Pyrexia of unknown origin: a paradigm for investigation

Pyrexia of unknown origin encompasses a heterogenous group of conditions that occur in patients presenting to both primary care and emergency departments. Despite advancements in diagnostic investigations and imaging, diagnosis can often remain elusive. A thorough approach, serial clinical reviews and timely discussion with physicians experienced in appropriate workup may help GPs in rationalising investigations and subsequent management.
- Pyrexia of unknown origin (PUO) is defined by the presence of persisting fever above 38.3°C on at least three occasions for longer than three weeks, without a diagnosis despite basic diagnostic evaluation.
- Classic PUO is typically subdivided into infectious, noninfectious inflammatory, malignant and miscellaneous aetiologies.
- A thorough history and examination are paramount, and red flags for potentially life-threatening conditions should be actively excluded.
- Basic investigations can be complemented by targeted investigations following the history and examination; more advanced imaging and molecular testing may be useful but should be considered in consultation with infectious diseases or other specialists.
- Delay treatment in the stable immunocompetent patient until a diagnosis is reached, so management can be tailored appropriately.
Pyrexia of unknown origin (PUO; or fever of unknown origin, FUO) refers to a heterogeneous group of otherwise unrelated conditions that is seen in patients presenting with persistent fever and typically eludes diagnosis, despite basic diagnostic testing. Although inclusive of uncommon conditions, PUO more frequently involves common conditions that present atypically. Aetiologies pertain to a number of medical specialties; therefore, a thorough and persistent approach to clinical review, sequential clue-driven investigations and management is required.
History and criteria
‘Fever’ and ‘pyrexia’ have been described since at least the 10th century BCE, where an elevated body temperature associated with tachycardia was considered a sign of disease and, often, infection. An interesting and more complete history of PUO is summarised by Wright and Auwaerter.1 Through to the present day, presentation of patients with PUO remains a common clinical issue in both primary and emergency care settings.
The term PUO was first described in 1961 in a case series of 100 patients presenting with:
- at least three recorded fevers measuring higher than 38.3°C
- febrile illness of at least three weeks’ duration
- no diagnosis despite one week of inpatient investigations.2
Although defined primarily for research settings, the criteria aimed to exclude self-limiting viral infections and diurnal temperature variations, and allow adequate time for investigations. Given the potential for outpatient investigations, the time criterion was revised to three inpatient days’ equivalence.3 Subsequent revision of the criteria for PUO shows that it varies by geographical location, proportion of the population that is ageing and the availability of imaging, including CT and positron emission tomography. At its core, a true PUO refers to a significant febrile illness that persists for a prolonged period of time, without any clear cause demonstrated despite extensive investigations.4
This article focuses on ‘classic PUO’ investigations; however, a wider range of diagnoses should be considered for patients who are immunosuppressed and those recently hospitalised. Other recent reviews also highlight the nuances of ‘travelassociated’ and ‘periodic’ fevers of unknown origin, which shift the proportion of potential aetiologies.5 Classic PUO is traditionally grouped as infectious, noninfectious inflammatory (connective tissue) disorders, malignant or miscellaneous.
The febrile response
A brief understanding of the body’s temperature regulation mechanisms assists with understanding the varied causes of febrile illnesses. Thermoregulation occurs in response to pyrogens, which may include hormones (thyroid hormone, catecholamines, glucocorticoids) or cytokine production from tumours, micro-organisms or tissue necrosis.6 Broadly speaking, exogenous pyrogens (such as endotoxins from bacteria and viruses) cause the production of endogenous pyrogens (interleukin-1, interleukin-6, tumour necrosis factor, interferons) from innate immune cells, which act on endothelial and neuronal cells of the preoptic region of the anterior hypothalamus. This produces local prostaglandin E2, thus resetting the body’s temperature ‘setpoint’. What ensues includes noradrenaline and acetylcholine release causing vasoconstriction and brown adipose tissue thermogenesis, respectively, manifesting as myocyte-induced shivering, piloerection and heat- or cool-seeking behaviours, among other effector responses.5,7 Involvement anywhere along this pathway can produce a febrile response.
