Fever in the returned child traveller: assessing for imported causes

Determining the cause of fever in a child returning from international travel can be challenging. The clinical features of many imported infections are nonspecific, making them hard to distinguish from common childhood febrile illnesses coincidentally acquired during or soon after travel. Judicious assessment, starting with a detailed travel history, is key to guiding appropriate investigations. Imported disease may require a notification to a public health unit and should be managed in consultation with an infectious diseases specialist.
- Fever is common in children after overseas travel and requires careful assessment to exclude serious infection.
- A focused history, examination and consideration of destination-specific health risks will guide investigations.
- Malaria, dengue and enteric fever are the most common imported infections in child travellers and can be life-threatening if the diagnosis is delayed or missed. Children visiting extended family in endemic countries are more commonly infected than tourists.
- Malaria can present up to two years after travel to an endemic area.
- Any child with post-travel fever who is systemically unwell or at high risk for falciparum malaria should be assessed in hospital.
- Imported infections in returned child travellers should be managed in consultation with a local infectious diseases physician, paediatrician or the on-call paediatric infectious diseases service for your region.
International travel by Australian residents, including children, has been steadily increasing since outbound travel restrictions imposed during the COVID-19 pandemic were lifted in April 2022.1 A corresponding progressive rise in presentations to general practice for post-travel illness is expected. Fever is common in children and, even after international travel, is often due to a viral or self-limiting illness. However, some febrile returned child travellers will have a serious imported infection (i.e. an infectious disease not endemic to Australia), such as malaria, dengue or enteric (typhoid or paratyphoid) fever. Child travellers presenting with undifferentiated fever to the emergency department of the Children’s Hospital at Westmead (CHW) in Sydney had an incidence of imported infection of around 30%.2 This incidence is likely to be lower in general practice; however, unpublished data from CHW (Mazzocato, et al.) show that children with serious imported infections were often first seen in primary care: 30% of children treated for enteric fever at CHW between November 2018 and April 2020 were diagnosed through a positive blood or stool culture ordered by their GP.
This article presents an approach to assessing post-travel fever in children to help GPs perform an appropriate work up and decide who to refer for further investigation or treatment. The most common causes are discussed.
Evaluation of fever in the returned child traveller
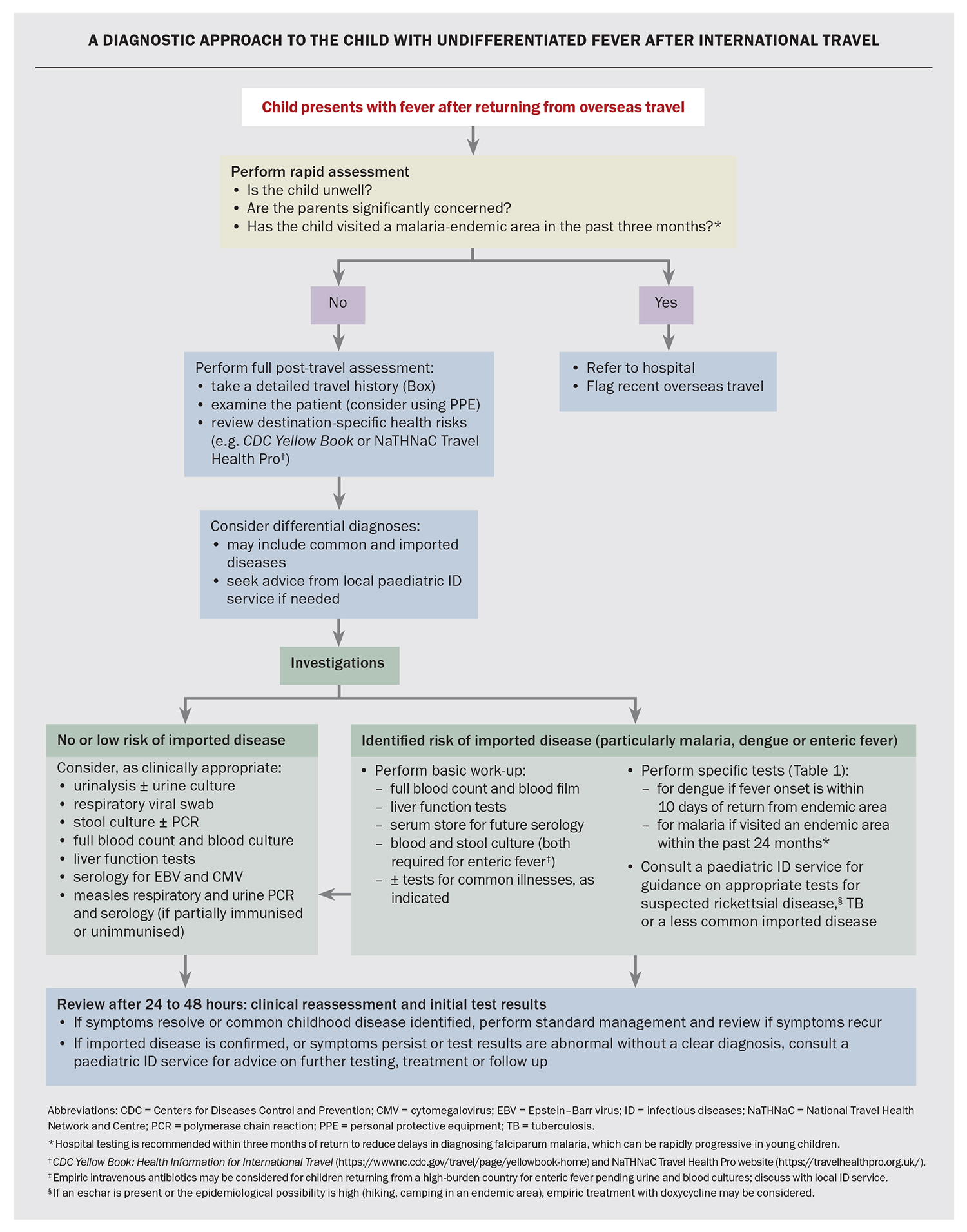
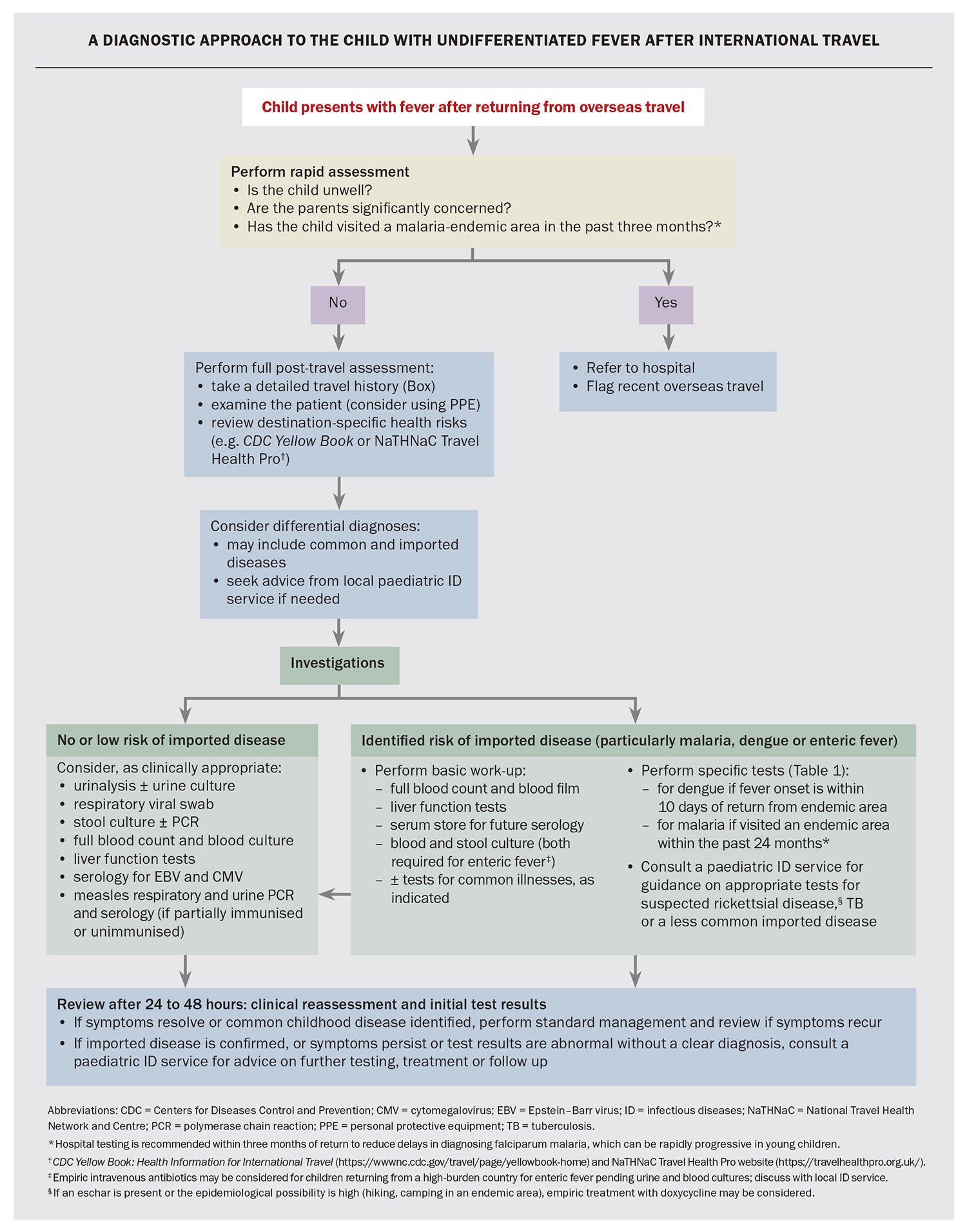
