Skin surgery in general practice: delivering the optimal outcome
Delivering the optimal surgical outcome requires the GP to provide near painless local anaesthesia, to remain sensitive and empathetic to the patient throughout the procedure and immediate postoperative period and to ensure a long-lasting imperceptible scar.
Excisional skin surgery is performed for many benign and malignant cutaneous lesions. Such surgery can be a source of significant anxiety in patients for reasons such as pre-existing needle phobia, algophobia and concern about the cosmetic outcome (resultant scar).
Analogous to an elephant never forgetting, a patient undergoing skin surgery will always remember three things:
- Did it hurt?
- Did it scar?
- How was I made to feel?
Therefore, it is the responsibility of the general practitioner performing the surgery to deliver the optimal surgical outcome by providing the patient with compassion, pain-free anaesthesia, meticulous wound closure and detailed after care. Anything less risks a traumatic experience and the potential for unsightly scarring. Unsightly scars can have a significant impact on a patient’s self-esteem and can cause physical dysfunction in severe cases.1 An ideal scar should be virtually undetectable. It should be flat, on the same plane as the adjacent tissue, lie parallel to relaxed skin tension lines and be camouflaged in the surrounding skin.2
We recommend following a simple sequential 10-step checklist throughout the preoperative, operative and postoperative stages to deliver the optimal surgical outcome (Box).
1. Choose the correct equipment
Before commencing the excision, it is important to carefully explain the procedure, including risks and complications, to the patient when obtaining written consent. Although the goal of any excision is to obtain the optimal outcome and achieve the best cosmesis possible, addressing patients’ questions and expectations before the procedure can allow patients to better understand the process and expected outcomes.
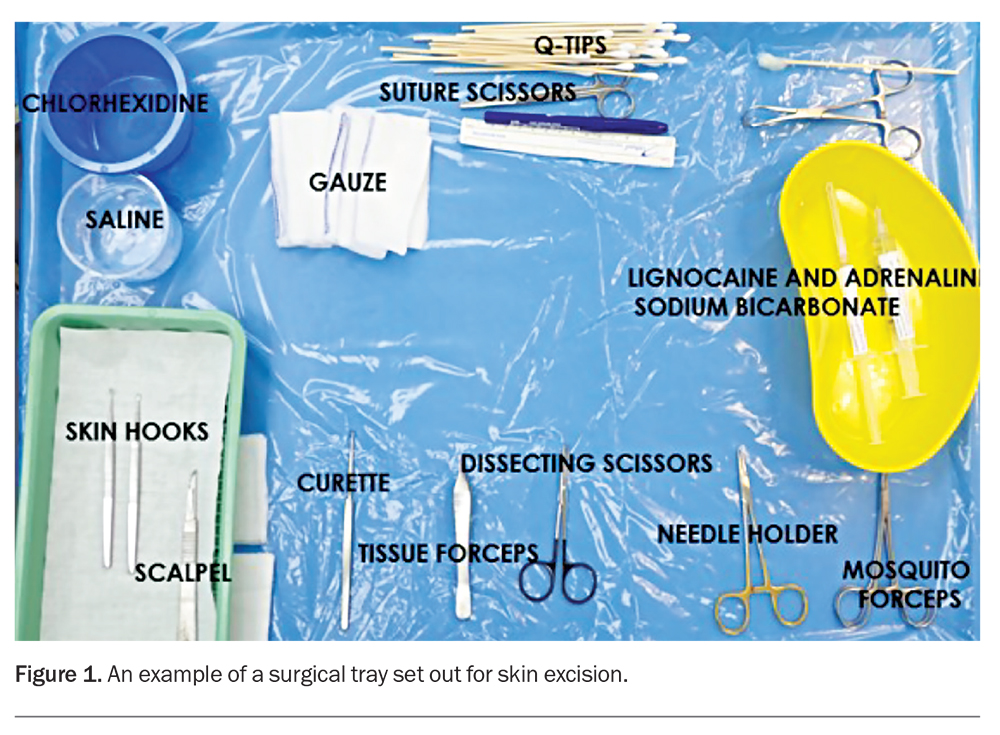
Appropriate magnification, lighting and surgical instruments are required to decrease the amount of effort required by the GP, to protect the patient’s skin during surgery and to facilitate optimal wound closure. We recommend surgical loupes of 2.5 magnification (working distance 420 mm, field of view 130 mm) with an attached LED headlight as the best and simplest option for optimal magnification and lighting. The surgical instruments required include a surgical marking pen, a scalpel holder, toothed forceps, a needle holder, suture scissors, tissue dissecting scissors and sterile drapes and gauze. Additional instruments that will be helpful in achieving the optimal results include an electrosurgery device, Q-tips, a cat paw skin retractor and mosquito forceps (Figure 1).
Serrated needle holders have jaws with teeth, and nonserrated needle holders have smooth jaws.3 Although serrated needle holders are better for securing the suture needle, they are less suitable for finer sutures (6-0 and above) as their teeth increase the risk of sutures breaking.3 Toothed forceps such as Adson tissue forceps with 1 mm tip size are ideal for holding tissue for skin closure. Quality dissecting scissors include tungsten carbide reinforced scissors, which ensure not only a smoother cut but greater durability, and ‘supercut’ scissors, which, due to their design of a sharper upper blade and a microserrated lower blade, allow a clean cut while keeping the tissue secured, thereby minimising tissue trauma during dissection.
High frequency (radiofrequency) electrosurgery uses an electric current which is passed through tissue.4 The lowest wattage waveform setting should be used in order to reduce any direct or collateral thermal damage.5 Additionally, it is preferable to use instruments with a finer tip rather than a broader tip, to ensure more focal and accurate electrocautery.6,7
The Q-tip is an inexpensive tool that can be used for a variety of purposes. It can be used to precisely roll over a ‘bleeder’, allowing pinpoint cauterisation by the GP, and the bulbous tip can be aligned along sutures, allowing the surgical assistant to use the Q-tip as a guide to facilitate the cutting of uniform suture lengths.
