol 2021; 116: 1036-1043.
91. Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol 2007; 5: 445-450.
92. Crepaldi M, Palo M, Maniero D, et al. Emerging pharmaceutical therapies to address the inadequacy of a gluten-free diet for celiac disease. Pharmaceuticals (Basel) 2023; 17: 4.
93. Karinen H, Kärkkäinen P, Pihlajamäki J, et al. Gene dose effect of the DQB1*0201 allele contributes to severity of coeliac disease. Scand J Gastroenterol 2006; 41: 191-199.
94. Carroccio A, Ambrosiano G, Di Prima L, et al. Clinical symptoms in celiac patients on a gluten-free diet. Scand J Gastroenterol 2008; 43: 1315-1321.
95. Ilus T, Kaukinen K, Virta LJ, et al. Refractory coeliac disease in a country with a high prevalence of clinically-diagnosed coeliac disease. Aliment Pharmacol Ther 2014; 39: 418-425.
96. Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut 2010; 59: 547-557.
97. Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut 2007; 56: 1373-1378.
98. Mearin ML, Catassi C, Brousse N, et al.; Biomed Study Group on Coeliac Disease and Non-Hodgkin Lymphoma. European multi-centre study on coeliac disease and non-Hodgkin lymphoma. Eur J Gastroenterol Hepatol 2006; 18: 187-194.
99. Daum S, Cellier C, Mulder CJ. Refractory coeliac disease. Best Pract Res Clin Gastroenterol 2005; 19: 413-424.
100. Cil T, Altintaş A, Işikdoğan A, et al. Screening for celiac disease in Hodgkin and non-Hodgkin lymphoma patients. Turk J Gastroenterol 2009; 20: 87-92.
101. Roshan B, Leffler DA, Jamma S, et al. The incidence and clinical spectrum of refractory celiac disease in a north american referral center. Am J Gastroenterol 2011; 106: 923-928.
102. Tennyson CA, Simpson S, Lebwohl B, Lewis S, Green PH. Interest in medical therapy for celiac disease. Therap Adv Gastroenterol 2013; 6: 358-364.
103. Aziz I, Evans KE, Papageorgiou V, Sanders DS. Are patients with coeliac disease seeking alternative therapies to a gluten-free diet? J Gastrointestin Liver Dis 2011; 20: 27-31.
104. Lähdeaho ML, Kaukinen K, Laurila K, et al. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology 2014; 146: 1649-1658.
105. Murray JA, Syage JA, Wu TT, et al.; CeliacShield Study Group. Latiglutenase protects the mucosa and attenuates symptom severity in patients with celiac disease exposed to a gluten challenge. Gastroenterology 2022; 163: 1510-1521.e6.
106. Syage JA, Murray JA, Green PHR, Khosla C. Latiglutenase improves symptoms in seropositive celiac disease patients while on a gluten-free diet. Dig Dis Sci 2017; 62: 2428-2432.
107. Pultz IS, Hill M, Vitanza JM, et al. Gluten degradation, pharmacokinetics, safety, and tolerability of TAK-062, an engineered enzyme to treat celiac disease. Gastroenterology 2021; 161: 81-93.e3.
108. Tack GJ, van de Water JM, Bruins MJ, et al. Consumption of gluten with gluten-degrading enzyme by celiac patients: a pilot-study. World J Gastroenterol 2013; 19: 5837-5847.
109. Guzmán-López MH, Sánchez-León S, Marín-Sanz M, et al. Oral consumption of bread from an RNAi wheat line with strongly silenced gliadins elicits no immunogenic response in a pilot study with celiac disease patients. Nutrients 2021; 13: 4548.
110. Gujral N, Löbenberg R, Suresh M, Sunwoo H. In-vitro and in-vivo binding activity of chicken egg yolk immunoglobulin Y (IgY) against gliadin in food matrix. J Agric Food Chem 2012; 60: 3166-3172.