As with any child presenting to general practice with fever, a rapid visual assessment of the febrile traveller will identify concerning features that warrant immediate referral to hospital, including poor perfusion; drowsiness, lethargy or irritability; pallor or jaundice; tachypnoea; nonblanching rash, petechiae or mucosal bleeding; and focal neurological signs. Alert the admitting physician of the child’s impending arrival and their recent travel. Hospital referral for children who have visited a high-risk malaria area within the past three months (especially Sub-Saharan Africa, Papua New Guinea or the Solomon Islands) is also recommended, even if prophylaxis was taken. Falciparum malaria presents within this timeframe and can rapidly become life-threatening. Inpatient testing reduces delays, allowing for clinical status to be monitored while awaiting results and earlier treatment if positive.
The initial evaluation of all other febrile child travellers can generally occur in primary care, and an approach to their assessment is presented in the Flowchart. A thorough patient travel history, careful examination and knowledge of destination- specific health risks are key to narrowing the differential diagnoses and ensuring the most appropriate tests are performed.
History
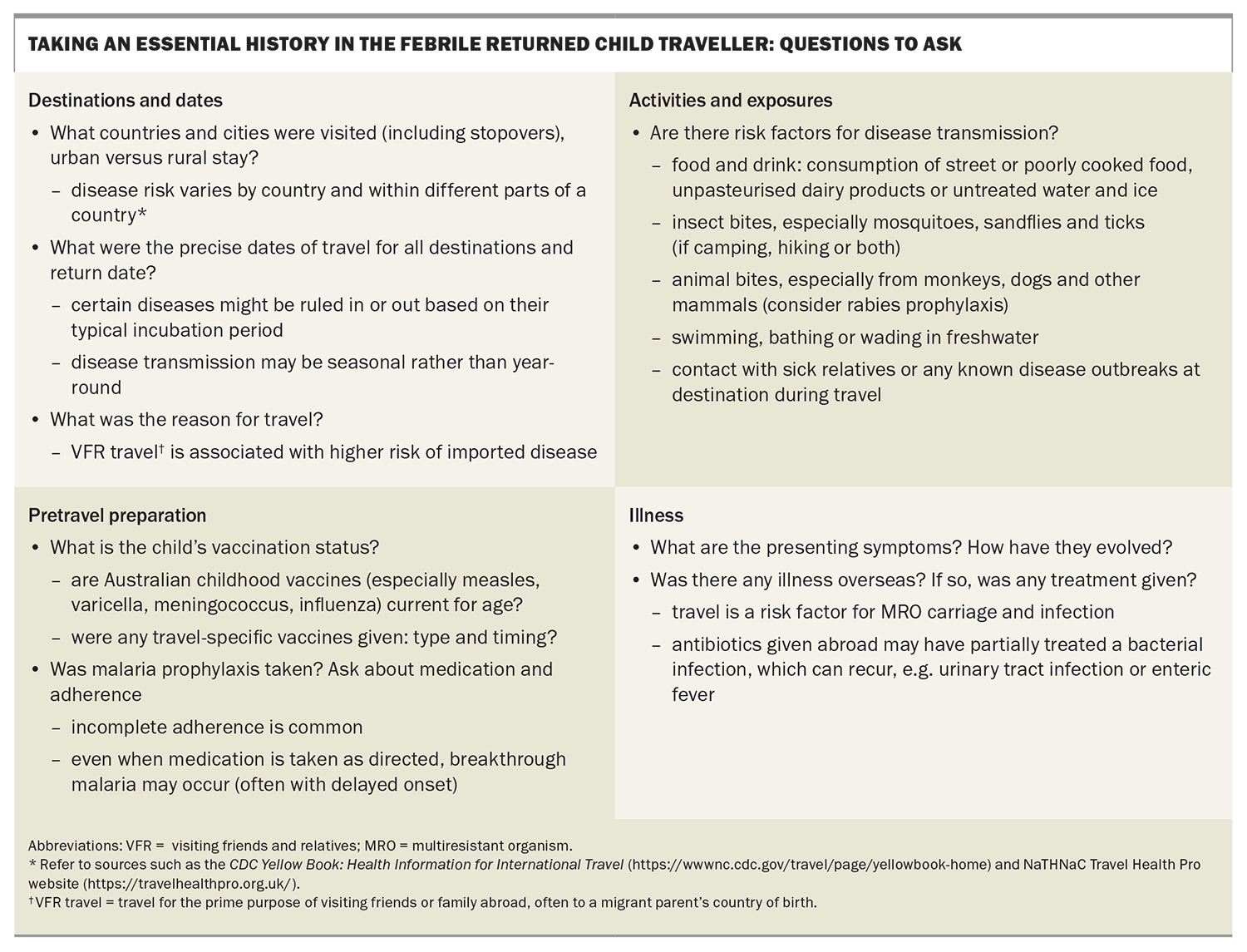
Taking a travel history is essential and includes recording the exact travel dates, destination(s) and activities performed during travel, as well as any pretravel vaccines or prophylaxis (Box). The reason for travel is also significant: diseases such as malaria and enteric fever are more common in travellers visiting friends and relatives (VFR) in endemic countries than in tourists.3 This is because VFR travellers (including children) are more likely to travel without seeking health advice and their exposure is often greater due to longer stays, having closer contact with local populations, visiting nonurban areas and eating riskier foods.4
Some travel-acquired infections can have long incubation periods (e.g. some forms of malaria). For any child presenting with unexplained fever, ask specifically about travel, both recent and past. Parents do not always volunteer this history, and travel may be forgotten by the time the child develops fever. Also check whether household members have travelled, as both tuberculosis (TB) and enteric fever have been reported in non-travelling children in close contact with infected adult travellers, commonly relatives visiting Australia from endemic countries.5,6
Take a precise history of any symptoms accompanying the fever. Even when fever is the only reported symptom, asking parents to review the timeline of illness may reveal a transient feature, such as a rash or red eyes, which can aid the diagnosis. Apparent localising symptoms do not rule out a systemic disease. Fever with florid diarrhoea or prominent cough and coryza are likely to be infectious gastroenteritis and acute respiratory infection, respectively. However, diarrhoea and cough can also occur in malaria, dengue and enteric fever, as can vomiting and headache.