2. Do not discriminate
Delivering the optimal surgical experience demands the same level of care, attention to detail and meticulous anaesthetic and wound closure techniques for all procedures regardless of the age of the patient, anatomical site of the lesion or nature of the lesion (malignant or benign).
Before the procedure, it is important to assess the risk of surgical site infection (SSI). SSI is associated with worsened patient outcome, morbidity and poor wound healing.8 Risk factors for SSI include patient factors such as older age, history of diabetes, smoking, Staphylococcus aureus nasal colonisation and comorbidities including immunosuppression; the site of the excision, particularly the groin and lower limb; and the presence of skin ulceration and/or inflammatory skin disease.8-14
Infected sites should not be operated on and should be adequately treated before surgery. There is no universal consensus on the use of decolonisation therapy and/or prophylactic antibiotic to reduce the risk of SSI. In general, assess on a case-by-case basis and consider decolonisation therapy and/or prophylactic antibiotic for high-risk patients while weighing the benefits and risk of bacterial resistance, allergies, adverse reactions and cost.15-18 Patients who are at risk of infective endocarditis or prosthetic joint infections should have prophylactic antibiotics to prevent bacteraemia.15
3. Plan meticulously
Planning the optimal surgical excision includes attention to the location of the excision in relation to relaxed skin tension lines (RSTL) as well as planning the dimensions of the surgical ellipse.
Tension mostly follows in the direction of the RSTL.19 The RSTL can be visualised as the wrinkles formed on relaxation of the skin, which can be observed by gently pinching the skin perpendicular to the RSTL.19 Excisions should be made parallel to RSTL as the skin perpendicular to the RSTL is most flexible, allowing less tension when approximating wound edges.20 Minimal wound tension allows easy apposition of wound edges and also reduces the risk of adverse scarring such as hypertrophic scar formation.20
The surgical ellipse is a fusiform-shaped excision most commonly used to remove skin lesions.21 A surgical ellipse should be marked out with appropriate dimensions before commencing the surgery. An excisional ellipse usually has a length-to-width ratio of 3:1 with the ends of the excision forming an angle of 30 degrees or less.21,22 Such dimensions are used to allow closure of the defect while minimising the scar length and reducing the risk of coning at the ends of the ellipse.21,22
The skin should be prepared before surgery using any form of antiseptic such as chlorhexidine, povidone-iodine or isopropyl alcohol, as long as it is not a formula that the patient is allergic to. Drapes with or without adhesives can be used to prevent contamination from surrounding skin and maintain a sterile field.
4. Administer painless anaesthesia
Local anaesthetic (LA) injection is often the most painful aspect of excisional skin surgery. Pain from LA infiltration may result from needle insertion through the skin, reaction to the anaesthetic solution and/or distention of the skin.23 Maximising patient comfort and reducing pain can improve the patient’s overall experience of surgery.
Every patient is unique, and variable factors such as age, sex, past experiences and culture can impact on a patient’s perception and reaction to pain.24 Careful explanation, encouragement and reassurance of patients before commencing any procedure helps to reduce their level of anxiety, which can contribute to the level of pain experienced.25 Distraction methods such as conversation (talk-anaesthesia) and the patient listening to their preferred music can minimise anxiety.25,26 A calm, collected bedside manner can also enhance confidence and help patients to relax.26
For patients who are particularly apprehensive about injections, application of topical aesthetic, vibrational analgesia and/or cryoanalgesia before LA injections should be offered for pain relief and psychological comfort. Topical anaesthetic can be used to target dermal free nerve endings to reduce painful sensations.27 Various topical anaesthetic formulations are available over the counter or can be compounded according to patients’ individual requirements.