Causes
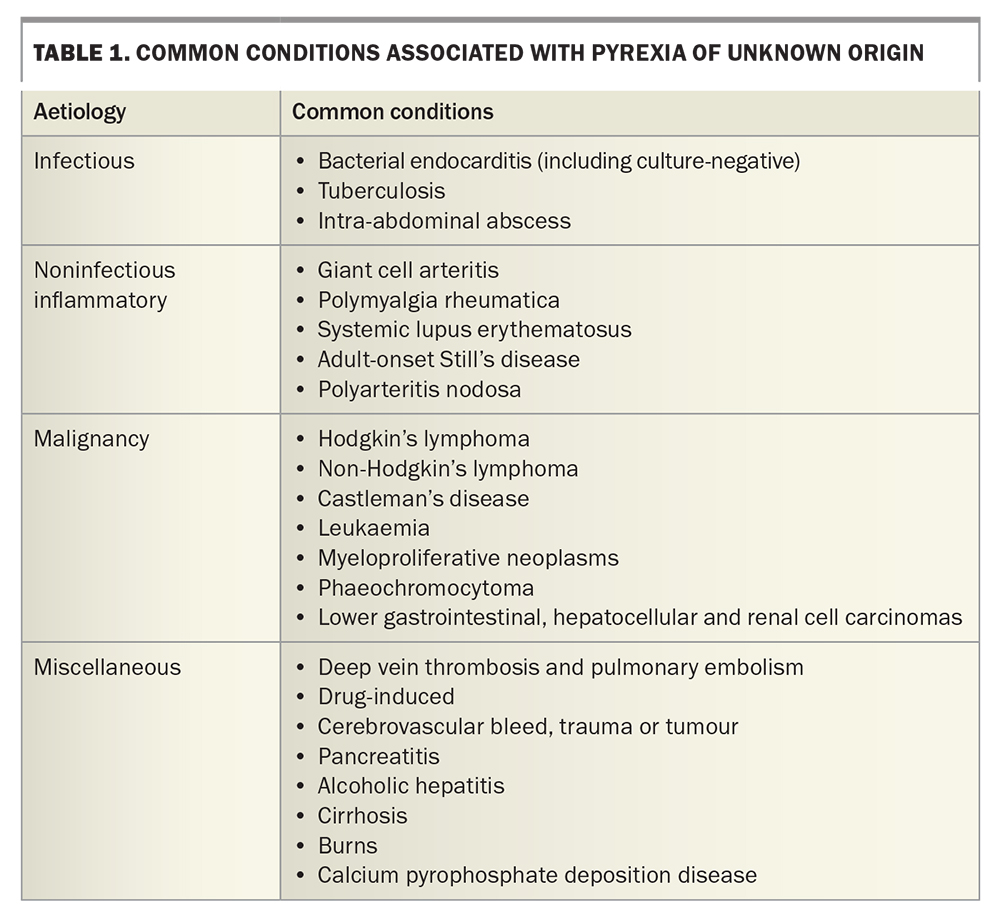
As mentioned above, aetiologies of classic PUO are subdivided into four groups (Table 1). The proportion of each group has changed with time and depends on patient age, relevant risk factors and the availability of imaging and other investigations. Since PUO was first described, there has been an overall trend towards a greater proportion of miscellaneous and undiagnosed causes, with a recent study quoting up to 50% of PUO as being undiagnosed.8
Infectious causes of PUO include occult abscess, endocarditis, tuberculosis and complicated urinary tract infections.4,7 In a returned traveller, common infectious causes should be considered alongside typhoid fever, acute HIV infection, gastroenteritis and amoebic liver abscesses.5 Of noninfectious inflammatory causes, polymyalgia rheumatica, giant cell arteritis, adult-onset Still’s disease and systemic lupus erythematosus should be considered, given that delays in diagnosis may be potentially organ- or life-threatening.9 Haematological malignancies (Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, myeloproliferative neoplasms) are represented more often than solid organ tumours; lower gastrointestinal, hepatocellular and urinary tract tumours are prominent in the latter group.10
Medications can also cause fevers, which can be present alongside peripheral eosinophilia and abnormal liver function tests. Fever may resolve within seven days of drug cessation. Other miscellaneous causes should be considered if there is any suspicion of abnormal hypothalamic function (cerebrovascular bleed, trauma, tumour), decreased heat loss (anticholinergic medications, drug abuse, heat stroke) or increased heat production (malignant hyperthermia, serotonin syndrome, thyrotoxicosis). Venous thromboembolic disease, pancreatitis, burns and factitious fever are important differentials.6
Presentation
A careful history and examination looking for so-called ‘potentially diagnostic clues’ is recommended. PUO literature often suggests that clinicians follow Sutton’s Law (after Willie Sutton, who robbed banks because ‘that’s where the money is’), which refers to pursuing investigations in order of diagnostic probability – consider the most likely diagnosis first.2 Patients with PUO may not necessarily have a rare condition, rather, otherwise common conditions may present atypically as a PUO.
