Type 1 diabetes: reducing cardiovascular risk
Cardiovascular risk is greatly increased in people with type 1 diabetes. Optimising glycaemic control and managing traditional risk factors, such as hypertension and dyslipidaemia, are the mainstay of treatment. In future, adjunctive noninsulin-based therapies may also have a role; however, these medications currently remain off label for type 1 diabetes, with more evidence needed.
- Cardiovascular risk is substantially increased in people with type 1 diabetes.
- Optimal management of blood glucose levels, as well as traditional cardiovascular risk factors, such as hypertension and dyslipidaemia, is the mainstay of management. There is evidence of undertreatment of cardiovascular risk factors in type 1 diabetes, particularly in younger age groups.
- Unique cardiovascular risk factors in type 1 diabetes include glycaemic variability and hypoglycaemia, as well as overall hyperglycaemia.
- Insulin resistance is increased in people with type 1 diabetes due to peripheral hyperinsulinaemia from subcutaneous insulin treatment, even at a lower body mass index.
- The pathophysiology of cardiovascular damage in type 1 diabetes is different from type 2 diabetes, and reliance on risk calculators designed for other patient groups can be misleading.
- Although there is potential for the use of adjunctive therapies such as sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists in type 1 diabetes, their use remains off label at this stage, and evidence for their effectiveness in reducing cardiovascular risk in this cohort is lacking.
Despite advances in the management of cardiometabolic disease in recent decades, premature cardiovascular disease (including coronary artery disease, acute myocardial infarction, stroke and heart failure) remains the greatest cause of mortality in people with type 1 diabetes. People with type 1 diabetes have at least three times the cardiovascular mortality risk of those without diabetes, and this is even more pronounced in people with onset of disease at a young age.1,2 Further, female sex should not be considered to offer protection against cardiovascular disease in those with type 1 diabetes. Studies show a higher relative rate of cardiovascular disease in women compared with men with type 1 diabetes.3
This article explores the important differences in the pathophysiology of cardiovascular disease in type 1 diabetes, options for cardiovascular risk estimation in this cohort and treatment of traditional risk factors.
Pathophysiology of cardiovascular disease in type 1 diabetes
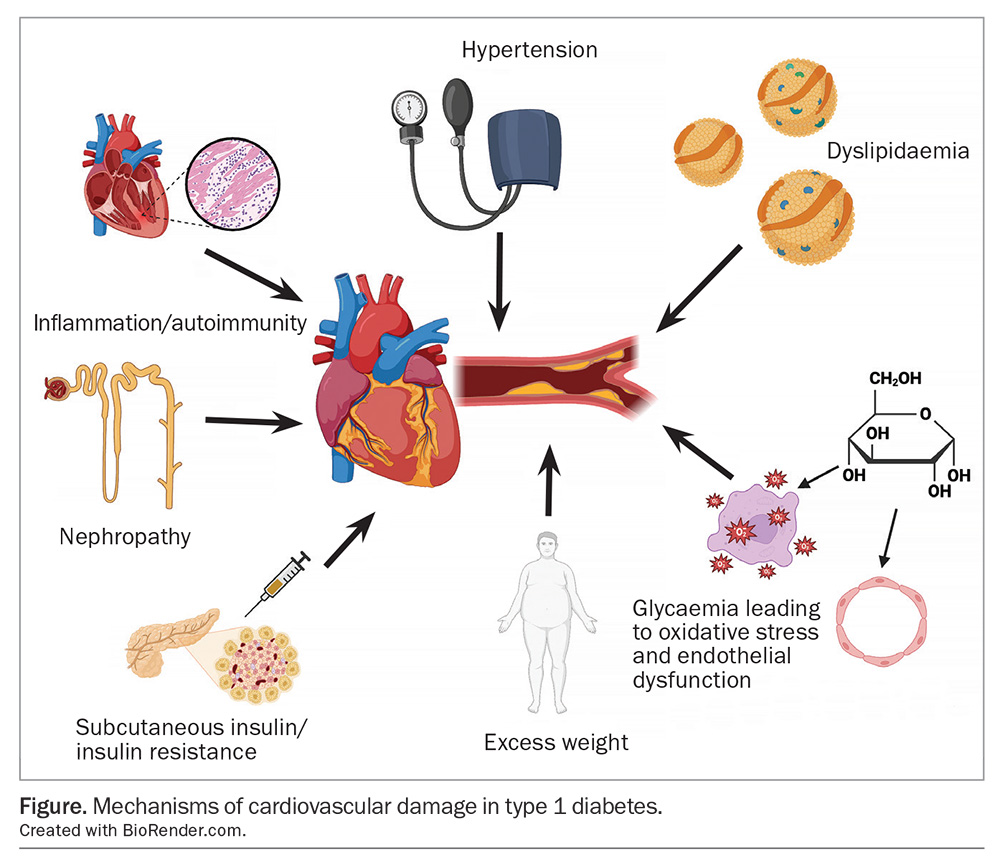
Several mechanisms contribute to cardiovascular damage in type 1 diabetes (Figure). These include traditional cardiovascular risk factors, such as hypertension, dyslipidaemia and excess weight, and there is evidence these are undertreated in people with type 1 diabetes. However, there are also cardiovascular risk factors unique to those with type 1 diabetes, such as glycaemic variability, hypoglycaemia, hyperglycaemia and insulin resistance.
Blood glucose management