Vibrational analgesia is another noninvasive method used to reduce the painful sensation from needle insertion. Various commercially available vibrational anaesthetic devices are available. They are placed on the skin 1 to 2 cm from the injection site and activated for a few seconds before the needle insertion and continued until the injection is complete.28,29 Alternatively, a readily available device that also induces vibrational analgesia is an electric toothbrush.30 The toothbrush can be covered with a disposable glove and used by applying the vibrating bristles above the injection site until the injection is complete.30
Vibrational analgesia is based on concepts of the gate control theory of pain, which involves afferent nerve fibres including A-alpha, A-beta, A-delta and C-nerve fibres. A-alpha fibres transmit information regarding proprioception, A-beta transmit touch and vibration, and A-delta and C fibres transmit pain and temperature.31-33 The gate control theory of pain suggests that when larger fibres are stimulated, smaller fibres are inhibited.31-33 Therefore, when larger A-beta fibres are stimulated from vibration, smaller nociceptive A-delta and C-fibres are inhibited from transmitting painful stimuli to the brain.31-33
Cryoanalgesia can also be used to reduce pain from needle insertion and involves application of an icepack on the skin for 1 to 2 minutes before injection.34 The mechanism is not completely understood; inhibition of nociceptors, slowing of nerve conduction, activation of gate control or release of endorphins (or a combination of these) may be responsible for reducing pain.34
The LA solution itself can also contribute to the pain due to its acidity.26,35 A systematic review and meta-analysis concluded that warming the LA solution to body temperature (37°C) and buffering with 8.4% sodium bicarbonate (1 mL/9 mL LA) can reduce the pain during injection.35 Warming of the LA solution works by reducing nociceptive fibre stimulation.35 It is also postulated that by increasing the pH of the LA solution, LA molecules can rapidly diffuse into cells, allowing more rapid onset.26,35
Meticulous technique for injection of LA can also reduce the degree of pain. For small lesions, a 30-gauge 12 mm (half inch) needle is recommended, and for large lesions a long 25-gauge 40 mm (1.5 inch) needle. LA should be injected slowly and using the lowest volume required to form a field block around the planned excision.25,36 The needle should be inserted bevel up into the upper subcutis rather than intradermally as there are fewer pain receptors and less tissue distension in the subcutis.25 A single entry point is recommended, advancing anterograde (i.e. forward), after pulling back the plunger to prevent inadvertent intravascular injection, and allowing 5 to 10 seconds after each infiltration for the local anaesthetic to reduce the pain from the subsequent needle advancement and anaesthetic infiltration.25
5. Stretch the skin
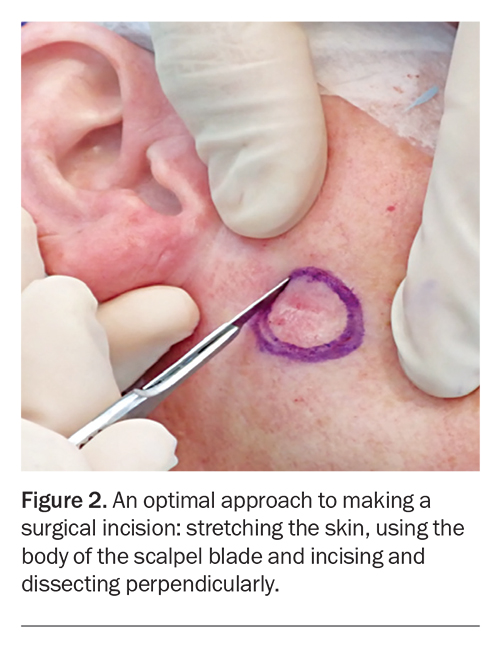
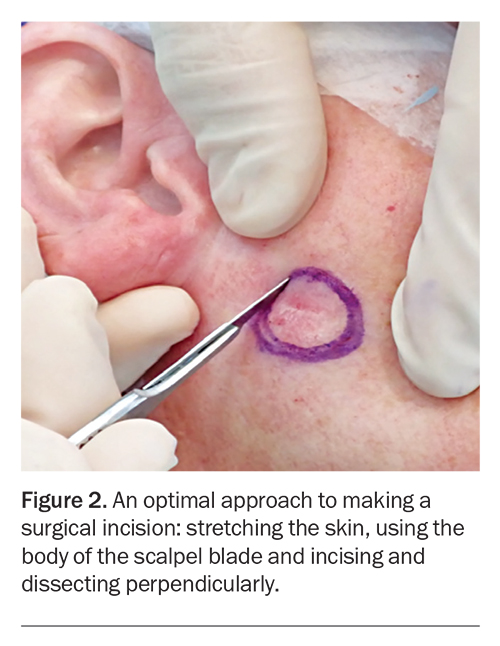
Before incision, the planned excision margins and surrounding skin should be stretched by the GP using their nondominant hand, or by an assistant placing their fingers perpendicular and parallel to the marked incision line (Figure 2).37 A stretched cutting surface provides stability and enables accurate clean incision lines, better control of scalpel depth and reduced wound bleeding due to pressure and contraction on surrounding blood vessels.37
6. Utilise the body of the scalpel blade
Scalpel blades have a sharp tip and a curved blade body. An incision should be made using the curved part of the scalpel (body) to ensure greater surface area contact, which allows better control and uniform dermal penetration (Figure 2).37 Incisions made using the tip of the scalpel should be avoided as using the tip can result in poor penetration, unnecessary multiple incisions and a resultant ‘stair casing’ of the wound edges, leading to poor scar outcome.21
7. Incise and dissect perpendicularly
