Genetic risk screening for type 1 diabetes. A model for early detection in children?

Type 1 diabetes is one of the most common childhood chronic conditions. Early diagnosis is challenging as the condition is asymptomatic in the early stages, and when symptoms do appear, they are initially vague. However, a delayed diagnosis increases the risk of developing life-threatening diabetic ketoacidosis. Genetic screening offers an opportunity to identify children at risk and provide ongoing monitoring to improve immediate and long-term health outcomes.
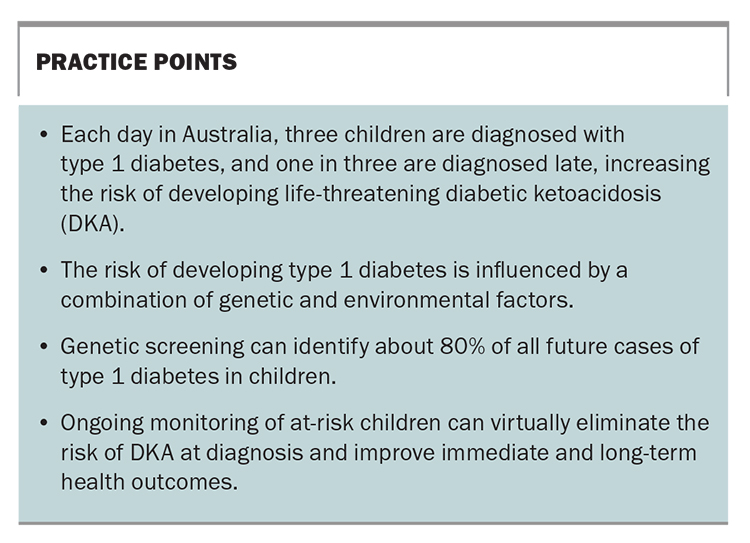
In Australia, three children are diagnosed with type 1 diabetes every day, making it one of the most common childhood chronic conditions. It is an autoimmune disorder characterised by the destruction of insulin-producing beta cells in the pancreas and a lifelong reliance on insulin therapy for survival. This article provides an overview of genetic screening for type 1 diabetes in children.
Why screen for type 1 diabetes?
Establishing a clinical diagnosis of type 1 diabetes can be challenging, given the initial symptoms are vague and easily missed or attributed to other pathologies. These common symptoms are known as the four T’s: thirsty (increased thirst), toilet (frequent urination), thinner (rapid or unexplained weight loss) and tired (fatigued or weak). Almost all children present with at least two of the four T’s, but this clinical practice challenge for primary healthcare is evident, considering about one-quarter of children are brought in for multiple healthcare visits in the lead up to diagnosis.1 As a result, children are often diagnosed late, with one in three not being diagnosed until they present with life-threatening diabetic ketoacidosis (DKA) and require emergency hospitalisation or intensive care. This delay in diagnosis can have lifelong implications, including the development of cognitive impairment, an increased future DKA risk and lower long-term glycaemic control, and thus an increased risk of serious diabetes-related complications from early adulthood.2-5 DKA education and awareness campaigns have had some short-term success but lack sustained outcomes.6,7
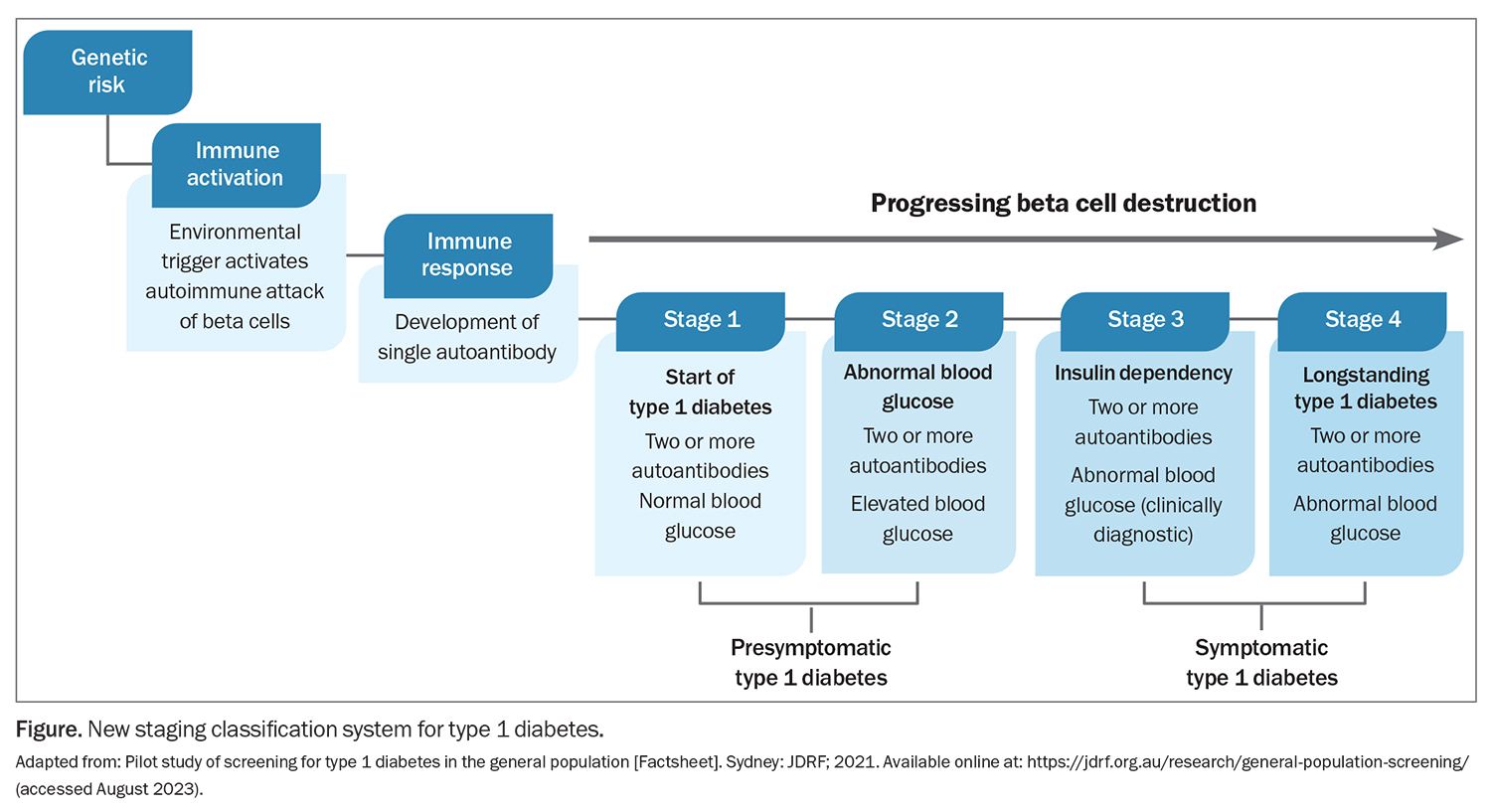
