Continuous glucose monitoring technology – understanding the benefits

Optimising glycaemic control is crucial to preventing diabetes-associated complications. Continuous glucose monitoring (CGM) technologies offer advantages over the standard method of self- monitoring blood glucose levels. It is important to understand the features of each CGM system to select the most appropriate device for each individual. CGM generates large quantities of data that require careful interpretation to effectively inform therapeutic decision making.
- Continuous glucose monitoring (CGM) has become state of the art in the management of type 1 diabetes.
- CGM is subsidised in Australia through the National Diabetes Services Scheme for all people with type 1 diabetes.
- There is strong evidence for the benefits of CGM in type 1 and insulin-treated type 2 diabetes, including reductions in HbA1c levels, increased time in range and reduced hypoglycaemia.
- CGM can detect clinically significant events that may be missed using traditional monitoring methods, such as asymptomatic hypoglycaemia, nocturnal hypoglycaemia, and unrecognised postprandial hyperglycaemia.
- Standardisation of glucose reports into an ambulatory glucose profile assists clinicians in analysing key aspects of glycaemic targets.
- Most CGM systems are now compatible with insulin pumps, allowing for integration into automated insulin delivery or closed-loop devices.
Importance of improved glycaemic control
Optimising glycaemic control remains the cornerstone to preventing microvascular and macrovascular complications of diabetes. Landmark studies, such as the Diabetes Control and Complications Trial (DCCT)1 and the UK Prospective Diabetes Study (UKPDS),2 provide compelling evidence that intensive glucose-lowering therapy reduces complications, including retinopathy, nephropathy, neuropathy and cardiovascular disease. Implementing intensive glucose-lowering regimens to achieve tight glycaemic targets is a significant challenge because of the practical burden of frequent capillary glucose monitoring and the increased risk of hypoglycaemia with more intensive treatment.
For the past five decades, measuring glycated haemoglobin (HbA1c) levels and self-monitoring blood glucose (SMBG) have been the standard methods of evaluating glycaemic status and adjusting therapy. HbA1c has been recognised as the most important surrogate index of glycaemia, given its correlation with diabetes-associated complications.3 However, the use of these tools has recognised limitations. HbA1c levels can be used to estimate the average glucose level over the preceding three months but do not reflect glucose variability or capture information on glycaemic excursions. SMBG provides a snapshot of the blood glucose level at a single point in time without indicating the directionality of glucose changes or the variations in glucose since the last finger prick. The utility of HbA1c is limited in settings of altered red blood cell turnover, haemoglobinopathies, pregnancy, advanced renal disease, nutritional deficiencies, recent blood loss or transfusions and certain medications. Relying solely on measuring HbA1c and infrequent SMBG may fail to detect events of significance, particularly asymptomatic hypoglycaemia, nocturnal hypoglycaemia and unrecognised postprandial hyperglycaemia. Continuous glucose monitoring (CGM) has emerged over the past 15 years as a valuable adjunct to HbA1c testing and SMBG. CGM devices use a subcutaneous sensor to measure interstitial glucose levels, providing detailed insights into 24-hour glucose profiles and allowing for the evaluation of clinically important glycaemic metrics, such as time in range (TIR), the degree of glucose variability and the amplitude of glucose excursions.