The correct technique when incising the skin is to maintain the scalpel blade perpendicular to the skin surface while moving the body of the blade in a continuous fashion along the marked excision outline, using enough pressure to fully penetrate the dermis (Figure 2).37 Thereafter, dissecting scissors should be used to dissect perpendicularly to the desired depth and then across the base of the excision parallel to the skin surface. Angling the scalpel/dissecting scissors inwards in an oblique manner can risk bevelling and wedge dissection leading to incomplete excision margins and inverted wounds. Inversion of wound edges risks poor healing and unsightly scarring.21
Danger zones for underlying neurovascular damage need to be identified and avoided. Description of these landmarks is beyond the purpose of this article; however, before performing dermatological surgery it is important for GPs to be familiar with the surface anatomy landmarks for the following structures. On the face this includes the superficial temporal artery, branches of the facial nerve (temporal, zygomatic, buccal and marginal mandibular) and the supraorbital, infraorbital and supratrochlear nerves. There are many important landmarks also on the neck, such as the external jugular vein, great auricular nerve and spinal accessory nerve. On the limbs, the ulnar nerve, common fibular and pretibial nerve need to be identified.38,39
8. Choose the correct suture
An ideal suture should exhibit just enough tensile strength to bring the edges of the tissue together while minimising tissue trauma and ischaemia.40 Various factors such as the surgical site, layer and depth of closure as well as tension of the wound should be considered when choosing the size (diameter) and material of the suture and the needle shape.41
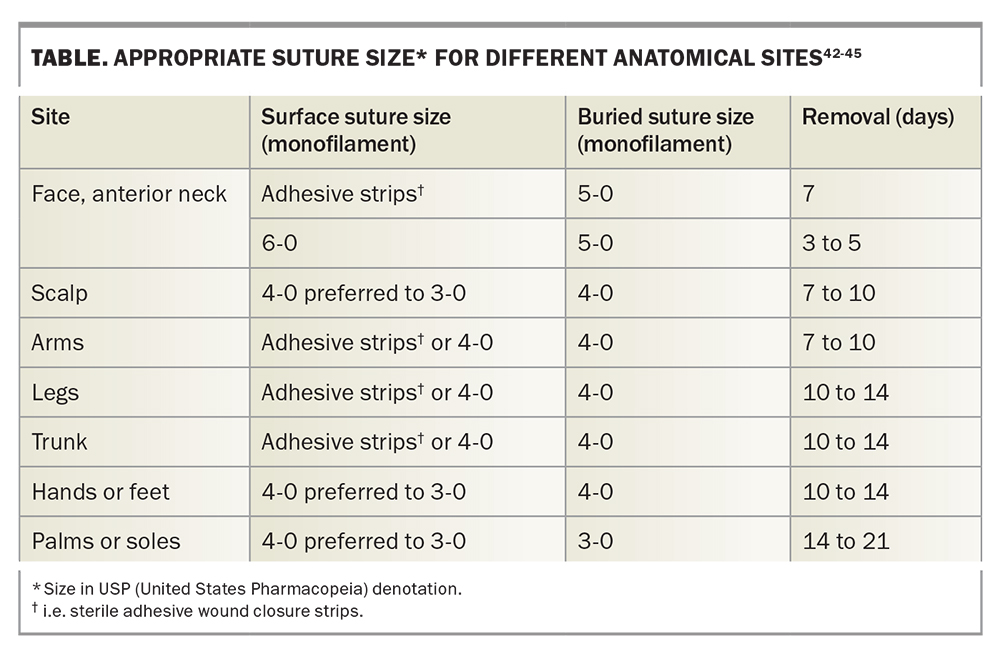
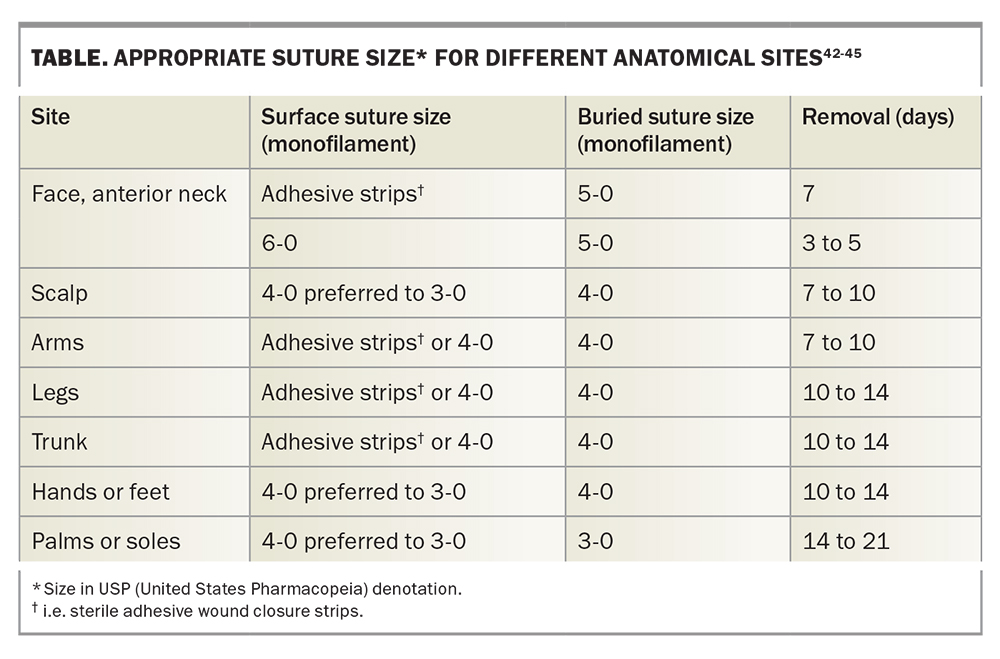
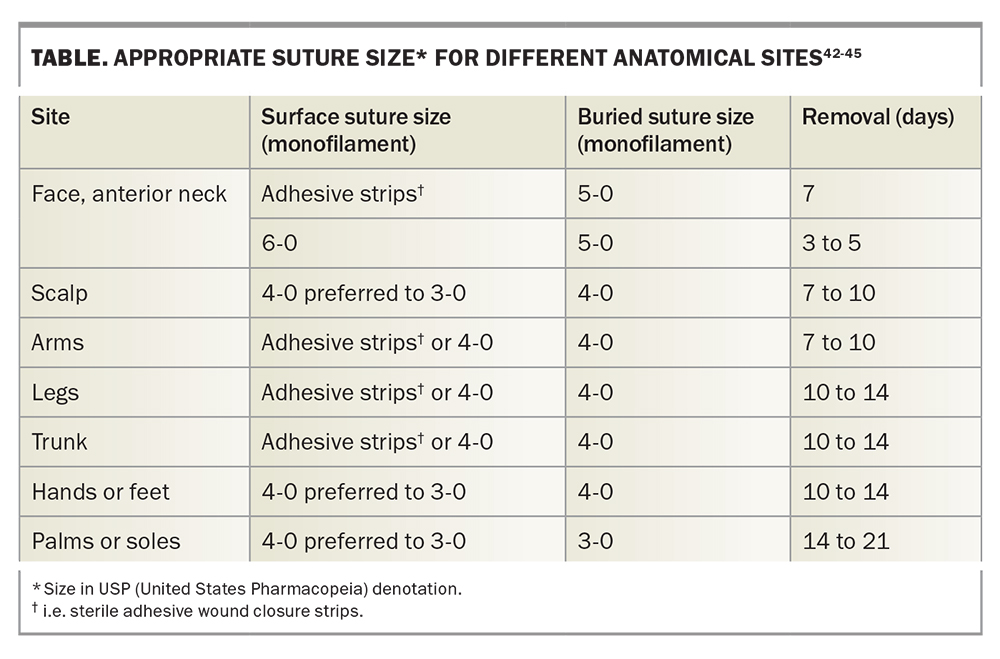
It is important to note that the suture tensile strength is less as the diameter of the suture decreases. Suture size is dependent on the anatomical site of the excision (Table). The smallest suitable suture diameter with enough tensile strength to close the wound edges should be chosen in order to minimise tissue injury.40,41
Sutures are broadly categorised as absorbable or nonabsorbable. Absorbable sutures are often used to close the lower dermis and subcutaneous tissue to minimise the dead space and wound tension during healing.46 Absorbable deep sutures are designed to reduce surface tension on a wound until they lose their inherent tensile strength and dissolve after a certain time. If absorbable sutures are inserted too close to the epidermis however, persistence of the suture can risk suture tracks and unsightly scar formation.46 Nonabsorbable sutures, on the other hand, are used to temporarily approximate superficial wound edges in the absence of tension and are removed as soon as possible.47
Absorbable and nonabsorbable sutures are available in monofilament and polyfilament materials. Polyfilament sutures are made up of multiple filaments braided together. The single stranded structure of monofilaments provides less reactivity, minimal tissue injury due to less tissue resistance, and lower risk of harbouring bacteria; however, monofilaments have more memory and therefore have lower knot security.40,46 Polyfilaments are more pliable and can hold knots more securely. They may result in more tissue resistance and increased risk of tissue injury, reactivity and infection; however, some have an outer coating for a smoother track through tissue and reduced risk of infection.40,46 Types of commonly used absorbable monofilament sutures include poliglecaprone and polydioxanone sutures; nonabsorbable monofilament sutures include polypropylene and nylon sutures. Absorbable polyfilament sutures include plain cat gut, chromic cat gut and polyglactin 910 sutures; nonabsorbable polyfilament sutures include silk and polyester sutures. We recommend absorbable deep monofilament sutures and nonabsorbable monofilament sutures for the best cosmetic outcome. Whenever possible, it is best to avoid absorbable surface sutures as the suture may degrade and lose its tensile strength before the wound is fully healed (Table).