Hyperglycaemia is a clear contributor to cardiovascular risk in type 1 diabetes. Likely mechanisms include oxidative stress and endothelial dysfunction caused by generations of damaging substances, such as advanced glycation end products, and inhibition of protective factors, such as endothelial nitric oxide synthase.4
Robust clinical trial evidence supports the benefits of optimising glycaemia in people with type 1 diabetes. The Diabetes Control and Complications Trial (DCCT), a landmark trial in type 1 diabetes management, showed that intensive glycaemic control over a 6.5-year period reduced the risk of developing cardiovascular disease over the next 10 years. These benefits persisted with follow up for 30 years in the Epidemiology of Diabetes Intervention and Complications (EDIC) study, showing management of glycaemia has ongoing effects or metabolic memory.5
With more widespread use of continuous glucose monitoring, variation in blood glucose levels has become easier to identify and study. Physiological studies in type 2 diabetes suggest oscillating blood glucose levels cause greater vascular dysfunction compared with stable levels of hyperglycaemia.6 In type 1 diabetes, glycaemic variability and hypoglycaemia have been associated with endothelial injury and increased inflammation, as well as increased coronary artery calcium scores.7-9 Dysglycaemia has also been shown to lead to autonomic neuropathy, which predicts cardiovascular mortality in type 1 diabetes.10,11
Optimising glycaemic control is therefore key to reducing cardiovascular risk in people with type 1 diabetes. Targets for glycated haemoglobin (HbA1c) levels are usually less than 7%, however, they should be individualised. Time in range (3.9 to 10 mmol/L) is increasingly used as an alternative measure if continuous glucose monitoring is available, which is now subsidised under the National Diabetes Services Scheme for all people with type 1 diabetes.12 Other factors, such as psycho-social issues, comorbidity and frailty, should also be considered in setting glycaemic targets for an individual.13,14
Insulin pump therapy may assist in improving overall glycaemia and reducing glycaemic variability. A large Swedish observational study showed that use of insulin pump therapy was associated with improved cardiovascular mortality when compared with multiple daily insulin injections.15
Insulin resistance
Insulin resistance, more commonly associated with type 2 diabetes, is under-recognised as a driver of cardiovascular disease in type 1 diabetes. It is partially induced by the necessity of subcutaneous insulin delivery for treatment, bypassing hepatic insulin clearance and leading to peripheral hyperinsulinaemia.16 Intensive insulin therapy can lead to a vicious cycle of weight gain, increased insulin resistance and higher doses of insulin. The term ‘double diabetes’ has been coined to signify the occurrence of insulin resistance in people with type 1 diabetes and is estimated to occur in 30% of adults with type 1 diabetes.17
Currently, there are limited therapeutic interventions with evidence for directly decreasing insulin resistance. Lifestyle measures to manage weight and increase physical activity are likely to be of benefit. Optimising insulin therapy, including consideration of use of diabetes technologies (e.g. insulin pumps), to minimise overtreatment is also important.