The presence of objective fevers should first be confirmed, ideally with a recording taken by the patient or in the clinic room, and a fever diary may be helpful to comment on the degree, duration and timing.
History
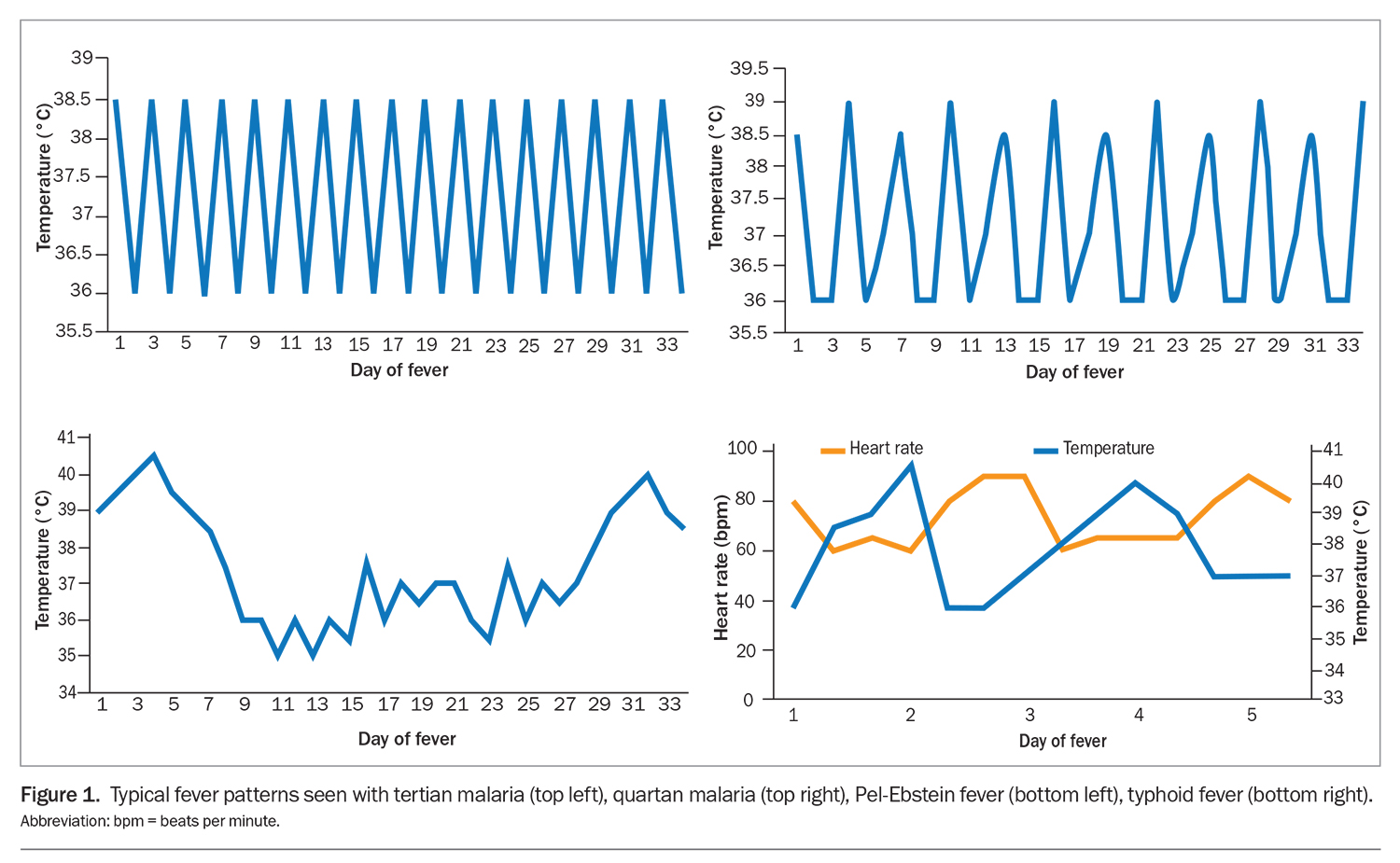
Note the onset, acuity, duration and timing of fevers. Although not specific, consider particular fever patterns, such as the tertian or quartan fevers of malaria, cyclical Pel-Ebstein fevers of lymphoproliferative malignancies or pulse-temperature dissociation seen in conditions such as typhoid fever (Figure 1). Similarly, establish the presence and duration of sweats, rigors or weight loss.7
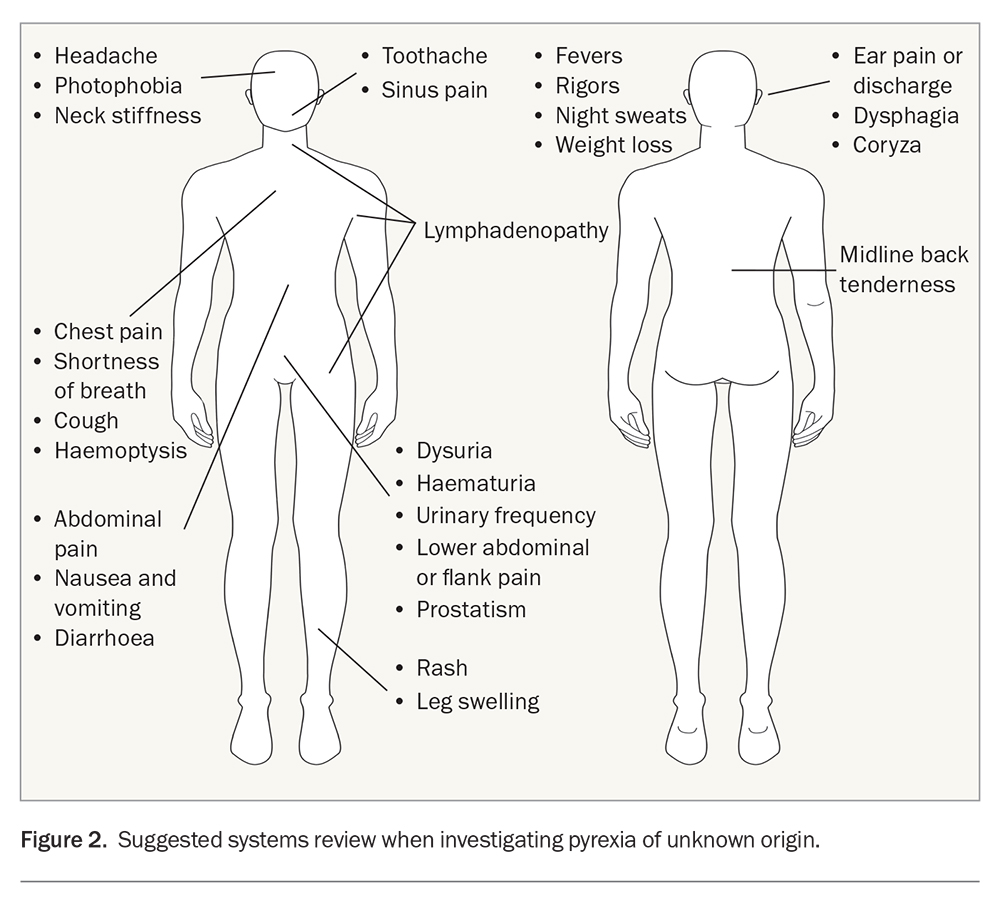