Examination
A full systems examination is essential, although physical findings are often nonspecific for many imported and common childhood febrile illnesses. In particular, look for conjunctival signs (pallor, jaundice, injection), skin rash or mucosal lesions (appearance and distribution, including the presence of an eschar or insect-bite marks), organomegaly (particularly liver and spleen), lymphadenopathy (localised or generalised) and joint pain or swelling.
Consider destination-specific health risks
Expertise in tropical and travel medicine varies among medical practitioners and some may feel uncertain about which diseases need to be considered according to travel destination(s). The most common imported febrile infections seen in travellers from Australia are acquired in the tropics or subtropics (Table 1). However, exposure to novel diseases can also occur in temperate regions; for instance, hiking in forests or reserves in parts of Europe, North America and southern Africa is a risk factor for tick-borne infections (rickettsial and flaviviral). Disease risk within a region or country is often focal rather than uniform and may also vary over time due to changing economic, political or climatic conditions. For example, periodic dengue outbreaks have occurred over the past decade in common tourist areas in the Mediterranean because of the expanding geographical range of Aedes mosquitoes.7
Two useful online resources that provide current information about countryspecific disease risks are the US Centers for Disease Control and Prevention’s (CDC) CDC Yellow Book: Health Information for International Travel (https://wwwnc.cdcgov/travel/page/yellowbook-home) and the UK National Travel Health Network and Centre’s (NaTHNaC) Travel Health Pro website (https://travelhealthpro.org.uk/). Advice is usually available from local travel medicine or infectious diseases (ID) services; the major metropolitan children’s hospitals provide on-call paediatric ID support.
Investigations
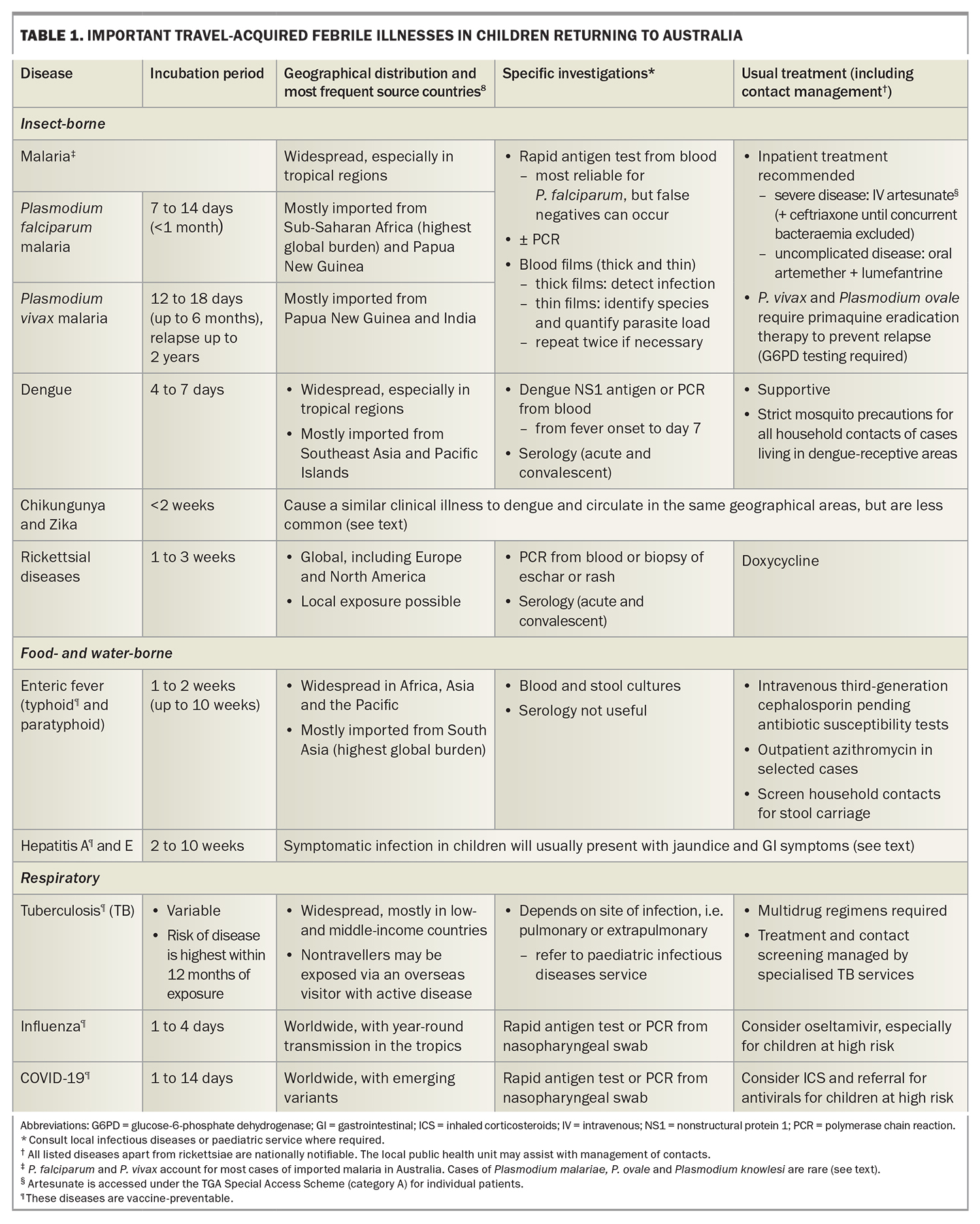
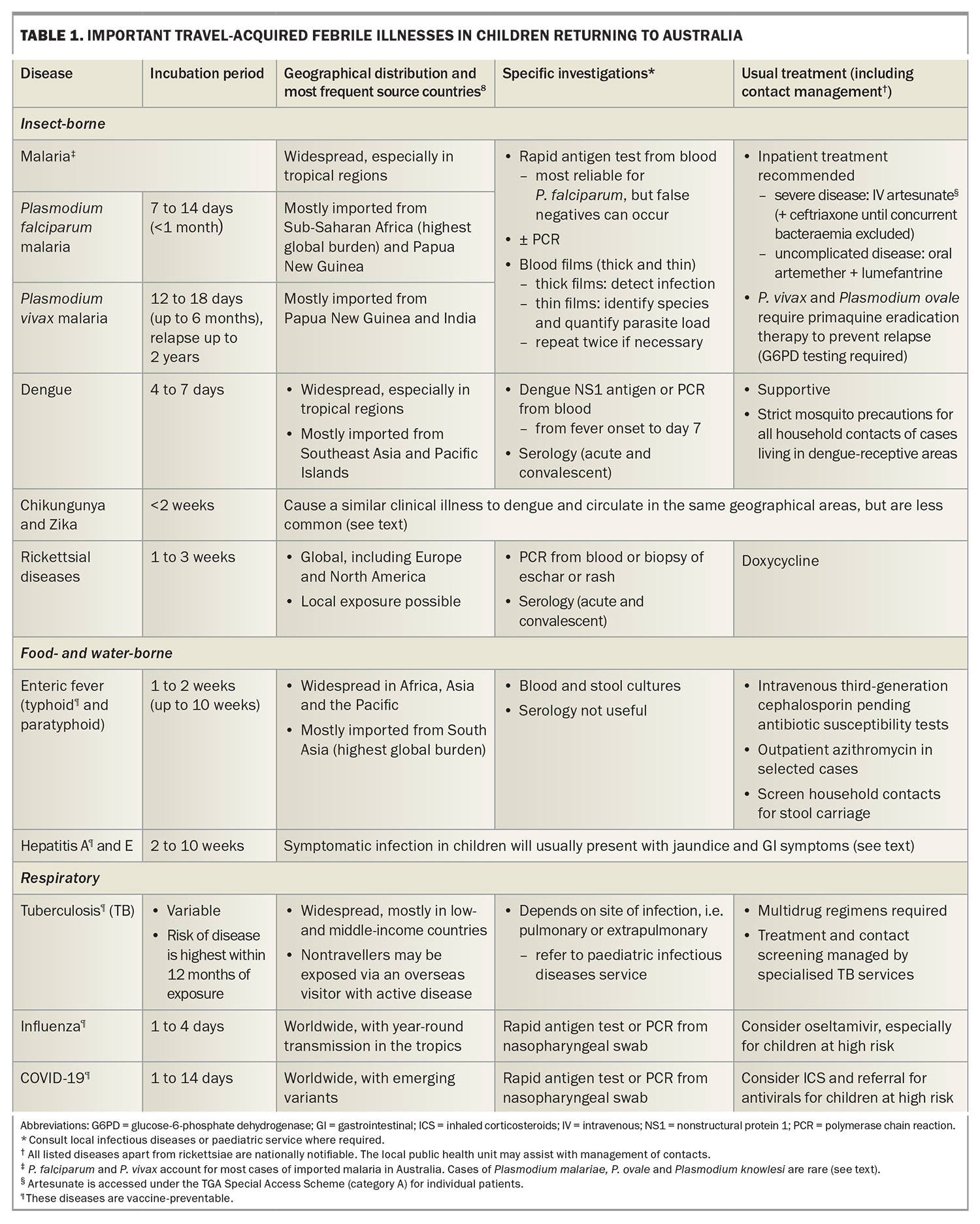
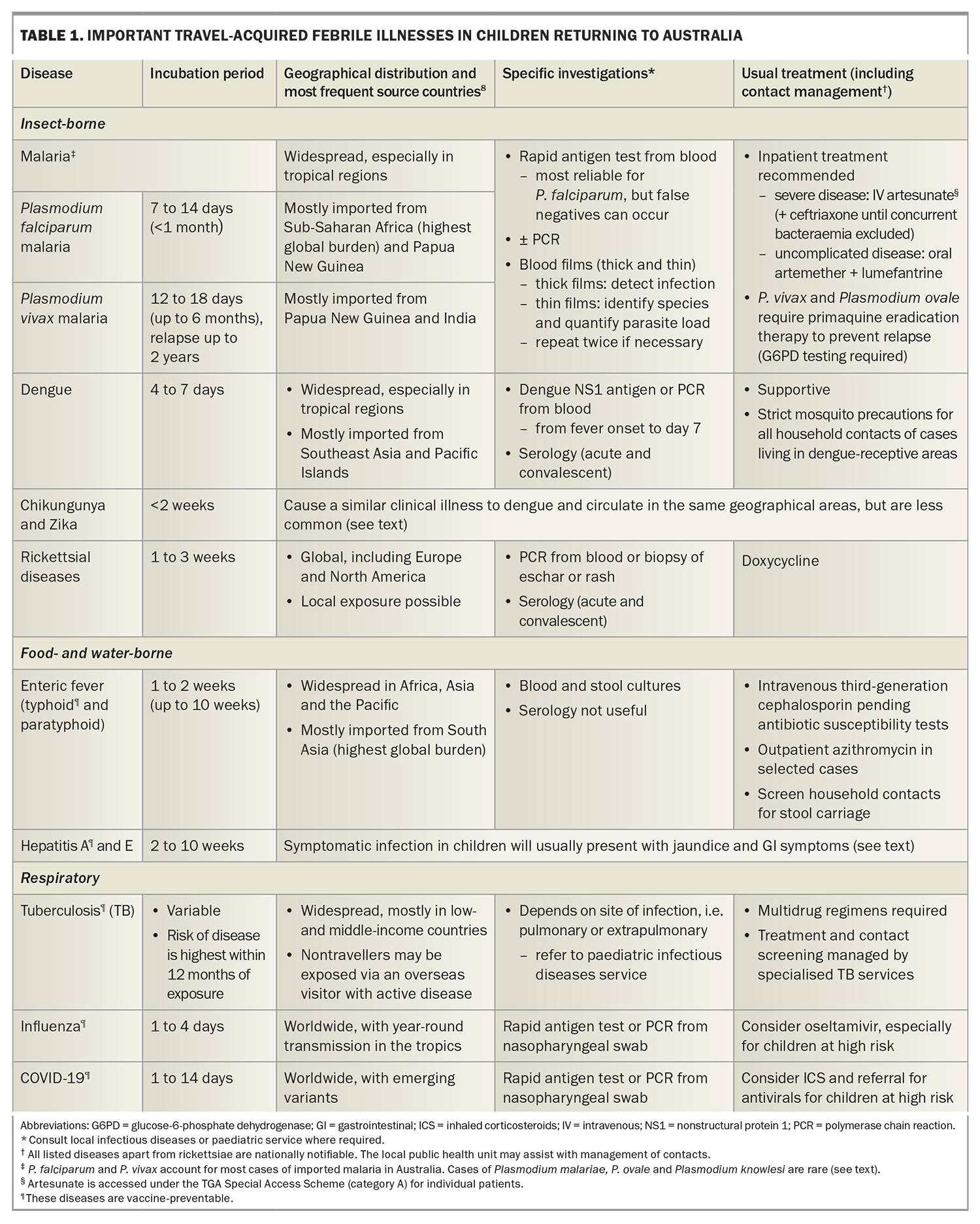
The approach to investigations for a child with unexplained fever after international travel is summarised in the Flowchart. For children returning from low-risk travel, such as a European winter holiday, watchful waiting and review may be appropriate initially, with investigations guided by symptom evolution or fever duration. However, for children at risk of serious imported infections (including most travellers to the tropics), the threshold for investigation is low, even for fully vaccinated children who do not appear unwell. Appropriate early tests ensure prompt diagnosis and treatment, and a timely public health response. Important imported febrile diseases to consider in child travellers are summarised in Table 1.8
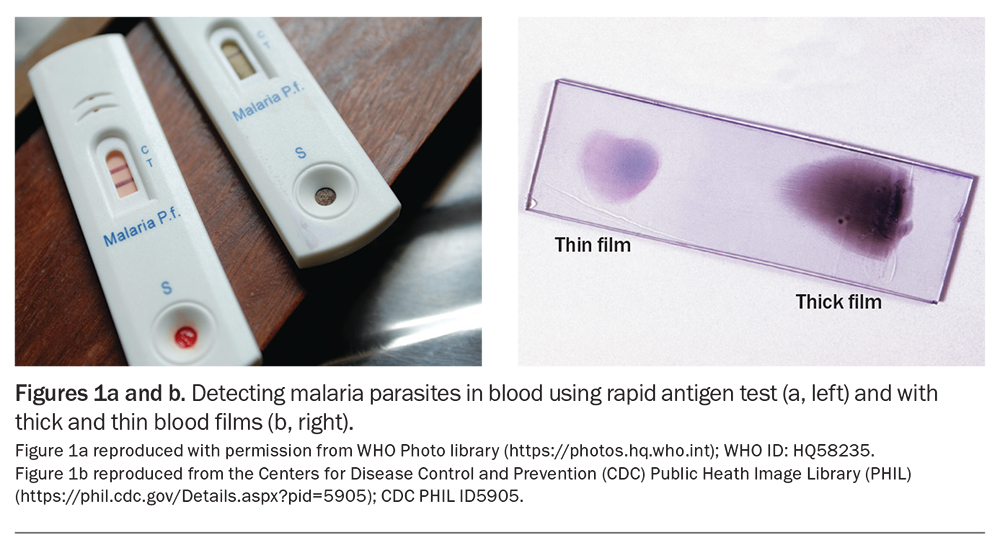
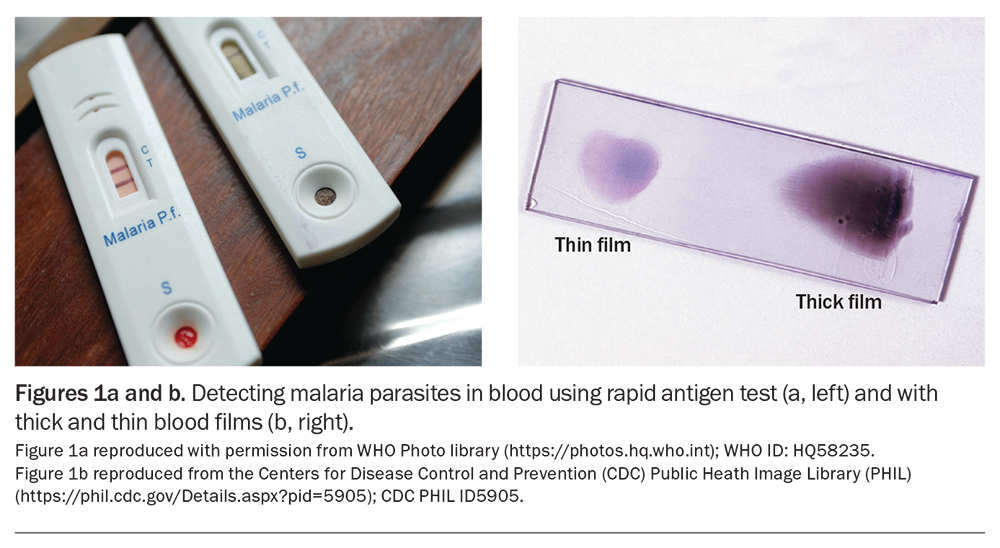