Suture needles vary in size, shape and tip.46 Selection of the appropriate needle is dependent on the tissue type and site of closure.48 An ideal suture needle should be sharp and easily handled by the holder in order to penetrate the skin with minimal tissue damage. Common needle shapes range from straight to J shaped, compound curve and needles of various curvature (one-quarter, three-eighths, half or five-eighths of a circle).41 Curved needles taking the shape of three-eighths of a circle are most commonly used for skin closure.41 Generally, the more limited the accessibility to the tissue, the more curved the needle will need to be.48
Finally, attention to the tip of the needle, also known as the ‘needle point’ is decided based on the tissue type. For skin closures, conventional cutting needles and reverse cutting needles are used due to their fine triangulated body and ability to sharply penetrate the skin. Conventional cutting needles have the cutting edges in the inner curvature and reverse cutting needles have the cutting edge along the outside of the curvature. As the cutting edge is restricted to the outer curvature, the reverse cutting needles hold the advantage of reducing the risk of accidental suture tear, and we prefer to use these. 41,48
9. Insert the perfect suture
The purpose of a perfect suture is to approximate wound edges sufficiently to avoid wound dehiscence while also minimising the risk of tissue injury, which could potentially lead to wound tension, ischaemia and adverse scarring.22,40 Conventionally, eversion of tissue edges is recommended during closure as it is thought to improve cosmetic outcome and reduce the risk of scar depression with retraction over time.21,49,50
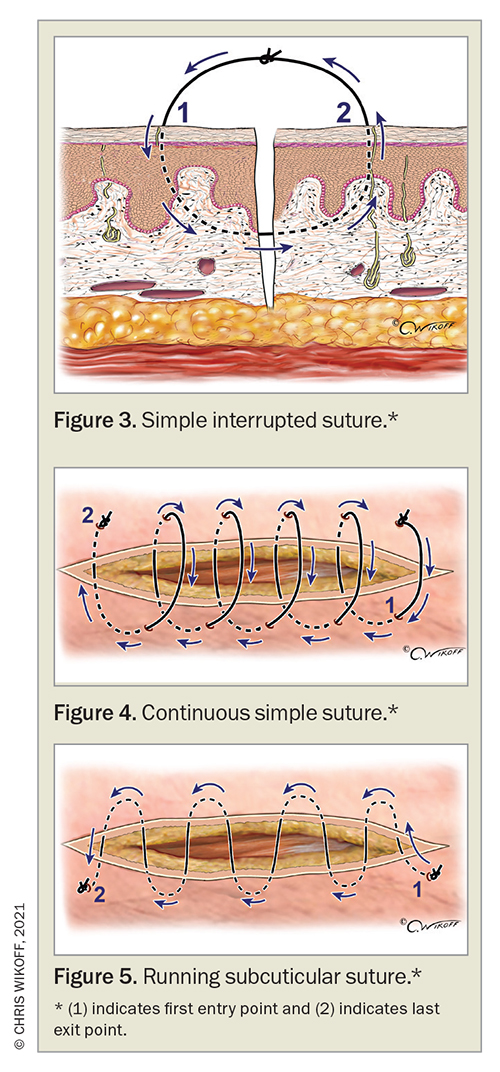
Wound closure can be achieved with various suturing techniques, including the simple interrupted, continuous and running subcuticular suture. The most commonly used suture type is the simple interrupted suture whereby multiple sutures are placed 3 to 5 mm from the wound edges (Figure 3) Each suture is placed with the needle pointing in a perpendicular fashion to the skin. Sutures are positioned from the middle of the wound and evenly spaced about 0.5 to 1 cm apart depending on the anatomical location and tissue. Interrupted sutures offer high tensile strength and the advantage of being able to remove individual sutures, for example if infection occurs.41
Continuous sutures have some similarities to simple interrupted sutures; however, rather than having multiple sutures, a single running suture is used instead, which can offer the benefit of reducing suturing time (Figure 4). Sutures are placed 3 to 5 mm from the wound edge similarly to the simple interrupted technique, although starting at the apex of the wound. The sutures are placed about 1 cm apart and continued until the end of the wound. This suture technique carries risks of wound dehiscence if the suture breaks, and tissue strangulation resulting in ischaemia and necrosis if too much tension is created.41
A running subcuticular suture is placed in the dermis along the wound edges starting from one apex of the wound, taking alternate horizontal bites of the dermis until the wound is closed (Figure 5). As this suture technique involves absorbable sutures and minimal puncturing of the epidermis there is no risk of crosshatch-like scarring, therefore it can be used on areas such as the face where cosmetic outcome is most important.41 Alternatively, a zipper stitch, which is a running buried vertical mattress stitch, can be used to close subcutaneous wounds using absorbable sutures.51