111. McCarville JL, Nisemblat Y, Galipeau HJ, et al. BL-7010 demonstrates specific binding to gliadin and reduces gluten-associated pathology in a chronic mouse model of gliadin sensitivity. PLoS One 2014; 9: e109972.
112. Pinier M, Fuhrmann G, Galipeau HJ, et al. The copolymer P(HEMA-co-SS) binds gluten and reduces immune response in gluten-sensitized mice and human tissues. Gastroenterology 2012; 142: 316-325.e1-12.
113. Daveson AJM, Stubbs R, Polasek TM, et al. Safety, clinical activity, pharmacodynamics, and pharmacokinetics of IMU-856, a SIRT6 modulator, in coeliac disease: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Gastroenterol Hepatol 2025; 10: 44-54.
114. Hoilat GJ, Altowairqi AK, Ayas MF, et al. Larazotide acetate for treatment of celiac disease: a systematic review and meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol 2022; 46: 101782.
115. Machado MV. New developments in celiac disease treatment. Int J Mol Sci 2023; 24: 945.
116. Schuppan D, Mäki M, Lundin KEA, et al.; CEC-3 Trial Group. A randomized trial of a transglutaminase 2 inhibitor for celiac disease. N Engl J Med 2021; 385: 35-45.
117. Lähdeaho ML, Scheinin M, Vuotikka P, et al. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: a phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol Hepatol 2019; 4: 948-959.
118. Cellier C, Bouma G, van Gils T, et al.; RCD-II Study Group Investigators. Safety and efficacy of AMG 714 in patients with type 2 refractory coeliac disease: a phase 2a, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Gastroenterol Hepatol 2019; 4: 960-970.
119. Wesley Research Institute. TEVA Study. Auchenflower: Wesley Research Institute; 2024. Available online at: https://www.wesleyresearch.org.au/clinical-trial/teva-study/ (accessed June 2025).
120. Okura Y, Ikawa-Teranishi Y, Mizoroki A, et al. Characterizations of a neutralizing antibody broadly reactive to multiple gluten peptide:HLA-DQ2.5 complexes in the context of celiac disease. Nat Commun 2023; 14: 8502.
121. Dieckman T, Schumann M, Beaumont H, Bontkes HJ, Koning F, Bouma G; RCDII Tofacitinib Consortium. Enduring clinical remission in refractory celiac disease type II with tofacitinib: an open-label clinical study. Clin Gastroenterol Hepatol 2024; 22: 2334-2336.
122. Buriánek F, Gege C, Marinković P. New developments in celiac disease treatments. Drug Discov Today 2024; 29: 104113.
123. Tye-Din JA, Daveson AJM, Goel G, et al. Efficacy and safety of gluten peptide-based antigen-specific immunotherapy (Nexvax2) in adults with coeliac disease after bolus exposure to gluten (RESET coeliac disease): an interim analysis of a terminated randomised, double-blind, placebo-controlled phase 2 study. Lancet Gastroenterol Hepatol 2023; 8: 446-457.
124. Daveson AJ, Jones DM, Gaze S, et al. Effect of hookworm infection on wheat challenge in celiac disease—a randomised double-blinded placebo controlled trial. PLoS One 2011; 6: e17366.
125. Croese J, Miller GC, Marquart L, et al. Randomized, placebo controlled trial of experimental hookworm infection for improving gluten tolerance in celiac disease. Clin Transl Gastroenterol 2020; 11: e00274.
126. Freitag TL, Podojil JR, Pearson RM, et al. Gliadin nanoparticles induce immune tolerance to gliadin in mouse models of celiac disease. Gastroenterology 2020; 158: 1667-1681.e12.
127. Kelly CP, Murray JA, Leffler DA, et al.; TAK-101 Study Group. TAK-101 nanoparticles induce gluten-specific tolerance in celiac disease: a randomized, double-blind, placebo-controlled study. Gastroenterology 2021; 161: 66-80.e8.