Children returning from areas endemic for malaria, enteric fever or dengue fever should have full blood count and liver function tests, a blood and stool culture collected, a malaria rapid antigen test (Figure 1a) and blood films (thick and thin; Figure 1b). The appropriate dengue test(s) will depend on the duration of fever (Table 1). Consider requesting serum storage in case paired acute and convalescent sera are later required for serological confirmation of an infection. If there is concern for TB, rickettsial disease or a rare imported infection (Table 2), seek ID specialist advice.
For most children, the work up can occur in the community, with scheduled follow up within 48 hours. However, for children returning from a highly endemic malarious area within the past three months, investigation in a hospital setting is recommended to expedite results.
Infections such as influenza, COVID-19, measles, varicella and invasive meningococcal disease can be travel-associated and should be tested for as indicated. Influenza in travellers may present interseasonally and be acquired in the northern hemisphere winter (Australian summer) or in the tropics, where transmission is year-round. Fever, vomiting and myalgia can precede respiratory symptoms. Measles and varicella are still endemic in many countries and unvaccinated infants are susceptible. Partially vaccinated children may experience milder disease, with more subtle clinical features. Travel to the ‘meningitis belt’ of Africa or participation in international events, such as the World Scout Jamboree, World Youth Day, the Hajj or Umrah, is a risk factor for invasive meningococcal disease. Although the quadrivalent meningococcal ACWY vaccination is mandatory to attend some events (e.g. Hajj or Umrah), clusters of meningococcal B and meningococcal X disease have occurred at mass gatherings.9
Finally, regardless of the destination travelled, and particularly in young children, consider testing for other common childhood febrile illnesses that are incidental to travel (e.g. urinary tract infection and glandular fever), as well as noninfective causes of persistent fever, such as Kawasaki disease, paediatric inflammatory multisystem syndrome temporally related to SARS-CoV-2 infection (PIMS-TS) and rheumatological conditions.
Even in children without a fever, testing for the underlying microbial cause for an obvious focal infection is recommended as travel can be associated with unusual or multidrug-resistant organisms, and standard empiric therapy may prove ineffective. Examples include skin infection in a child visiting the Pacific Islands caused by Haemophilus ducreyi, or a urinary tract infection caused by extended-spectrum beta-lactamase- producing Escherichia coli. For infections that are managed symptomatically, such as gastroenteritis, recent travel also increases the likelihood of a cause for which treatment might be considered, such as Giardia duodenalis or Shigella.
Review and management: specialist referral and public health notification