Dyslipidaemia
The lipid profile in type 1 diabetes is altered, and the alteration is likely caused by portal insulin deficiency. HDL-cholesterol levels are often normal or even elevated, and triglyceride and LDL-cholesterol levels are at normal levels. Standard lipid testing can therefore be falsely reassuring in some cases, as increases in intermediate-density lipoprotein and small-dense LDL levels lead to an overall increase in atherogenesis.18 In addition, elevated cardiovascular risk occurs at younger ages than screening and treatment would normally be considered.
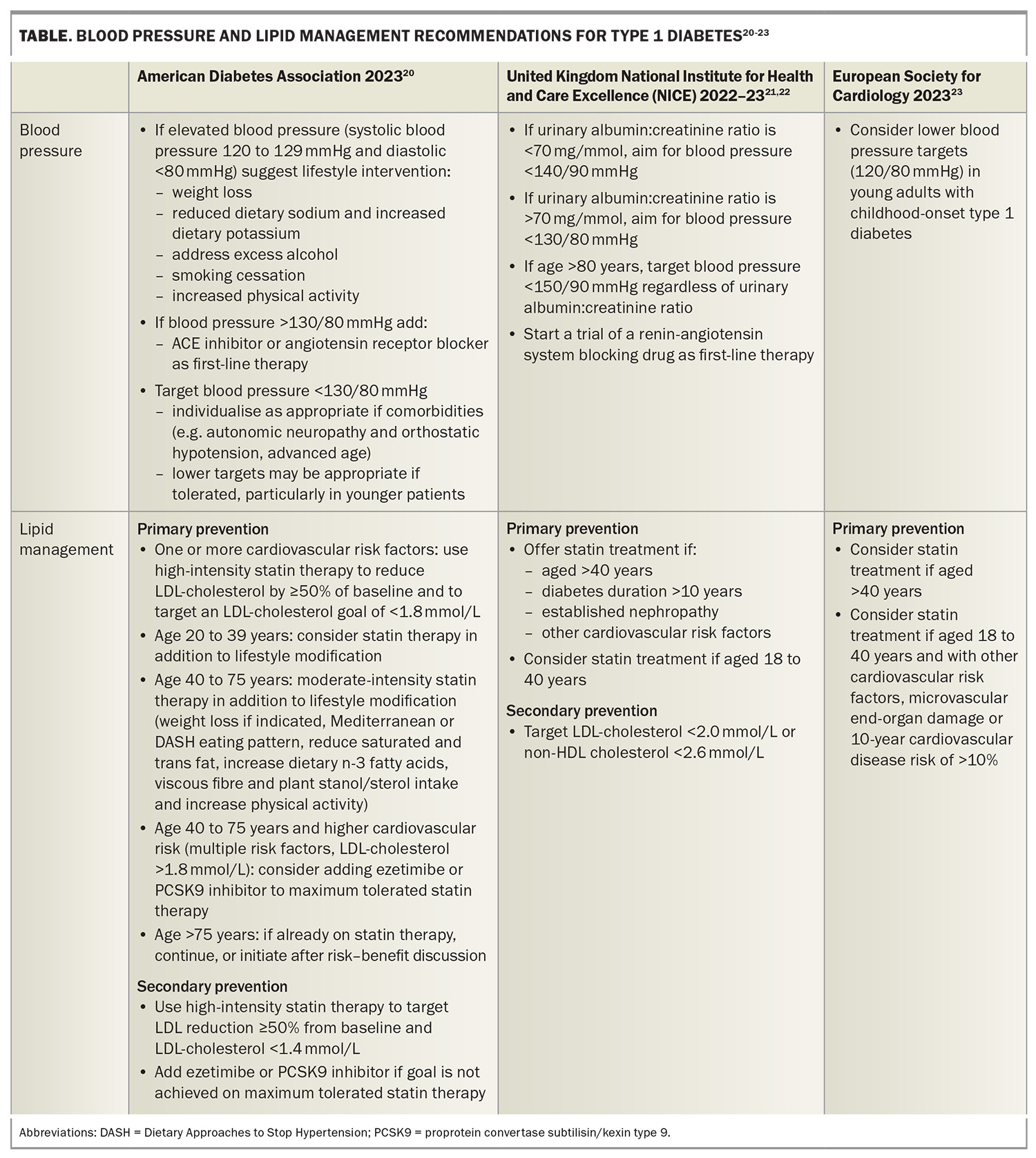
Global lipid management recommendations vary for people with type 1 diabetes and no established cardiovascular disease (primary prevention); however, overall, statin therapy is recommended for all people with type 1 diabetes over 40 years of age, regardless of other cardiovascular risk factors. Statin therapy should also be considered in those under 40 years of age if additional cardiovascular risk factors are present. In a randomised controlled trial in people aged 40 to 80 years with type 1 or type 2 diabetes, statin therapy improved cardiovascular outcomes, including in those without elevated HDL-cholesterol levels or known cardiovascular disease at baseline. Overall, the result was not significant for the type 1 diabetes subgroup although the trend was similar to those with type 2 diabetes.19 Lipid management recommendations from major international guidelines are summarised in the Table.20-23 Statins should be avoided in pregnant or breastfeeding women.