With the aid of screening and ongoing monitoring, healthcare professionals and families can now be informed early if a child carries a genetic risk factor for type 1 diabetes and if onset of the autoimmune condition has been triggered. In turn, screening has been shown to virtually eliminate the risk of DKA in international clinical trials, with DKA rates consistently around 5%.8 Traditionally, type 1 diabetes was thought to exhibit a severe, rapid onset, giving healthcare professionals and families little to no warning or chance to establish an early diagnosis. However, a new staging system identifies two early presymptomatic stages (stages 1 and 2) that mark the onset of type 1 diabetes, rather than the traditional late-stage, symptomatic clinical diagnosis (stages 3 and 4; Figure).9 Identifying these presymptomatic stages of type 1 diabetes allows for screening to identify children at increased risk of the condition and, in turn, early detection and monitoring.
Furthermore, the first preventative therapy, teplizumab, a monoclonal antibody, has been approved by the US Food and Drug Administration for stage 2 type 1 diabetes to delay the onset of stage 3 insulin-requiring type 1 diabetes.10 Although teplizumab is not yet available in Australia, efforts are underway to bring the therapy to Australia and additional therapies are being investigated in Australian and international clinical trials. Therefore, screening provides the opportunity to use preventative therapies in children as they become available in Australia, in addition to increasing the speed and efficiency of clinical trials by widening the pool of potential participants.
Genetic risk of type 1 diabetes
The risk of developing type 1 diabetes is influenced by a combination of genetic and environmental factors. First-degree relatives of individuals with type 1 diabetes have a 15 times greater risk than the general population (about 5% vs 0.3%). Screening for type 1 diabetes in first-degree relatives is well established, based on the Environmental Determinants of Diabetes Islet Autoimmunity (https://www.endia.org.au/) and Type1Screen (https://type1screen.org/) studies in Australia. However, 90% of individuals do not present with a family history of the disease at the time of diagnosis.9 Thus, the only way to capture the vast majority of children who will develop type 1 diabetes is to offer routine, population-wide screening.