Travellers investigated in the community who return a positive blood or stool culture for Salmonella enterica serovars Typhi or Paratyphi should be referred to hospital for intravenous antibiotic treatment pending antibiotic susceptibility results. Some children with confirmed nonfalciparum malaria or dengue infection may continue to be managed in the community in consultation with local paediatric ID services. Although many children with persistent fever and negative initial screening test results will have a nonspecific and self-limiting illness, these cases should also be discussed with a specialist; investigations for less common conditions may be suggested.
Many imported infections are notifiable, with variations in reporting between state health departments. Most are laboratory-reportable; however, if strong clinical suspicion of a transmissible or notifiable infection exists, early contact with the local public health unit before the diagnosis is confirmed is recommended to expedite follow up of the patient and their contacts.
Common imported febrile illnesses in children
Malaria
Malaria is caused by the parasitic protozoan Plasmodium and spread by infected Anopheles mosquitoes, which usually bite from dusk to dawn. The highest global burden of disease is in Sub-Saharan Africa, but malaria is also endemic in parts of Asia, the Pacific Islands (west of Fiji), and Central and South America. Five Plasmodium species cause malaria in humans: P. falciparum and P. vivax are responsible for most cases imported into Australia; P. malariae and P. ovale are less common; and P. knowlesi, a zoonotic form from Southeast Asia, is rare.
Although childhood malaria vaccination has commenced in parts of Africa, prevention in travellers is still through mosquito avoidance and chemoprophylaxis, with effective medications available for children of all ages. Malaria is more common in newly arrived migrants and returned VFR travellers from endemic countries, who are less likely to take prophylaxis. In 2016, 305 cases of malaria were imported into Australia, most frequently from travellers returning from countries in Sub-Saharan Africa (54%), Papua New Guinea and the Solomon Islands (18%), India and Pakistan (11%) and Indonesia (5%).8
Symptoms are nonspecific and include fever, headache, chills, vomiting and, sometimes, cough or diarrhoea. Pallor and splenomegaly may be present. Symptoms usually start seven to 40 days after the infective mosquito bite, depending on the species. The use of prophylaxis can delay symptoms. P. falciparum has the shortest incubation period (usually one to two weeks) and is the most severe form. Untreated, it can rapidly progress and cause severe anaemia, metabolic acidosis, hypoglycaemia, seizures, coma or death, particularly in children under 5 years of age. For this reason, in-hospital malaria testing is recommended for children returning from a malaria-endemic area within the past three months.