For people with type 1 diabetes and established cardiovascular disease (secondary prevention), recommendations for lipid-lowering therapy are the same as for the general population. Therapy consists of high-intensity statin therapy as first line, with ezetimibe and a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor as needed to reach LDL-cholesterol targets. Options include monoclonal antibodies (evolocumab and alirocumab) or inclisiran, a small interfering ribonucleic acid PCSK9 inhibitor. In Australia, the accepted LDL-cholesterol target for secondary prevention is 1.8 mmol/L, although US guidelines suggest a lower target of 1.4 mmol/L.20
Hypertension
Hypertension is common in people with type 1 diabetes.24 Major guidelines advocate for blood pressure targets in line with other high-risk groups. The American Diabetes Association notes the lack of specific evidence to inform blood pressure targets in people with type 1 diabetes, however, suggests a target of less than 130/80 mmHg (Table).20-23
Individualisation of blood pressure targets is important. Autonomic neuropathy in people with type 1 diabetes can lead to postural hypotension, supine hypertension or loss of circadian blood pressure rhythm. This may mean the 130/80 mmHg target is unable to be achieved. In others, a more aggressive target may be achievable and potentially lead to better cardiovascular outcomes. If blood pressure exceeds 120/80 mmHg, lifestyle interventions, including weight loss, increased physical activity, reduced sodium intake, increased potassium intake, limited alcohol intake, smoking cessation and increased physical activity, should be encouraged. For those with established cardiovascular disease or diabetic nephropathy with albuminuria, first-line hypertension treatment should be an ACE inhibitor or angiotensin receptor blocker, although these medications should be avoided in pregnant women. Blockade of the renin-angiotensin-aldosterone system offers additional benefits in reducing albuminuria and cardiovascular risk. Furthermore, microalbuminuria in type 1 diabetes is clearly linked with increased cardiovascular risk.25 If beta-blocker therapy is indicated, clinicians should caution patients that its use may reduce perception of hypoglycaemia symptoms.