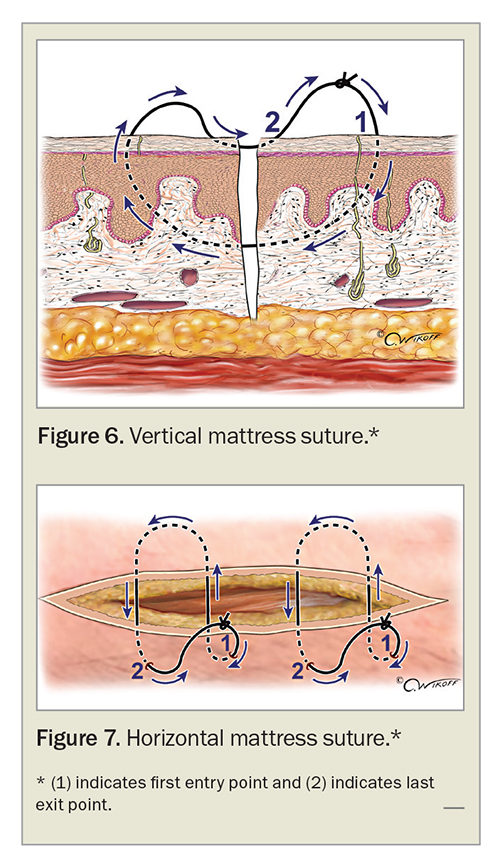
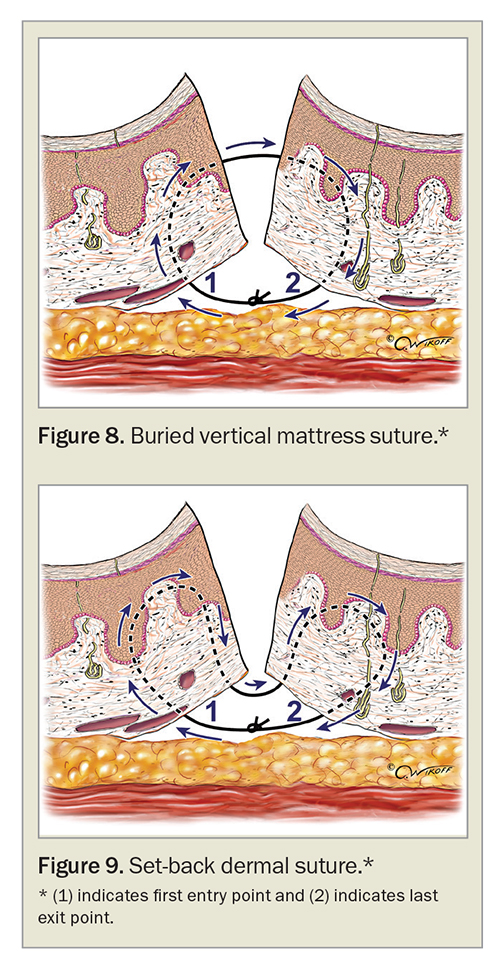
Where nonabsorbable interrupted surface sutures do not provide adequate tension reduction and/or eversion, special suture techniques such as horizontal and vertical mattress sutures can be used, although we favour them less due to the risk of necrosis and scarring from epidermal compression if sutured too tightly (Figures 6 and 7).49 Absorbable suture techniques such as the buried vertical mattress suture and the set-back dermal suture are preferred for eversion of wound edges without the risk of epidermal compression and surface suture marks (Figures 8 and 9).41,42,49,52
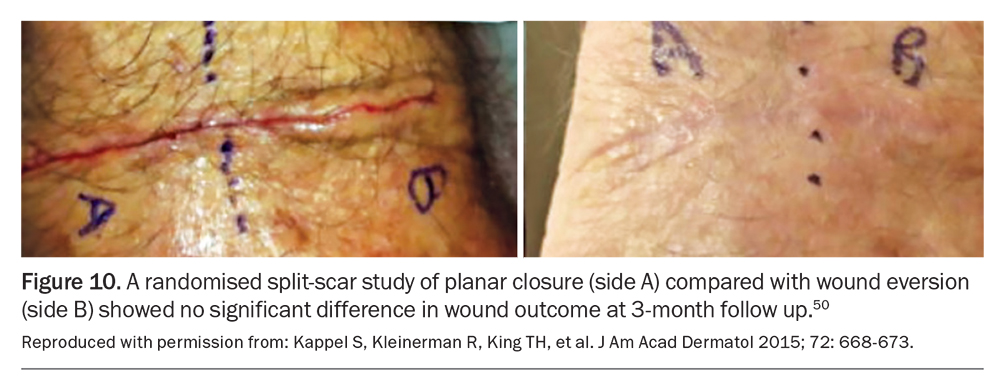
Although everting wound edges has been the gold standard approach in wound closure, recent studies have challenged this traditional surgical dogma. A randomised split-scar study in 60 patients suggested there were no significant differences in the scar outcome when comparing everted wounds formed by dermal set-back or inverted vertical mattress sutures with planar wounds formed by simple buried sutures (Figure 10).50 However, another randomised study comparing set-back buried dermal sutures with buried vertical mattress sutures in 42 patients showed superior wound eversion after surgery and associated better cosmetic outcome three months after the procedure for wounds with set-back sutures.53 Further studies comparing different suture techniques in variable anatomical positions for the best scar result and its correlation to postoperative eversion height may be required before definitive recommendations can be made.
It is the second author’s opinion after 20 years as a Mohs surgeon that tension from sutures is the enemy of wound healing and the major impediment to creating an imperceptible scar. In the absence of tension, neither planar nor everted closure makes any significant positive or negative contribution to the outcome. Excessive tension created by sutures can cause localised tissue injury and necrosis due to ischaemia and pressure exerted by the suture. Such tissue damage can increase the risk of infection, depigmentation and dermal inflammation, which can ultimately lead to hypertrophic and keloid scar formation.20,54 Particular attention and care is required at certain sites, such as wounds overlying joints, where closure of defects is associated with significant tension.20
Methods for decreasing suture tension include planning excisions along relaxed skin tension lines, increasing the surgical ellipse ratio to 4:1 or 5:1, using absorbable buried sutures, using sterile adhesive wound closure strips rather than nonabsorbable surface sutures whenever possible and early removal of surface sutures.55,56 Application of glue instead of adhesive tape can also be considered, although it is generally avoided due to risk of contact dermatitis and allergic reactions.57 Timely removal of surface sutures is important in reducing the risk of cross-hatch-like scarring from sutures that are overdue for removal, or, conversely, wound dehiscence from removing stitches too early.49 A guide to suture selection and timing of removal is provided in the Table.