Severe disease is uncommon with P. vivax or P. ovale, but relapses can occur months to years after the first infection due to the reactivation of dormant liver forms of the parasite known as hypnozoites (absent in P. falciparum and P. malariae). Any patient with a history of unexplained prolonged or recurrent fever who has spent time in a malaria-endemic region within the past two years should be tested for malaria, even if they are afebrile at the time of review.
Anaemia and thrombocytopenia are common on full blood count (FBC) examination. Specific diagnosis is by detection of Plasmodium-specific DNA on a polymerase chain reaction (PCR) or rapid antigen test and microscopy of thick and thin blood films (gold standard, Figure 1b) from whole blood. Negative initial results should be repeated if there is strong clinical suspicion of malaria.
The current Australian recommendation for treatment for all malaria species is intravenous artesunate (sourced through the TGA Special Access Scheme for individual patients) for severe infection (with ceftriaxone until coexistent bacteraemia has been excluded), and oral artemether- lumefantrine for uncomplicated disease.10 Initial inpatient treatment of P. falciparum is recommended in children, regardless of severity. For P. vivax and P. ovale malaria, treatment is followed with a course of primaquine to eradicate liver hypnozoites and requires prior exclusion of glucose- 6-phosphate dehydrogenase deficiency to avoid haemolysis which may occur with primaquine administration.
Dengue
Dengue is caused by a flavivirus spread via Aedes mosquitoes (chiefly Ae. aegypti and Ae. albopictus), which are peridomestic and bite during the daytime, mostly in the morning and afternoon. Dengue is endemic to tropical countries in the Asia-Pacific region and Central and South America, and also occurs in Africa. In 2016, 80% of the 2193 dengue infections imported into Australia were acquired in Southeast Asia or the Pacific, with Indonesia the most frequent specific country (60%), followed by Papua New Guinea and Thailand (5% each).8 However, the geographical range of Aedes mosquitoes is expanding into subtropical and temperate regions, such as North Queensland and the Mediterranean, and local clusters can occur in these ‘dengue-receptive’ areas if dengue is introduced by a viraemic traveller.
Four dengue serotypes (DENV-1 to 4) infect humans. Infection induces life-long type-specific immunity but reinfection with a new serotype can result in more severe disease due to a process called antibody-dependent enhancement (ADE). ADE can also occur in dengue-naïve individuals who are vaccinated and subsequently encounter natural infection, which is why the current dengue vaccine is unsuitable for most Australian travellers. Prevention is through mosquito avoidance measures.
Dengue has a short incubation period (four to seven days) and patients usually present with fever within a week of returning from an endemic area. Young children often have nonspecific symptoms; older children may report more typical symptoms, including headache (retro-orbital), vomiting and myalgia or arthralgia, often associated with a maculopapular rash. A minority develop haemorrhagic complications or vascular leakage with shock, which typically begins 48 hours after fever defervescence and can be fatal. Infants are at higher risk of severe disease.
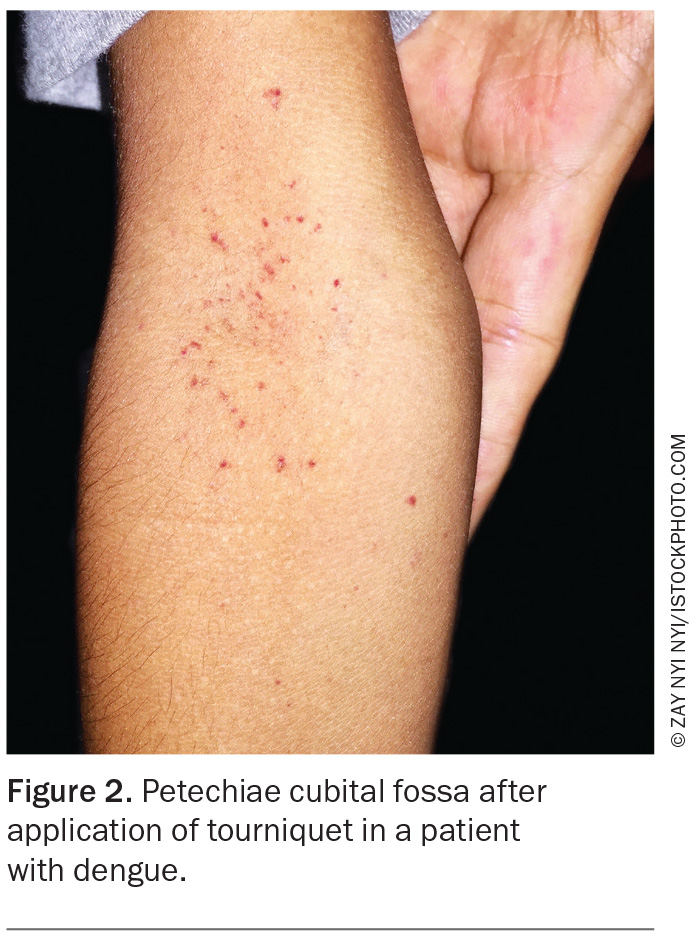
Leucopenia and thrombocytopenia are common on FBC, and the appearance of crops of petechiae on the skin distal to the tourniquet after venepuncture is suggestive of infection (Figure 2). Clinical diagnosis can be confirmed in the acute febrile phase by detecting dengue RNA (via PCR) or antigen (nonstructural protein 1 [NS1] protein) in blood. Serology (dengue IgM) may not be positive until the convalescent phase, and careful interpretation is needed to exclude false positives from prior exposure to other flaviviruses through infection (e.g. Zika or Murray Valley encephalitis viruses) or immunisation (e.g. Japanese encephalitis or yellow fever vaccines).
Treatment is supportive. Mild cases resolve spontaneously, but vigilance is required for warning signs (severe abdominal pain, persistent vomiting, mucosal or gastrointestinal bleeding, tachypnoea, fatigue or irritability), even after the fever resolves, as this is when complications often occur. Patients with complications need to be closely monitored in hospital, with special attention to fluid management.
Enteric fever (typhoid and paratyphoid infection)
Nontyphoidal Salmonella infections primarily cause gastroenteritis, whereas infection with S. Typhi (causing typhoid fever) and serovars Paratyphi A, B and C (causing paratyphoid fever) results in a bacteraemic febrile illness. Transmission is via the faecal–oral route, mostly through contaminated food and water, rather than direct person-to-person contact. The peak incidence worldwide occurs in the 5- to 9-year age group.11 Enteric fever is endemic throughout Asia, Oceania and Africa, particularly where sanitation is poor and access to clean drinking water limited. In Australia, most cases occur in returned VFR travellers from South Asia, which has the highest global burden of disease.12
The incubation period is usually between one to two weeks but can be as long as 60 days. Persistent fever is the most common presentation, often with intermittent and variable gastrointestinal symptoms (abdominal pain, vomiting, constipation or diarrhoea). Other nonspecific symptoms include headache, lethargy, cough and muscle and joint pains. Encephalopathy and gastrointestinal perforation are late complications. The characteristic ‘rose spot’ rash (2 to 4 mm blanching erythematous maculopapular lesions, usually on the trunk) is rarely seen.