Excess weight
The prevalence of overweight and obesity is increasing in the type 1 diabetes population, and may now exceed general population trends.26 However, the issue is compounded in people with type 1 diabetes with the necessity of subcutaneous insulin treatment, leading to insulin resistance and weight gain. This creates a difficult balance between managing glycaemia and limiting total daily insulin dose. Excessive weight gain during the DCCT/EDIC trial was associated with an increase in cardiovascular events, which partially offset the benefit derived from intensive glycaemic control.27
Although lifestyle interventions, such healthy eating, physical activity and behavioural and psychological support, are the basis of any weight loss intervention, these pose additional challenges in the context of type 1 diabetes. Changes in diet and levels of physical activity require insulin dose adjustment, and during development of new habits this can be an additional barrier to change. A recent Australian study found a prevalence of disordered eating of 31% in a cohort of people with type 1 diabetes attending a metropolitan hospital clinic.28 Insulin omission is often employed for weight loss, leading to safety concerns.29
Limited medication options are approved by the TGA for obesity treatment in Australia.30 Phentermine, which reduces appetite by stimulating neurotransmitter release, is contraindicated in people with coronary artery disease. Orlistat inhibits lipases and therefore fat absorption, and can be used in type 1 diabetes; however, it is poorly tolerated due to side effects and cases of diabetic ketoacidosis have been reported.31 A naltrexone and bupropion combination medication may be useful in regulating appetite and reward circuits in the brain, although, typically, weight loss is modest. Adjunctive therapies have been trialled in people with type 1 diabetes to reduce insulin requirements. These include glucagon-like peptide-1 (GLP-1) receptor agonists (liraglutide, semaglutide) and the dual GLP-1/gastric inhibitory polypeptide receptor agonist (tirzepatide). Liraglutide is approved by the TGA for a weight loss indication in Australia, as is 2.4 mg weekly semaglutide (although currently this dose is not available in Australia). Neither are approved by the TGA for use in type 1 diabetes. The potential use of adjunctive therapies in type 1 diabetes is discussed in more detail below.
Cardiovascular risk assessment
The Australian Chronic Disease Prevention Alliance recently updated the Australian cardiovascular disease risk guidelines. These guidelines include the Australian cardiovascular disease risk calculator, which uses a comprehensive equation to account for diabetes-specific risk factors, such as time since diagnosis, HbA1c level, estimated glomerular filtration rate, urinary albumin:creatinine ratio, body mass index and insulin use.32 However, although these risk factors have all been shown to contribute to cardiovascular risk in type 1 diabetes, the calculator is not validated for this group. Risk calculators specifically developed for type 1 diabetes include the Steno T1 Risk Engine, derived from studies performed in Denmark, and a risk calculator based on Scottish and Swedish registries.33-35 It is unclear whether the data may be extrapolated to the Australian population.
Newer imaging techniques, such as coronary artery calcium scores and CT coronary angiography for risk stratification, have been explored in type 1 diabetes. Coronary artery calcium scores have demonstrated validity in type 1 diabetes but there is insufficient evidence to recommend they be used for routine screening.36,37
Role of adjunctive agents in reducing cardiovascular risk
With the increasing availability of multiple agents shown to improve cardiovascular outcomes in type 2 diabetes, interest in repurposing these medications to improve outcomes in type 1 diabetes is increasing. The 2024 American Diabetes Association guidelines recommend treatment with a sodium-glucose cotransporter-2 (SGLT-2) inhibitor and/or GLP-1 receptor agonist for people with type 2 diabetes and high cardiovascular risk or heart failure.20 However, at this stage there are no noninsulin medications approved by the TGA for type 1 diabetes, although many are used off label in this context.38
Metformin
Evidence for cardiovascular protection with use of metformin is mixed in the general population, in those with type 2 diabetes, and in small trials with surrogate cardiovascular endpoints in type 1 diabetes.39 A meta-analysis of trials in type 1 diabetes found that use of metformin reduces insulin dose requirements.40
The Reducing With Metformin Vascular Adverse Lesions (REMOVAL) study in adults with type 1 diabetes and more than three cardiovascular risk factors followed progression of common carotid intima-media thickness (cIMT) as a surrogate measure of cardiovascular risk for three years. Progression of mean cIMT was not significantly reduced with use of metformin but maximal cIMT, a tertiary endpoint, was significantly reduced.39 A subgroup analysis by smoking status found that progression of mean cIMT (per year) was reduced by use of metformin versus placebo in never-smokers.41
Overall, evidence does not support use of metformin for cardiovascular risk reduction; however, it is relatively safe and may be useful for insulin dose reduction on a case-by-case basis.
SGLT-2 inhibitors
Although originally developed for their glycaemic effects in people with type 2 diabetes, SGLT-2 inhibitors are now approved by the TGA and listed on the PBS for both heart failure and chronic kidney disease, independent of diabetes status. Trials in people with type 1 diabetes have shown a glycaemic benefit with reduced HbA1c, increased time in range, reduced glycaemic variability, weight loss and decreased blood pressure with use of SGLT-2 inhibitors, all of which theoretically translate into reduced cardiovascular risk.42 However, these trials also demonstrated an increased risk of diabetic ketoacidosis. Trials of SGLT-2 inhibitors at lower than standard therapeutic dose did not show an increased risk of ketoacidosis or hypoglycaemia, although did show benefit for glycaemia and weight.43 It is unclear whether cardioprotective benefit is maintained at this dose and it should also be noted that people with type 1 diabetes were excluded from trials showing cardiovascular benefit.