In those individuals who proceed to develop type 1 diabetes, the most significant genetic predisposition is the presence of variations in the human leucocyte antigen (HLA) gene, which accounts for around 50% of the genetic risk. However, type 1 diabetes is polygenic in nature, with more than 70 genetic variants involved. This collective genetic risk of type 1 diabetes can be captured in a polygenic risk score, a weighted sum of the risk associated with each variant in an individual. A high polygenic risk score indicates an increased genetic risk of type 1 diabetes in childhood. The area under the receiver-operating characteristic curve for type 1 diabetes is 0.96, which is one of the most accurate polygenic risk scores for any condition.11 However, genes alone do not determine a patient’s outcome in type 1 diabetes, and polygenic risk scores should be considered to reflect a risk factor rather than as a definitive diagnostic tool.
Genetic risk-stratified screening for type 1 diabetes
Polygenic risk scores can be used at the population level to identify children at increased risk of developing type 1 diabetes, as those who would benefit the most from ongoing antibody monitoring to detect presymptomatic disease. In practice, 10% of children would be identified as being at risk of type 1 diabetes, capturing around 80% of future cases of type 1 diabetes.11 Given the autoimmune condition is triggered by an environmental factor, not all children with a genetic predisposition will proceed to develop the condition. Of the 10% of children at risk of the condition, one in 40 children will develop type 1 diabetes by 16 years of age.11 This is a substantially higher risk than the general population background risk (2.4% vs 0.3%).9 At-risk children should be monitored for the development of type 1 diabetes autoantibodies, indicating the initiation of the autoimmune disease.
Genetic screening in practice: the Australian Type 1 Diabetes National Screening Pilot
The Type 1 Diabetes National Screening Pilot aims to compare the feasibility, acceptability and cost of three screening models for type 1 diabetes in the Australian paediatric general population. Two of these screening models are genetic risk-stratified screening models, whereas the third skips this risk stratification, instead offering islet autoantibody-only screening to all children aged 2, 6 and 10 years. In the first model, newborns are offered genetic screening for type 1 diabetes via a heel prick blood sample collected at the same time as in the existing National Newborn Screening Program. The second model is aligned with the National Immunisation Program Schedule. Infants aged 6 to 12 months are offered genetic screening via a saliva swab either at home or at their local GP clinic or pharmacy. In both screening models, it is anticipated that one in 10 children will receive an ‘increased chance’ risk result and be offered islet autoantibody monitoring, with referral to a paediatric endocrinologist if type 1 diabetes (stages 1 to 3) is identified. These screening models are designed to be sustainable, scalable, accessible and cost-effective as a national screening program for the benefit of all Australian children in the future.
As part of the Pilot, data on screening uptake rates and parental anxiety, as well as the beliefs, attitudes and experiences with screening of both family members and healthcare professionals are collected to help inform if, and how, screening should be offered in the future. Screening is due to close at the end of 2023, and the results will be available by the end of 2024, following the first follow-up recall for
at-risk children. For more information about this Pilot, see www.KidsDiabetesScreen.com.au or contact the Screening Helpline on 1300 505 909.
Considerations for population genetic screening
Although genetic screening is promising, there are considerations to be made before its implementation in clinical practice. First, the score is developed from a largely Caucasian population dataset and has not been validated in the multicultural Australian population. This is not a unique problem for type 1 diabetes testing and, reassuringly, the genetic risk score has been shown to perform well across genetic ancestry groups relevant to Australia, including East Asian, Southeast Asian and Middle Eastern populations.12-14 Nonetheless, further validation work is required, and this should be taken into account when counselling families about their child’s individualised genetic risk.
Second, knowledge of a child’s risk of type 1 diabetes may cause anxiety for the child and family members. Emphasising an accurate perception of their own risk and their child’s risk, providing access to information and support and establishing actionable follow-up and management pathways are important to manage and alleviate anxiety. International data indicate that although families experience increased anxiety at the time of antibody detection (stages 1 and 2), anxiety at the time of stage 3 diagnosis and insulin initiation is reduced, suggesting a ‘softer’ introduction to type 1 diabetes may be beneficial.15,16
Lastly, GPs are ideally situated to offer infants screening for type 1 diabetes, given their role and experience in implementing screening programs and risk counselling for other conditions. Similarly, pharmacies are experienced with type 1 diabetes through the dispensing of insulin and diabetes-related consumables through the National Diabetes Services Scheme. Genetic counselling services may also be helpful in assisting families to understand their risk and adapt to this information. A genetic counsellor is available to families and healthcare professionals through the Type 1 Diabetes National Screening Pilot via the dedicated screening helpline. However, the genetic counselling workforce is not currently equipped to manage a nationwide population screening program. Therefore, upskilling of primary healthcare professionals is essential and access to genetic counsellors should be considered in the mainstreaming of genomic healthcare.