Current CGM systems
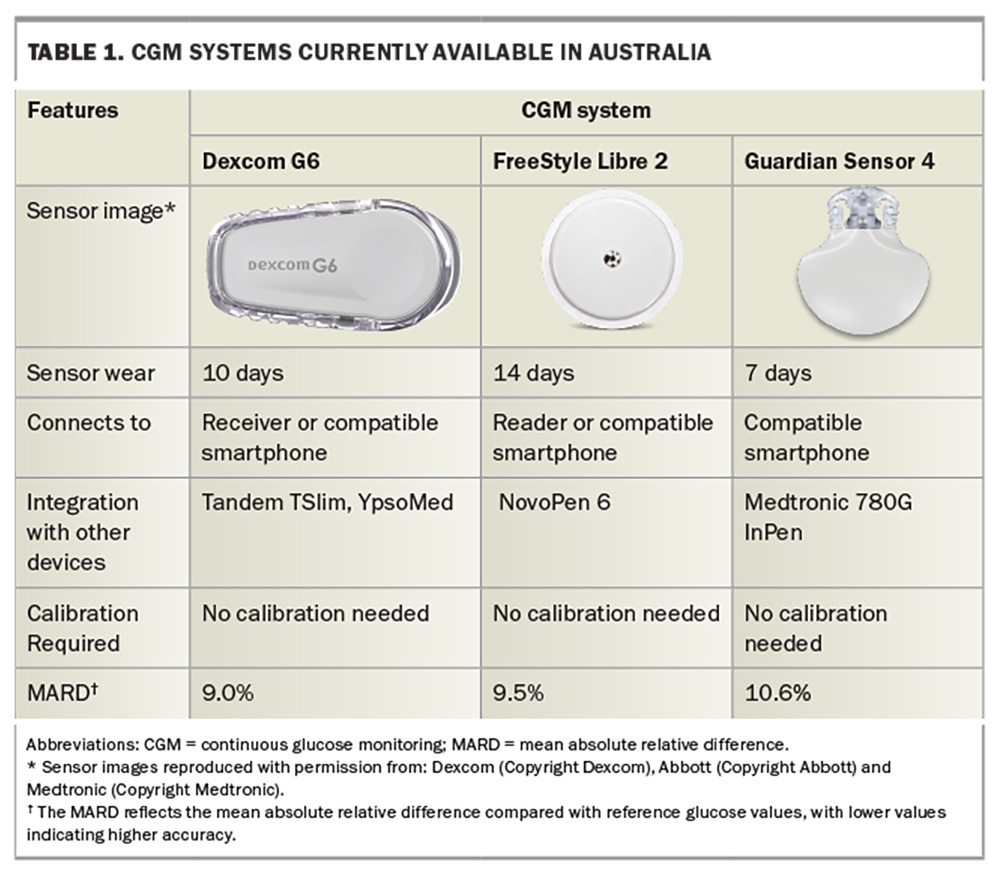
CGM systems comprise a transcutaneous sensor inserted into the subcutaneous tissue, usually on the abdomen or arm. The sensor contains an enzyme-based biosensor that measures interstitial fluid glucose concentrations at one- to five-minute intervals via an amperometric method. A mathematical algorithm converts the measured interstitial levels to calibrated blood glucose values. Data can be viewed in real-time on standalone readers, integrated into insulin pumps or transmitted to a mobile phone. Users can set alarms to alert when glucose levels rise or fall below specific thresholds. Certain CGM systems feature hypo-prediction capabilities, providing users with alerts for predicted hypoglycaemia. Historically, CGM devices required regular calibration with fingerprick testing for accuracy; however, most current devices are factory-calibrated and do not require user calibration.
Mean absolute relative difference (MARD) is a metric used to assess the accuracy of CGM systems. It measures the average difference between CGM readings and the reference values obtained from a laboratory device across a range of glucose levels. A lower MARD indicates higher accuracy. Typically, CGM systems with a MARD of about 10% are regarded as having acceptable performance.4
Clinical benefits of CGM
The large quantity of glucose readings collected by CGM systems provides a comprehensive profile of glycaemic status over the entire day, including the TIR (usually 3.9 to 10.0 mmol/L), time spent in hypo- and hyperglycaemia and measures of glucose variability. Numerous studies have demonstrated the clinical advantages of CGM compared with SMBG. CGM is associated with reductions in HbA1c levels of about 0.3 to 0.5% in individuals with type 1 diabetes and 0.2 to 0.3% in those with insulin-treated type 2 diabetes.5 It has been found to increase TIR while reducing hypoglycaemia by 23 to 43%.6 The benefits of CGM are consistent across diabetes populations, including in children, adolescents, adults, pregnant women and older people.7-11 In addition to improving glycaemic control, CGM is associated with reduced diabetes-associated stress, increased treatment satisfaction and better quality of life.12 The likelihood of achieving an HbA1c level below 7% is 2.5 times greater after the introduction of CGM, and the benefit is maintained in the long term.13 Diabetic ketoacidosis was also reduced by 49% in people using a CGM device.13 Younger people with diabetes, who are less likely to achieve treatment targets and may be more inclined to adopt the technology, are likely to see the greatest benefits from CGM.14 Some CGM devices integrate with smart insulin pens, allowing doses and administration times to be automatically displayed on 14-day glucose patterns without requiring manual entry by the patient. CGM devices currently available in Australia are presented in Table 1.