If SGLT-2 inhibitors are used in people with type 1 diabetes, careful attention to individual risk of ketoacidosis, education regarding symptoms and potential precipitants, and availability of ketone strips for testing are all crucial and likely require input from an endocrinologist.
GLP-1 and GLP-1/GIP receptor agonists
Large clinical trials in type 2 diabetes have demonstrated a reduction in major adverse cardiovascular events with incretin-based medications, and more recently in obese individuals without diabetes.44 Liraglutide, semaglutide and tirzepatide have all been shown to lead to weight loss and, in addition to effects on the gut, are thought to cause direct suppression of appetite.45
The incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) are secreted by the neuroendocrine cells in the intestinal epithelium, and promote insulin release in response to food intake, known as the ‘incretin effect’. The effects of GLP-1 or GIP agonism differ in type 1 diabetes because of impaired or absent insulin secretion. However, GLP-1 and GIP modulate glucagon as well as insulin release. Glucagon dysregulation in type 1 diabetes results in inappropriate postprandial glucose release (which raises blood glucose levels) and an inadequate response to hypoglycaemia. GLP-1 has been shown to reduce glucagon levels and increase insulin sensitivity in those with type 1 diabetes, may reduce postprandial hyperglycaemia and reduce insulin dose, in addition to any weight loss or direct cardiovascular effect.46,47
At this stage, use of incretin-based medications, such as semaglutide and tirzepatide, remain off label for use in type 1 diabetes; however, real-world use has shown promise and prospective clinical trials are needed and planned.48,49
Conclusion
Cardiovascular disease is the greatest contributor to the gap in mortality between people with type 1 diabetes and the general population. This risk can be reduced by optimising glycaemia and addressing traditional risk factors, such as hypertension, dyslipidaemia, smoking and excess weight. Much of the evidence for intervention to reduce cardiovascular risk in type 1 diabetes is extrapolated from studies performed in the general population or in people with type 2 diabetes. It is important to recognise that usual assessment tools have limitations in assessing risk in type 1 diabetes, and a high degree of vigilance is needed to prevent cardiovascular events in this high-risk group. MT
COMPETING INTERESTS: Dr Snaith and Dr Frampton are coinvestigators on the RESET1 study and Novo Nordisk A/S have provided study medication to support this trial.
ACKNOWLEDGEMENTS: Dr Frampton is supported by the University Postgraduate Award (University of New South Wales). Dr Snaith is supported by JDRF Australia (Grant# 3-SRA-2023-1296-M-N), the recipient of the Commonwealth of Australia grant for Accelerated Research under the Medical Research Future Fund.
References
1. Rawshani A, Sattar N, Franzén S, et al. Excess mortality and cardiovascular disease in type 1 diabetes in relation to age at onset: a nationwide study of 27,195 young adults with diabetes. Lancet 2018; 392: 477-486.
2. Harjutsalo V, Barlovic DP, Groop PH. Long-term population-based trends in the incidence of cardiovascular disease in individuals with type 1 diabetes from Finland: a retrospective, nationwide, cohort study. Lancet Diabetes Endocrinol 2021; 9: 575-585.
3. Huxley RR, Peters SAE, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2015; 3: 198-206.
4. Lespagnol E, Dauchet L, Pawlak-Chaouch M, et al. Early endothelial dysfunction in type 1 diabetes is accompanied by an impairment of vascular smooth muscle function: a meta-analysis. Front Endocrinol 2020; 11: 203.
5. The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care 2016; 39: 686-693.
6. Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008; 5: 1349-1354.
7. Hoffman RP, Dye AS, Huang H, Bauer JA. Glycemic variability predicts inflammation in adolescents with type 1 diabetes. J Pediatr Endocrinol Metab 2016; 29: 1129-1133.