The FBC may show mild anaemia, sometimes with mild leucopenia or thrombocytopenia. Liver function tests often show a mild transaminitis. Diagnosis relies on culturing the organism from blood or stool, and both specimens should be requested for any returned child traveller from South Asia with persistent unexplained fever, even if they otherwise appear well and have no diarrhoea. Sensitivity is poor (60% and 30% for blood and stool culture, respectively), but multiple samples can optimise the yield. The Widal serology test performs poorly and should not be used.
There is widespread resistance to historical first-line antibiotics, such as amoxicillin, cotrimoxazole and fluoroquinolones, and initial empiric treatment with an intravenous third-generation cephalosporin in hospital is recommended until the organism’s antibiotic susceptibility profile is known. Initial treatment with carbapenems is sometimes used for unwell children who have acquired enteric fever in Pakistan, where there is risk of contracting extensively drug-resistant S. Typhi.13 Once fever resolves, oral azithromycin is often used to complete the treatment course, but note that azithromycin resistance is also emerging in India.14 Asymptomatic chronic carriage occurs in 1 to 4% of those who are infected. ‘Clearance’ stool cultures may be required for some cases after treatment, and for asymptomatic cotravellers and close household contacts with high-risk occupations (food handlers and health, aged care and childcare workers).
Typhoid infection may be prevented with vaccination; however, current vaccines do not protect against paratyphoid infection. Conjugate typhoid vaccines used for infants overseas are not currently available in Australia. Here, an oral live attenuated vaccine is licensed for use in children from 6 years of age, and a parenteral polysaccharide vaccine from 2 years of age (but is recommended and safe in high-risk travellers from 18 months of age). Both vaccines are moderately protective and require revaccination after three to five years but should be offered to all travellers to high-risk areas, particularly VFR travellers to South Asia, where there is increasing drug resistance.
Hepatitis A and E
Hepatitis viral infections are endemic in most parts of the world, including some parts of Australia, and are usually contracted by exposure to contaminated food or water. In 2016, travellers from Australia most frequently acquired infection in South Asia.8 The median incubation period is 30 to 40 days (range two to 10 weeks). Infection is often subclinical in young children; older children may present with jaundice and gastrointestinal symptoms (abdominal pain, vomiting or diarrhoea), with or without fever. Transaminitis is usually not severe, although fulminant hepatitis can occur, especially in those with pre-existing hepatic disease. Detection of viral RNA in the blood or serology (detection of hepatitis A or E IgM) confirms the diagnosis. Treatment is supportive. Hepatitis A is preventable by a highly effective vaccine, which is available for children from 12 months of age. Nonimmune contacts of cases should be offered vaccination.
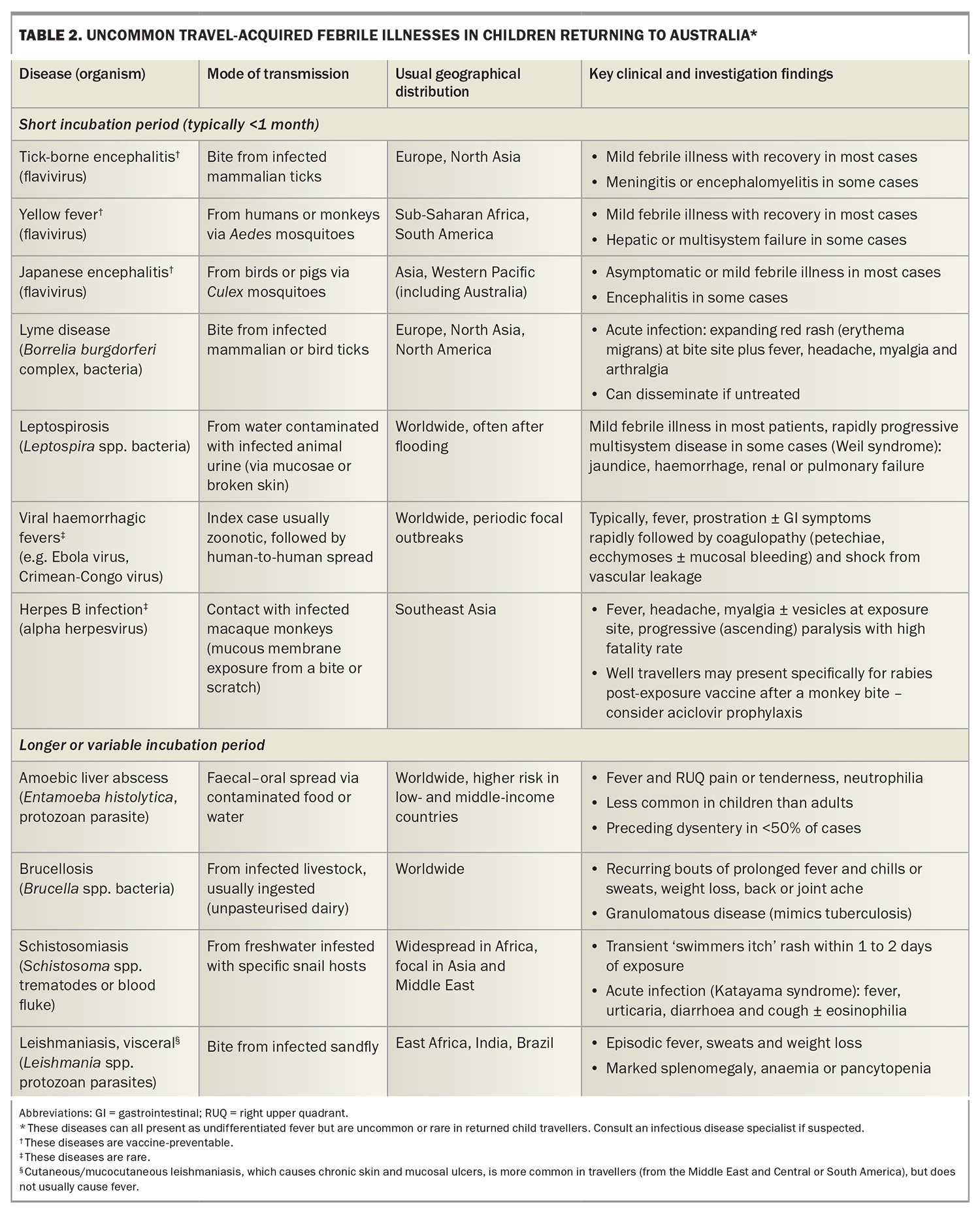
Rickettsial diseases
Rickettsial infections are caused by a group of closely related intracellular bacteria (Rickettsia spp. and Orientia spp.) that are found globally and transmitted by arthropods. They include the spotted fevers, which are spread by ticks (e.g. Rocky Mountain spotted fever, African tick-bite fever), and scrub typhus and murine typhus, which are spread by mites and fleas, respectively. Camping and hiking in national parks or game parks in endemic areas are particular risk factors for rickettsial infections, which can also be acquired domestically (e.g. Queensland tick typhus).