CGM data interpretation
CGM provides an abundance of information on glycaemia, necessitating structured approaches to data reporting and analysis, particularly for clinicians who may access many weeks or months of data at a consultation. The ambulatory glucose profile (AGP) format was developed to facilitate a consistent approach to visualising CGM data across different devices and healthcare settings.15 The Australasian Diabetes Society (ADS) released A Practical Guide of the Glucose Pattern Insights (GPI) Report in Primary Care to assist in interpreting these reports.16 This guide provides a standardised framework focused on the key CGM metrics and pattern recognition to facilitate decision making.
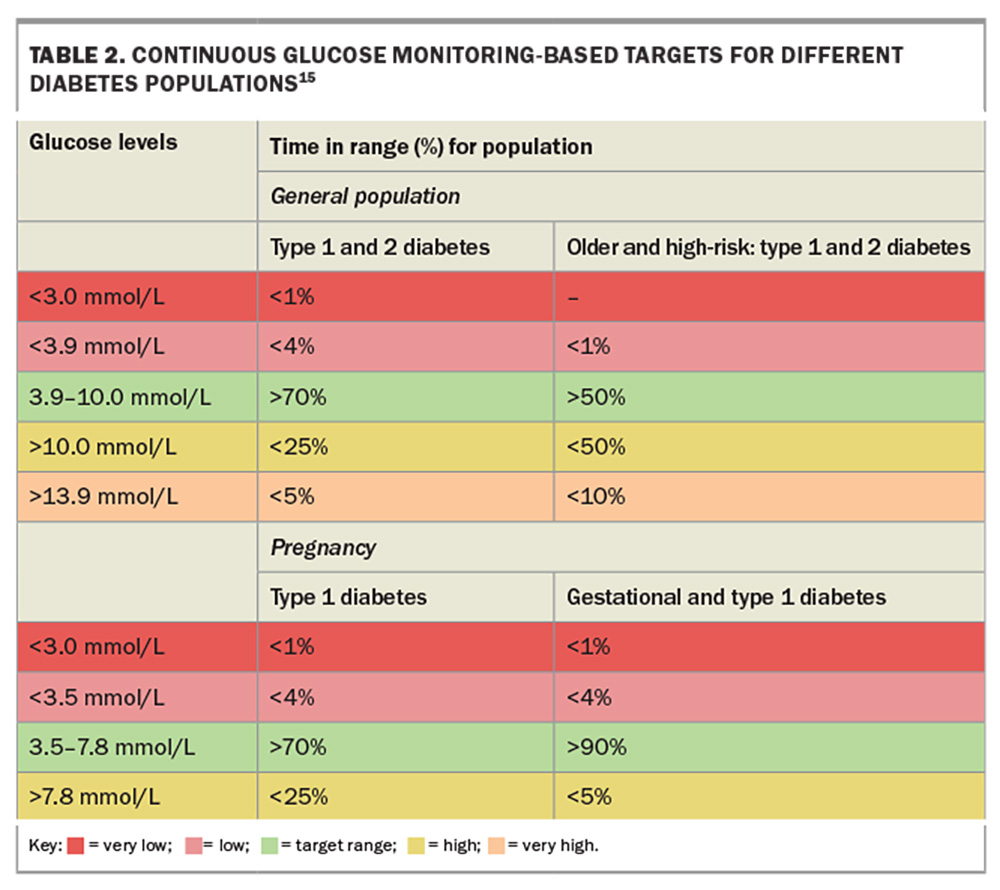
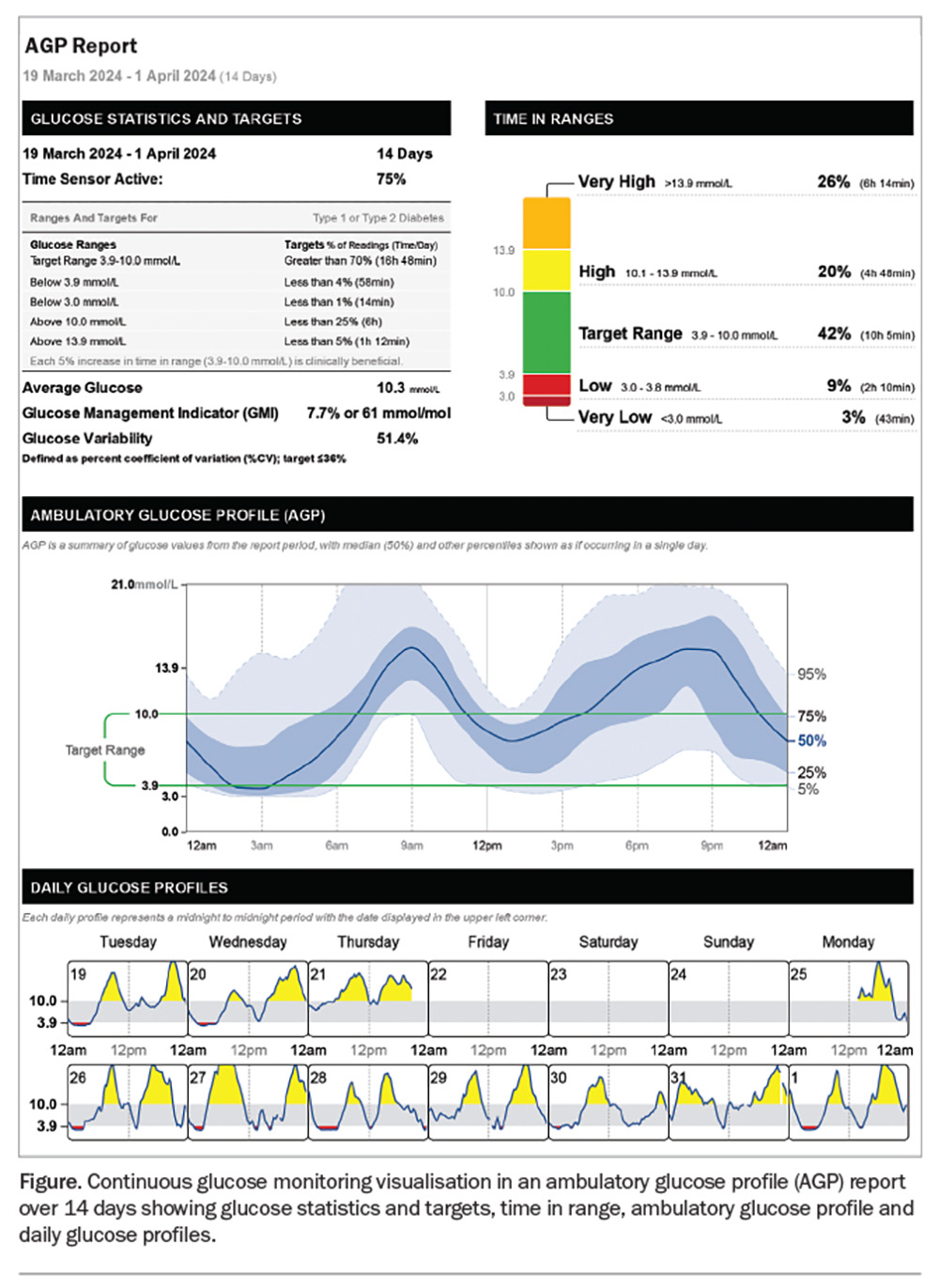
The AGP report consolidates 14 days of CGM data into a single-page display with three sections: glucose pattern insights, considerations for the clinician and 14-day glucose patterns (Figure). CGM should capture at least 70% of the 14-day period for the report to be considered valid and representative. The glucose management indicator (GMI) is a calculated HbA1c- equivalent value based on the mean glucose level over 14 days. A GMI below 7% (53 mmol/mol) is an appropriate goal for most patients. Recommended target TIRs are 70% (equivalent to an HbA1c level below 7%) for most adults with type 1 or 2 diabetes and 50% or higher for older people or individuals with impaired hypoglycaemia awareness to minimise the risk of hypoglycaemia (Table 2).15 Time below range (TBR) indicates the percentage of CGM readings below 3.9 mmol/L, reflecting the exposure to clinically significant hypoglycaemia. Minimising TBR to less than 4% is the highest priority to reduce the risk of severe hypoglycaemia. Time above range (TAR) denotes hyperglycaemic values higher than 10.0 mmol/L, with consensus TAR targets of below 25% recommended for most individuals.