8. Jamiołkowska M, Jamiołkowska I, Łuczyński W, et al. Impact of real-time continuous glucose monitoring use on glucose variability and endothelial function in adolescents with type 1 diabetes: new technology—new possibility to decrease cardiovascular risk? J Diabetes Res 2016; 2016: 4385312.
9. Snell-Bergeon JK, Roman R, Rodbard D, et al. Glycaemic variability is associated with coronary artery calcium in men with Type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes study. Diabet Med 2010; 27: 1436-1442.
10. Mizokami-Stout K, Bailey R, Ang L, et al. Symptomatic diabetic autonomic neuropathy in type 1 diabetes (T1D): Findings from the T1D exchange. J Diabetes Complications 2022; 36: 108148.
11. Poon MS, Chan AKF, Cusumano JM, Craig ME, Donaghue KC. Complications during adolescence predict mortality in young adults with childhood onset type 1 diabetes. Pediatric Diabetes 2024; 2024: e8194756.
12. National Diabetes Services Scheme (NDSS). Access CGM and Flash GM. The NDSS is administered by Diabetes Australia. Available online at: https://www.ndss.com.au/about-the-ndss/cgm-access/ (accessed June 2024).
13. Cheung NW, Conn JJ, d’Emden MC, et al. Position statement of the Australian Diabetes Society: individualisation of glycated haemoglobin targets for adults with diabetes mellitus. Med J Aust 2009; 191: 339-344.
14. Toschi E, O’Neal D, Munshi M, Jenkins A. Glucose targets using continuous glucose monitoring metrics in older adults with diabetes: are we there yet? J Diabetes Sci Technol 2024; 19322968241247568.
15. Steineck I, Cederholm J, Eliasson B, et al. Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18,168 people with type 1 diabetes: observational study. BMJ 2015; 350: h3234.
16. Gregory JM, Cherrington AD, Moore DJ. The peripheral peril: injected insulin induces insulin insensitivity in type 1 diabetes. Diabetes 2020; 69: 837-847.
17. Kietsiriroje N, Pearson S, Campbell M, Ariëns RAS, Ajjan RA. Double diabetes: a distinct high-risk group? Diabetes Obes Metab 2019; 21: 2609-2618.
18. Vergès B. Lipid disorders in type 1 diabetes. Diabetes Metab 2009; 35: 353-360.
19. Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361: 2005-2016.
20. American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of care in diabetes—2024. Diabetes Care 2023; 47(Suppl 1): S179-S218.
21. National Institute for Health and Care Excellence (NICE). Type 1 diabetes in adults: diagnosis and management. NICE; 2015. Available online at: https://www.nice.org.uk/guidance/NG17/chapter/recommendations#control-of-cardiovascular-risk (accessed June 2024).
22. National Institute for Health and Care Excellence (NICE). Cardiovascular disease: risk assessment and reduction, including lipid modification. NICE; 2023. Available online at: https://www.nice.org.uk/guidance/ng238/chapter/recommendations#people-with-type-1-diabetes (accessed June 2024).
23. Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes: Developed by the task force on the management of cardiovascular disease in patients with diabetes of the European Society of Cardiology (ESC). European Heart J 2023; 44: 4043-4140.
24. Lithovius R, Groop PH, FinnDiane Study Group. The many faces of hypertension in individuals with type 1 diabetes. diabetes Res Clin Pract 2023; 197: 110564.
25. Chaturvedi N, Bandinelli S, Mangili R, Penno G, Rottiers RE, Fuller JH. Microalbuminuria in type 1 diabetes: Rates, risk factors and glycemic threshold. Kidney International 2001; 60: 219-227.
26. Conway B, Miller RG, Costacou T, et al. Temporal patterns in overweight and obesity in type 1 diabetes. Diabetic Medicine 2010; 27: 398-404.
27. Purnell JQ, Braffett BH, Zinman B, et al. Impact of excessive weight gain on cardiovascular outcomes in type 1 diabetes: Results From the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes Care 2017; 40: 1756-1762.