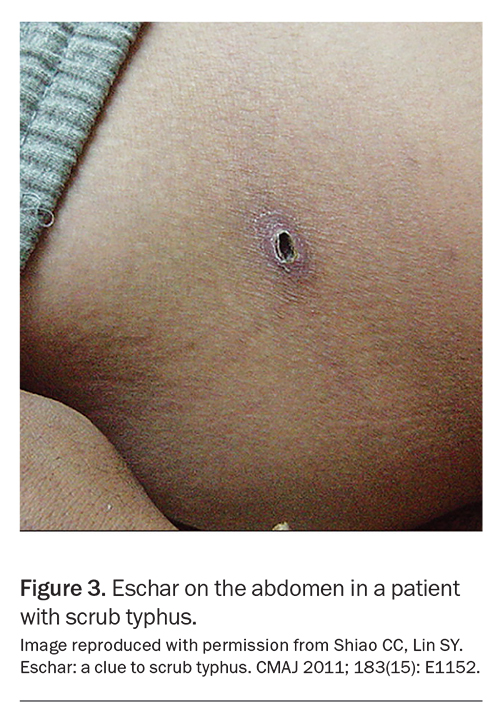
Patients typically present with fever, rash, headache and myalgia within five to 10 days of the bite. The rash is usually generalised (may involve the palms) and can be maculopapular, petechial or vesicular (or sometimes absent). There may be a necrotic scab (eschar) at the bite site (Figure 3) and localised or generalised lymphadenopathy. Severe infection can cause pneumonitis, encephalitis or multiorgan failure; thus, empiric antibiotics are often started based on high clinical suspicion, as early confirmation of diagnosis can be challenging.
Blood tests may show leucopenia or thrombocytopenia and biochemical hepatitis. Rickettsial DNA may be detected in a biopsy of the eschar or rash or, less commonly, in whole blood. Diagnosis is more often retrospective, by demonstrating seroconversion on acute and convalescent phase blood samples. Doxycycline is the most effective treatment and is recommended for children of all ages.
Tuberculosis
Mycobacterium tuberculosis infection has high prevalence in many parts of the world, with the risk of infection related to the proximity and duration of exposure to people who are infectious. Children, especially infants, are more susceptible than adults. Investigations for TB should be carried out in any child who has stayed in areas of high endemicity and presents with any symptom or sign compatible with TB, including fever, weight loss, lethargy, persistent lymph node enlargement or chronic cough. Discussion with a paediatric ID specialist or the local TB service is recommended to ensure appropriate testing and management. BCG vaccination reduces the risk of TB meningitis and disseminated TB in children younger than 5 years of age and is recommended if visiting a high-prevalence TB country for longer than a month.
Zika and Chikungunya infection
Zika (a flavivirus), and Chikungunya (an alphavirus), are spread by the same mosquito vectors as dengue and cause similar acute febrile illnesses. Although all three viruses circulate in the same geographical areas, Australian travellers most often acquire dengue in Southeast Asia, Zika in Polynesia and Chikungunya in South Asia.9 Prevention is by avoiding mosquito bites, although Zika virus is also sexually transmitted.
Zika virus has a longer incubation period than dengue (three to 14 days) and often causes prominent conjunctival injection in addition to nonspecific viral symptoms. Children usually experience very mild illness. Infection during pregnancy has been associated with severe birth defects such as microcephaly. Chikungunya has an incubation period of two to 12 days. It causes fever, rash and significant polyarthralgia, although joint signs, such as swelling and stiffness, are more common in adults than children.15 Joint pain may persist for months. PCR of viral RNA in blood (or urine for Zika) during the acute febrile phase, or by paired acute and convalescent serology thereafter, confirms the diagnosis. There is no specific treatment and most patients recover completely.
Conclusion
Fever is common in children after overseas travel. Although often due to a self-limiting or common childhood condition, it can signify a serious imported infection. A detailed travel history and examination guides appropriate investigations. Malaria, dengue and enteric fever are the most common imported infections in children and should be excluded in travellers returning from endemic areas; VFR travellers are at higher risk than tourists. Consultation with an ID physician or paediatrician is recommended if there is uncertainty about diagnosis, investigations or treatment, even for children well enough to be managed in the community. Pretravel health advice regarding safe eating and drinking and avoiding insect bites, and specific travel immunisation and malaria prophylaxis can help reduce febrile illness in child travellers. MT
COMPETING INTERESTS: Dr Mazzacato: None. Dr Khatami has received research grants from the Australia Medical Research Future Fund and the Australian National Health and Medical Research Council; holds unpaid positions as the Paediatric Representative for the Australasian Society for Infectious Diseases Clinical Research Network, Scientific Advisory Committee member for the Sydney Children’s Hospitals Network Human Research Ethics Committee, Sydney Children’s Hospitals Network Advanced Therapeutics Committee phage therapy content expert and Deputy Director (clinical) for Phage Australia.
References
1. Australian Bureau of Statistics (ABS). Overseas Arrivals and Departures, Australia. Canberra: ABS; 2023. Available online at: www.abs.gov.au/statistics/industry/tourism-and-transport/overseas-arrivals-and-departures-australia (accessed November 2023).
2. Chong CH, McCaskill ME, Britton PN. Pediatric travelers presenting to an Australian emergency department (2014-2015): a retrospective, cross-sectional analysis. Travel Med Infect Dis 2019; 31: 101345.
3. Heywood AE, Zwar N, Forssman BL, et al. The contribution of travellers visiting friends and relatives to notified infectious diseases in Australia: state-based enhanced surveillance. Epidemiol Infect 2016; 144: 3554-3563.
4. Hendel-Paterson B and Swanson S. Paediatric travelers visiting friends and relatives (VFR) abroad: Illnesses, barriers and pre-travel recommendations. Travel Med Infect Dis 2011; 9: 102-203.
5. Khatami A, Khan F, Macartney KK. Enteric fever in children in Western Sydney, Australia, 2003-2015. Pediatr Infect Dis J 2017; 36: 1124-1128.
6. Al Yazidi LS, Marais BJ, Wickens M, et al. Overview of paediatric tuberculosis cases treated in the Sydney Children’s Hospitals Network, Australia. Public Health Res Pract 2019; 29: e28231807.
7. European Centre for Disease Prevention and Control (ECDC). Autochthonous vectorial transmission of dengue virus in mainland EU/EEA, 2010-present. Frösunda: ECDC; 2023. Available online at: www.ecdc.europa.eu/en/all-topics-z/dengue/surveillance-and-disease-data/autochthonous-transmission-dengue-virus-eueea (accessed November 2023).
8. NNDSS Annual Report Working Group. Australia’s notifiable disease status, 2016: Annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell (2018). 2021; 27: 45.
9. Badahdah AM, Rashid H, Khatami A, Booy R. Meningococcal disease burden and transmission in crowded settings and mass gatherings other than Hajj/Umrah: a systematic review. Vaccine 2018; 36: 4593-4602.
10. Malaria. In: Therapeutic Guidelines. Melbourne: Therapeutic Guidelines Limited; April 2019; amended May 2022. Available online at: https://www.tg.org.au (accessed November 2023).
11. GBD 2017 Typhoid and Paratyphoid Collaborators. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 2019; 19: 369-381.
12. Forster DP, Leder K. Typhoid fever in travellers: estimating the risk of acquisition by country. J Travel Med 2021; 28: taab150.
13. Howard-Jones A, Kesson AM, Outhred AC, Britton PN. First reported case of extensively drug-resistant typhoid in Australia. Med J Aust 2019; 211: 286-286.e1
14. John J, Bavdekar A, Rongsen-Chandola T, et al; NSSEFI Study Team. Burden of typhoid and paratyphoid fever in India. N Engl J Med 2023; 388: 1491-1500.
15. Imad HA, Phadungsombat J, Nakayama EE, et al. Clinical features of acute chikungunya virus infection in children and adults during an outbreak in the Maldives. Am J Trop Med Hyg 2021; 105: 946-954.