The second section of an AGP contains the GPI report’s determination of the ‘Most Important Pattern’, based on a hierarchical algorithm that weighs the relative degrees of hypoglycaemia, hyperglycaemia and glucose variability. The three main patterns are persistent low glucose, mixed highs with some lows and persistent high glucose.
The third section contains the modal day graph, depicting the median and inter-decile glucose ranges for each hourly period. This graph can rapidly identify glucose patterns related to meals, activity, overnight periods or other daily routines.
The Figure presents an example of a report that shows a mixed pattern of hypoglycaemia and hyperglycaemia. In this report, GMI is 7.7%. The TBR is 9%, higher than the recommended range, and the TIR is 42%, lower than the target. The AGP identifies periods of hypoglycaemia in the early hours of the morning, as well as hyperglycaemia in the morning and evening, which can be observed in more detail in the daily patterns. This information can help guide changes in basal insulin reductions, identify different patterns of behaviour on different days and adjust prandial insulin. It is useful to look at individual days as well as the AGP report and identify any dosing or dietary issues that may contribute to hyper or hypoglycaemia. The initial course of action would be to address the issue of hypoglycaemia and its underlying causes. This entails examining whether the hypoglycaemia arises from basal insulin usage or from the administration of excessive correction doses at bedtime in response to elevated glucose levels. After this, the cause of hypo- and hyperglycaemia could be further established by discussing the timing of insulin and food intake. The use of CGM technology has precipitated a paradigm shift in glycaemic management, prioritising hypoglycaemia mitigation over hyperglycaemia reduction. This approach, contrary to the hyperglycaemia-centric focus fostered by HbA1c monitoring, is predicated on the understanding that addressing hypoglycaemia can paradoxically improve overall glycaemic control, by attenuating counter regulatory hormone responses, reducing glycaemic variability and optimising TIR. The AGP report reveals how seemingly acceptable HbA1c values can mask significant blood sugar fluctuations, showing the limitations of relying solely on HbA1cand the valuable insights provided by CGM data.
Intermittent CGM use
Ongoing CGM use may not be feasible or necessary for all patients. Intermittent CGM use for limited periods can assist in identifying patterns of glycaemic dysregulation, hypoglycaemic vulnerability, postprandial excursions, or the impact of therapeutic or behavioural adjustments in ways that isolated SMBG readings cannot. It is particularly helpful in patients with type 2 diabetes who are treated with insulin and not meeting glycaemic targets. Intermittent use of CGM has shown benefits in improving diet and exercise habits, thereby reducing body weight and prandial glucose and HbA1c levels.17 An HbA1c decrease of 0.8% has been observed with intermittent CGM use, persisting with long-term follow up.18
Clinical scenarios that may warrant consideration of intermittent CGM use for insulin-treated diabetes include:
- for temporary glucose pattern evaluation to identify factors contributing to high or low glucose levels
- to assess suspected nocturnal hypoglycaemia
- to elucidate the glycaemic impacts of implemented changes and identify persistent glycaemic patterns if initiating lifestyle or medication changes
- to assist management in those with existing complications such as gastroparesis
- to monitor fluctuating glycaemia in the setting of acute illnesses, bariatric surgery, chemotherapy, dialysis or steroid therapy
- for temporary glucose assessment, for example, medical assessment for specific activities.