Conclusion
With appropriate training and support, the primary healthcare system is well positioned to provide population-wide genetic risk-stratified screening for type 1 diabetes. The goal would be to identify children at increased risk of developing the condition; connect families to ongoing monitoring, education and support; and, eventually, be able to offer access to preventative therapies. As a future national screening program, early detection of type 1 diabetes could virtually eliminate the one-in-three risk of life-threatening DKA at diagnosis and, thus, the associated trauma and short- and long-term health consequences. Practice points for GPs are provided in the Box. MT
COMPETING INTERESTS: Ms Wadling has received support from the University of Sydney. Dr Bell has received support from the University of Sydney; has received research grants from JDRF Australia – Population Screening Fellowship (2020 to 2023) and JDRF Australia and International – Australian Type 1 Diabetes National Screening Pilot (2021 to 2024); and was a Scientific Reviewer for the American Diabetes Association (2022 and 2023).
References
1. Claessen FM, Donaghue K, Craig M. Consistently high incidence of diabetic ketoacidosis in children with newly diagnosed type 1 diabetes. Med J Aust 2012; 197: 216.
2. Ghetti S, Kuppermann N, Rewers A, et al. Cognitive function following diabetic ketoacidosis in children with new-onset or previously diagnosed type 1 diabetes. Diabetes Care 2020; 43: 2768-2775.
3. Ampt A, van Gemert T, Craig ME, Donaghue KC, Lain SB, Nassar N. Using population data to understand the epidemiology and risk factors for diabetic ketoacidosis in Australian children with type 1 diabetes. Pediatr Diabetes 2019; 20: 901-908.
4. Paes VM, Barrett JK, Taylor-Robinson DC, et al. Effect of early glycemic control on HbA1c tracking and development of vascular complications after 5 years of childhood onset type 1 diabetes: systematic review and meta-analysis. Pediatr Diabetes 2019; 20: 494-509.
5. Samuelsson J, Samuelsson U, Hanberger L, Bladh M, Åkesson K. Poor metabolic control in childhood strongly correlates to diabetes-related premature death in persons <30 years of age – a population-based cohort study. Pediatr Diabetes 2020; 21: 479-485.
6. Rabbone I, Maltoni G, Tinti D, et al. Diabetic ketoacidosis at the onset of disease during a national awareness campaign: a 2-year observational study in children aged 0-18 years. Arch Dis Child 2020; 105: 363-366.
7. Lansdown AJ, Barton J, Warner J, et al. Prevalence of ketoacidosis at diagnosis of childhood onset type 1 diabetes in Wales from 1991 to 2009 and effect of a publicity campaign. Diabet Med 2012; 29: 1506-1509.
8. Sims EK, Besser REJ, Dayan C, et al. Screening for type 1 diabetes in the general population: a status report and perspective. Diabetes 2022; 71: 610-623.
9. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015; 38: 1964-1974.
10. Herold KC, Bundy BN, Long SA, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019; 381: 603-613.
11. Sharp SA, Rich SS, Wood AR, et al. Development and standardization of an improved type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care 2019; 42: 200-207.
12. Wan R, Sharp SA, Tam CHT, et al. 108-OR: Application of a European genetic risk score for type 1 diabetes in a Han-Chinese Population. Diabetes 2020; 69: 108-OR.
13. Harrison JW, Tallapragada DSP, Baptist A, et al. Type 1 diabetes genetic risk score is discriminative of diabetes in non-Europeans: evidence from a study in India. Sci Rep 2020; 10: 9450.
14. Yaghootkar H, Abbasi F, Ghaemi N, et al. Type 1 diabetes genetic risk score discriminates between monogenic and Type 1 diabetes in children diagnosed at the age of <5 years in the Iranian population. Diabet Med 2019; 36: 1694-1702.
15. Johnson SB. Psychological impact of screening and prediction in type 1 diabetes. Curr Diab Rep 2011; 11: 454-459.
16. Ziegler AG, Kick K, Bonifacio E, et al. Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA 2020; 323: 339-351.