10. Implement meticulous aftercare
Aftercare starts immediately following the procedure and is vital to ensuring minimum scar production and an optimal surgical outcome. The importance of maximising nutrition and controlling diabetes is a message started preoperatively and continued during the aftercare period.56 Similarly, reducing the enemy of wound healing, smoking, should be emphasised at every opportunity. The vasoconstrictive effect from nicotine, reduction of oxygen delivery from carbon monoxide and inhibition of oxidative metabolism and cellular oxygen transport all contribute to poor wound healing.58
Postoperative pain is never forgotten (nor forgiven) by patients and can ruin the surgical experience no matter how perfect the resultant scar. Advice on rest, elevation and icepacks must be conveyed and appropriate analgesia prescribed.
Once the procedure is performed, maintaining a moist environment in the initial phase of wound healing is vital for improved scar formation. Options include application of a moisturising agent such as petrolatum three-times daily for one to three weeks after surgery, or alternatively a hypoallergenic, porous, stretchable, nonwoven tape can be applied and changed weekly for one to three weeks. Ensuring a humid environment can induce epithelisation, reduce associated redness and minimise the tendency for hypertrophic scarring.56 Consider the use of tape or sterile adhesive wound closure strips after suture removal for one to six weeks, to reduce shear force on the wound during scar formation and healing.56 Compression stockings can be considered for lower limb wounds, particularly in patients with venous insufficiency. Sunscreen and photoprotection are generally advised as part of wound care immediately after surgery for up to six to 12 months.56 Ultraviolet radiation exposure to wounds can cause scar hyperpigmentation as well as thickening due to change in collagenisation.56 Once wound edges are healed and a scar is formed (usually three weeks after the surgery), daily scar massage is suggested to break down any excess collagen and soften the scar.56 If patients are unsatisfied with the scar outcome, appropriate laser therapy can be considered.56
After the surgery, patients should be provided with written information on how to care for their wound, what to expect and when to seek medical attention, a plan for follow up and receiving pathology results and the contact details of the clinic and staff.
Conclusion
Achieving an optimal surgical outcome for patients always involves not only delivering an imperceptible scar but also providing near painless anaesthesia, while maintaining a caring and empathetic environment. Failure to provide all the necessary components to achieve this risks, not only an unsatisfactory scar, but also the generation of feelings of anxiety and fear that may have a long-term adverse effect on the patient. The above simple sequential 10-point checklist can assist GPs in achieving the best possible surgical outcome in patients of any age, at any site, and for any lesion whether benign or malignant. In tricky or difficult cases, referral to a dermatologist or surgeon is recommended. MT
COMPETING INTERESTS: None.
References
1. Brown B, McKenna S, Siddhi K, McGrouther D, Bayat A. The hidden cost of skin scars: quality of life after skin scarring. J Plastic Reconstr Aesthet Surg 2008; 61: 1049-1058.
2. Sharma M, Wakure A. Scar revision. Indian J Plast Surg 2013; 46: 408-418.
3. Stamp CV, McGregor W, Rodeheaver GT, Thacker JG, Towler MA, Edlich RF. Surgical needle holder damage to sutures. Am Surg 1988; 54: 300-306.
4. Hainer BL. Electrosurgery for the skin. Am Fam Physician 2002; 66: 1259-1266.
5. Cordero I. Electrosurgical units - how they work and how to use them safely. Community Eye Health 2015; 28: 15-16.
6. Chandra RV, Savitharani B, Reddy AA. Comparing the outcomes of incisions made by colorado microdissection needle, electrosurgery tip, and surgical blade during periodontal surgery: a randomized controlled trial. J Indian Soc Periodontol 2016; 20: 616-622.
7. Sharma R. Safety of colorado microdissection needle (stryker) for skin opening in craniomaxillofacial surgery. J Maxillofac Oral Surg 2012; 11: 115-118.
8. Heal CF, Buettner PG, Drobetz H. Risk factors for surgical site infection after dermatological surgery. Int J Dermatol 2012; 51: 796-803.
9. Gill JF, Yu SS, Neuhaus IM. Tobacco smoking and dermatologic surgery. J Am Acad Dermatol 2013; 68: 167-172.
10. Dixon AJ, Dixon MP, Askew DA, Wilkinson D. Prospective study of wound infections in dermatologic surgery in the absence of prophylactic antibiotics. Dermatol Surg 2006; 32: 819-826; discussion 26-27.
11. Amici JM, Rogues AM, Lasheras A, et al. A prospective study of the incidence of complications associated with dermatological surgery. Br J Dermatol 2005; 153: 967-971.
12. Kulichová D, Geimer T, Mühlstädt M, Ruzicka T, Kunte C. Surgical site infections in skin surgery: a single center experience. J Dermatol 2013; 40: 779-785.
13. Neumayer L, Hosokawa P, Itani K, El-Tamer M, Henderson WG, Khuri SF. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg 2007; 204: 1178-1187.
14. Wahie S, Lawrence CM. Wound complications following diagnostic skin biopsies in dermatology inpatients. Arch Dermatol 2007; 143: 1267-1271.
15. Lee MR, Paver R. Prophylactic antibiotics in dermatological surgery. Australas J Dermatol 2016; 57: 83-91.
16. Chan SA, Wernham AGH, Stembridge N, et al. Do perioperative antibiotics reduce the risk of surgical-site infections following excision of ulcerated skin cancers? A critically appraised topic. Br J Dermatol 2018; 178: 394-399.
17. Norman G, Dumville JC, Mohapatra DP, Owens GL, Crosbie EJ. Antibiotics and antiseptics for surgical wounds healing by secondary intention. Cochrane Database Syst Rev 2016; (3): CD011712.