Limitations and barriers of CGM devices
CGM has emerged as a revolutionary monitoring tool for optimising diabetes management; however, various limitations and barriers to widespread adoption persist.
Cost and access constraints
All CGM systems are now accessible and subsidised for patients with type 1 diabetes through the National Diabetes Services Scheme CGM Initiative. Department of Veterans’ Affairs cardholders with type 2 diabetes can access subsidised CGM products prescribed by their GP, diabetes educator or endocrinologist. Referral to a diabetes educator or endocrinologist for CGM device selection may be beneficial to determine the most suitable device for each patient depending on factors such as accuracy, cost, ease of use, phone compatibility, device integrations and size. CGM devices must be funded privately for those not eligible for subsidies. The price of these devices starts from about $50 a week. Some manufacturers offer discounted sensors for trial periods. Despite the government subsidy increasing the affordability of CGM, many eligible people have not yet adopted this life-changing technology. This underutilisation highlights the ongoing need to raise awareness, overcome barriers to adoption and ensure that more people with type 1 diabetes can experience the valuable insights and improved glycaemic management that CGM technology provides.
Accuracy limitations
Although the accuracy of modern CGM systems continues to improve, they remain indirect measurements compared with blood glucose measurements. CGM devices quantify interstitial glucose, which lags five to 20 minutes behind capillary glucose levels.19 Factors including body habitus, sensor location, sensor compression during sleep, exercise, rapid fluid shifts (e.g. during dialysis), certain medications (e.g. paracetamol, vitamin C) and fluctuating interstitial dynamics during periods of rapid blood glucose change can contribute to discrepancies between sensor measurements and plasma levels.
Skin issues, wearability and mental health
CGM devices may cause redness, itching or infection at insertion sites. CGM devices and sensor adhesives can also become unstable, peel off or dislodge during physical activities, sweating or exposure to moisture. Patches, wipes and creams are available to reduce these occurrences. People with impaired dexterity or neurocognition may find it challenging to self-insert CGM sensors, although SMBG may be even more challenging in this population. Many studies have shown improved quality of life with the use of CGM compared with SMBG. However, some individuals may experience increased anxiety and diabetes-associated distress from constantly seeing their CGM tracing. These people may benefit from counselling to understand and respond to their CGM data. Additionally, the physical presence of the CGM sensor may negatively affect body image and self-confidence.
The future landscape of CGM technology
Although contemporary CGM devices have transformed diabetes care, several promising developments on the horizon could further enhance CGM accuracy, convenience and clinical utility.
Automated insulin delivery integration
CGM combined with insulin pump delivery is the fundamental technology behind closed-loop insulin delivery systems, also known as the artificial pancreas. In these systems, CGM provides real-time glucose levels to an algorithm that calculates insulin dose adjustments using a compatible insulin pump, minimising glycaemic excursions. Several hybrid closed-loop systems are in clinical use in Australia, including the Medtronic MiniMed 770G and 780G pumps, Tandem Control-IQ, and CamAPS FX used in conjunction with the YpsoPump.
Implantable CGM sensors
Although not yet available in Australia, implantable CGM sensors may overcome the cost and inconvenience associated with the limited functional lifespan of transcutaneous CGM sensors. The Eversense system is inserted subcutaneously in a minor outpatient procedure and provides data for up to six months before needing replacement.
Advances in existing devices
The Dexcom G7 is currently used overseas but is not yet available in Australia. It is 60% smaller than the G6, has a 10.5-day lifespan and requires less than 30 minutes to warm up, compared with the two hours required for the G6. The G7 integrates the sensor and transmitter into a single piece.
The FreeStyle Libre 3 features a 70% smaller sensor, increased transmission distance of 10 meters and improved MARD. It has customisable alarms, can be worn on the arm or body and may be used with an automated insulin delivery system. It is not clear when it will be available in Australia.
Simplera is half the size of previous Guardian CGM systems and is expected to be available later this year. It has an updated user interface and a simple 10-second insertion process. It integrates with the InPen (a smart insulin pen that records insulin times and doses) and the MiniMed 780G insulin pump/automated insulin delivery system.