28. Watt A, Ng AH, Sandison A, Fourlanos S, Bramley A. Prevalence of disordered eating in adults with type 1 diabetes in an Australian metropolitan hospital. Health Soc Care Community 2022; 30: e974-e980.
29. Bryden KS, Neil A, Mayou RA, Peveler RC, Fairburn CG, Dunger DB. Eating habits, body weight, and insulin misuse. A longitudinal study of teenagers and young adults with type 1 diabetes. Diabetes Care 1999; 22: 1956-1960.
30. Walmsley R, Sumithran P. Current and emerging medications for the management of obesity in adults. Med J Aust 2023; Online first. Available online at: https://www.mja.com.au/journal/2023/218/6/current-and-emerging-medications-management-obesity-adults (accessed June 2024).
31. Azar S, Zantout M. Diabetic ketoacidosis associated with orlistat treatment. Diabetes Care 2001; 24: 602.
32. Overview | CVD Risk Guideline. Available online at: www.cvdcheck.org.au/overview (accessed June 2024).
33. Vistisen D, Andersen GS, Hansen CS, et al. Prediction of first cardiovascular disease event in type 1 diabetes mellitus: the Steno Type 1 Risk Engine. Circulation 2016; 133: 1058-1066.
34. McGurnaghan SJ, McKeigue PM, Read SH, et al. Development and validation of a cardiovascular risk prediction model in type 1 diabetes. Diabetologia 2021; 64: 2001-2011.
35. CVD Risk Prediction T1D. Available online at: https://diabepi.shinyapps.io/cvdrisk/ (accessed June 2024).
36. Budoff M, Backlund JYC, Bluemke DA, et al. The association of coronary artery calcification with subsequent incidence of cardiovascular disease in type 1 diabetes: The DCCT/EDIC Trials. JACC Cardiovasc Imaging 2019; 12(7 Pt 2): 1341-1349.
37. Guo J, Erqou SA, Miller RG, Edmundowicz D, Orchard TJ, Costacou T. The role of coronary artery calcification testing in incident coronary artery disease risk prediction in type 1 diabetes. Diabetologia 2019; 62: 259-268.
38. Forner P, Snaith J, Greenfield JR. Prescribing patterns of adjunctive therapy for the treatment of type 1 diabetes mellitus among Australian endocrinologists. Intern Med J 2023. doi: 10.1111/imj.16312. Online ahead of print.
39. Petrie JR, Chaturvedi N, Ford I, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2017; 5: 597-609.
40. Vella S, Buetow L, Royle P, Livingstone S, Colhoun HM, Petrie JR. The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia 2010; 53: 809-820.
41. Timmons JG, Greenlaw N, Boyle JG, et al. Metformin and carotid intima-media thickness in never-smokers with type 1 diabetes: The REMOVAL trial. Diabetes Obes Metab 2021; 23: 1371-1378.
42. Taylor SI, Blau JE, Rother KI, Beitelshees AL. SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits versus risks. Lancet Diabetes Endocrinol 2019; 7: 949-958.
43. Rosenstock J, Marquard J, Laffel LM, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: The EASE Trials. Diabetes Care 2018; 41: 2560-2569.
44. Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023; 389: 2221-2232.
45. Brierley DI, de Lartigue G. Reappraising the role of the vagus nerve in GLP-1-mediated regulation of eating. Br J Pharmacol 2022; 179: 584-599.
46. Creutzfeldt WOC, Kleine N, Willms B, Ørskov C, Holst JJ, Nauck MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide i(7–36) amide in type I diabetic patients. Diabetes Care 1996; 19: 580-586.
47. Gutniak M, Ørkov C, Holst JJ, Ahrén B, Efendić S. Antidiabetogenic effect of glucagon-like peptide-1 (7–36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 1992; 326: 1316-1322.
48. Australian New Zealand Clinical Trial Registry - Trial Review. Efficacy of REducing cardiometabolic risk with SEmaglutide in Type 1 diabetes. Available online at: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=386649&isReview=true (accessed June 2024).
49. Australian New Zealand Clinical Trial Registry - Trial Review. Tirzepatide in Type 1 Diabetes: Cardiometabolic Effects. Available online at: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=386985&isReview=true (accessed June 2024).