18. Cherian P, Gunson T, Borchard K, Tai Y, Smith H, Vinciullo C. Oral antibiotics versus topical decolonization to prevent surgical site infection after Mohs micrographic surgery - a randomized, controlled trial. Dermatol Surg 2013; 39: 1486-1493.
19. Borges AF. Relaxed skin tension lines (RSTL) versus other skin lines. Plast Reconstr Surg 1984; 73: 144-150.
20. Son D, Harijan A. Overview of surgical scar prevention and management. J Korean Med Sci 2014; 29: 751-757.
21. Zuber TJ. Fusiform excision. Am Fam Physician 2003; 67: 1539-1544, 1547-1548, 1550.
22. Garg S, Dahiya N, Gupta S. Surgical scar revision: an overview. J Cutan Aesthet Surg 2014; 7: 3-13.
23. Zilinsky I, Bar-Meir E, Zaslansky R, Mendes D, Winkler E, Orenstein A. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol 2005; 4: 212-216.
24. McGrath PA. Psychological aspects of pain perception. Arch Oral Biol 1994; 39 Suppl: 55S-62S.
25. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg 2013; 132: 675-684.
26. Quaba O, Huntley JS, Bahia H, McKeown DW. A users guide for reducing the pain of local anaesthetic administration. Emerg Med J 2005; 22: 188.
27. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol 2015; 31: 450-456.
28. Park KY, Lee Y, Hong JY, Chung WS, Kim MN, Kim BJ. Vibration anesthesia for pain reduction during intralesional steroid injection for keloid treatment. Dermatol Surg 2017; 43: 724-727.
29. Sharma P, Czyz CN, Wulc AE. Investigating the efficacy of vibration anesthesia to reduce pain from cosmetic botulinum toxin injections. Aesthet Surg J 2011; 31: 966-971.
30. Duplisea MJ, Flores K. Buzzing away the pain: using an electric toothbrush for vibration anesthesia during painful procedures. Pediatr Dermatol 2019; 36: 414-415.
31. Smith KC, Comite SL, Balasubramanian S, Carver A, Liu JF. Vibration anesthesia: a noninvasive method of reducing discomfort prior to dermatologic procedures. Dermatol Online J 2004; 10(2): 1.
32. Melzack R. From the gate to the neuromatrix. Pain 1999; Suppl 6: S121-S126.
33. Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965; 150: 971-979.
34. Algafly AA, George KP. The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br J Sports Med 2007; 41: 365-369; discussion 9.
35. Hogan ME, vanderVaart S, Perampaladas K, Machado M, Einarson TR, Taddio A. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med 2011; 58: 86-98.e1.
36. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med 1987; 5: 83-90.
37. Chandra S, Podder I, Chatterjee M, Field L. Anatomy and applications of the #15 scalpel blade and its variations. J Cutan Aesthet Surg 2018; 11: 79-82.
38. Birrell AL, Warren LR, Warren LJ. Hazards and danger zones in cutaneous surgery. Clin Skin Cancer 2017; 2: 1-4.
39. Aramrattana A, Sittitrai P, Harnsiriwattanagit K. Surgical anatomy of the spinal accessory nerve in the posterior triangle of the neck. Asian J Surg 2005; 28: 171-173.
40. Byrne M, Aly A. The surgical suture. Aesthet Surg J 2019; 39(Suppl 2): S67-S72.
41. Ethicon. Wound closure manual. Somervile, New Jersey: Johnson & Johnson; 2004.
42. Kantor J. Atlas of suturing techniques: approaches to surgical wound, laceration and cosmetic repair. First ed. McGraw-Hill Education; 2016. p. 24-26.
43. Forsch RT, Little SH, Williams C. Laceration repair: a practical approach. Am Fam Physician 2017; 95: 628-636.
44. Forsch RT. Essentials of skin laceration repair. Am Fam Physician 2008; 78: 945-951.
45. Trott AT. Wounds and lacerations: emergency care and closure. 4th ed. Philadelphia: Elsevier Saunders; 2012.
46. Kudur MH, Pai SB, Sripathi H, Prabhu S. Sutures and suturing techniques in skin closure. Indian J Dermatol Venereol Leprol 2009; 75: 425-434.
47. Lober CW, Fenske NA. Suture materials for closing the skin and subcutaneous tissues. Aesthetic Plast Surg 1986; 10: 245-248.
48. Ethicon. Selection and use of surgical needles. Somerville, New Jersey: Johnson & Johnson.
49. Zuber TJ. The mattress sutures: vertical, horizontal, and corner stitch. Am Fam Physician 2002; 66: 2231-2236.
50. Kappel S, Kleinerman R, King TH, et al. Does wound eversion improve cosmetic outcome? Results of a randomized, split-scar, comparative trial. J Am Acad Dermatol 2015; 72: 668-673.
51. Yag-Howard C. Zipper stitch: a novel aesthetic subcutaneous closure. Dermatol Surg 2013; 39: 1400-1402.
52. Vatanchi M, Brian Jiang SI. Achieving eversion utilizing topical tissue adhesive. J Am Acad Dermatol 2019; 81: e37-e38.
53. Wang AS, Kleinerman R, Armstrong AW, et al. Set-back versus buried vertical mattress suturing: results of a randomized blinded trial. J Am Acad Dermatol 2015; 72: 674-680.
54. Field LM. Suture marks: factors of causation and prevention. Dermatol Surg 2006; 32: 1425-1426; author reply 1426.
55. Watts GT. Sutures for skin closure [letter]. Lancet 1975; 1(7906): 581.
56. Commander SJ, Chamata E, Cox J, Dickey RM, Lee EI. Update on postsurgical scar management. Semin Plast Surg 2016; 30: 122-128.
57. Nakagawa S, Uda H, Sarukawa S, et al. Contact dermatitis caused by dermabond advanced use. Plast Reconstr Surg Glob Open 2018; 6: e1841.
58. Silverstein P. Smoking and wound healing. Am J Med 1992; 93(1A): 22S-24S.