Conclusion
CGM has revolutionised the monitoring, evaluation and optimisation of glycaemic control. CGM delivers continuous data on glucose levels, variability and trends. These data empower clinicians and people with diabetes with insights to inform therapeutic decisions to improve glycaemic outcomes while minimising hypoglycaemia risk. It is important to understand when SMBG should be used in preference to CGM and to understand the features of CGM devices to ensure the right device for each person.
Growing evidence shows the benefits of CGM across populations, including reductions in HbA1c level, increased TIR, reduced hypoglycaemia and improved treatment satisfaction. This state of the art technology has well-established benefits for type 1 diabetes and mounting evidence for insulin-treated type 2 diabetes. As utility and accuracy improve, costs decline and reimbursement broadens, CGM benefits will continue expanding across the diabetes spectrum. MT
COMPETING INTERESTS: Associate Professor Cohen has received consulting fees from Abbott and Lilly; honoraria for presentations for Astra Zeneca, Boehringer Ingelheim and Lilly; as well as grants from Boehringer Ingelheim, Novo Nordisk, Ypsomed and receipt of equipment. Dr Nanayakkara has received consulting fees and honoraria for presentations from Lilly.
References
1. Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977-986.
2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577-1589.
3. Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights 2016; 11: 95-1104.
4. Heinemann L, Schoemaker M, Schmelzeisen-Redecker G, et al. Benefits and limitations of MARD as a performance parameter for continuous glucose monitoring in the interstitial space. J Diabetes Sci Technol 2020; 14: 135-150.
5. Kieu A, King J, Govender RD, Ostlundh L. The benefits of utilizing continuous glucose monitoring of diabetes mellitus in primary care: a systematic review. J Diabetes Sci Technol 2023; 17: 762-774.
6. Maiorino MI, Signoriello S, Maio A, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care 2020; 43: 1146-1156.
7. Cardona-Hernandez R, Schwandt A, Alkandari H, et al. Glycemic outcome associated with insulin pump and glucose sensor use in children and adolescents with type 1 diabetes. Data from the international pediatric registry SWEET. Diabetes Care 2021; 44: 1176-1184.
8. Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA 2020; 323: 2388-2396.
9. Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr 2013; 5: 39.
10. O’Malley G, Wang A, Ogyaadu S, Levy CJ. Assessing glycemic control using CGM for women with diabetes in pregnancy. Curr Diab Rep 2021; 21: 44.
11. Toschi E, Slyne C, Sifre K, et al. The relationship between CGM-derived metrics, A1C, and risk of hypoglycemia in older adults with type 1 diabetes. Diabetes Care 2020; 43: 2349-2354.
12. Speight J, Choudhary P, Wilmot EG, et al. Impact of glycaemic technologies on quality of life and related outcomes in adults with type 1 diabetes: a narrative review Diabet Med 2023; 40: e14944.
13. Johnson SR, Holmes-Walker DJ, Chee M, et al. Universal subsidized continuous glucose monitoring funding for young people with type 1 diabetes: uptake and outcomes over 2 years, a population-based study. Diabetes Care 2022; 45: 391-397.
14. Nanayakkara N, Pease AJ, Ranasinha S, et al. Younger people with type 2 diabetes have poorer self-care practices compared with older people: results from the Australian National Diabetes Audit. Diabet Med 2018; 35: 1087-1095.
15. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019; 42: 1593-1603.
16. Australian Diabetes Society (ADS). A practical guide of the glucose pattern insights (GPI) report in primary care. ASD, 2023. Available online at: https://www.diabetessociety.com.au/wp-content/uploads/2023/11/ABB3368_ADS-GPI-Report-Summary_2pp_A4_R4_FA.pdf (accessed July 2024).
17. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract 2008; 82: 73-79.
18. Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 2012; 35: 32-38.
19. Moser O, Yardley JE, Bracken RM. interstitial glucose and physical exercise in type 1 diabetes: integrative physiology, technology, and the gap in-between. Nutrients 2018; 10: 93.