Screen for localising symptoms in a systems review (Figure 2); these may have occurred subtly early in the illness, prior to the onset of fevers. Never underestimate collateral history from family, friends or carers, particularly in the context of PUO.11
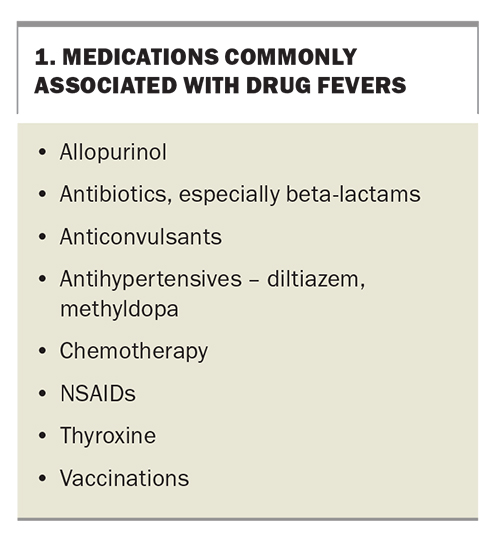
When taking a medical history, pay particular attention to previous fevers or infections, or known immunosuppression. Recent surgeries and the presence of drains, prostheses or other foreign material should be noted. Review all regular, over-the-counter and ‘as needed’ medications, alongside any herbal supplements and allergies. Common medications associated with drug fevers are listed in Box 1. A family history malignancy, autoimmunity, ethnicity and familial fevers should also be sought.
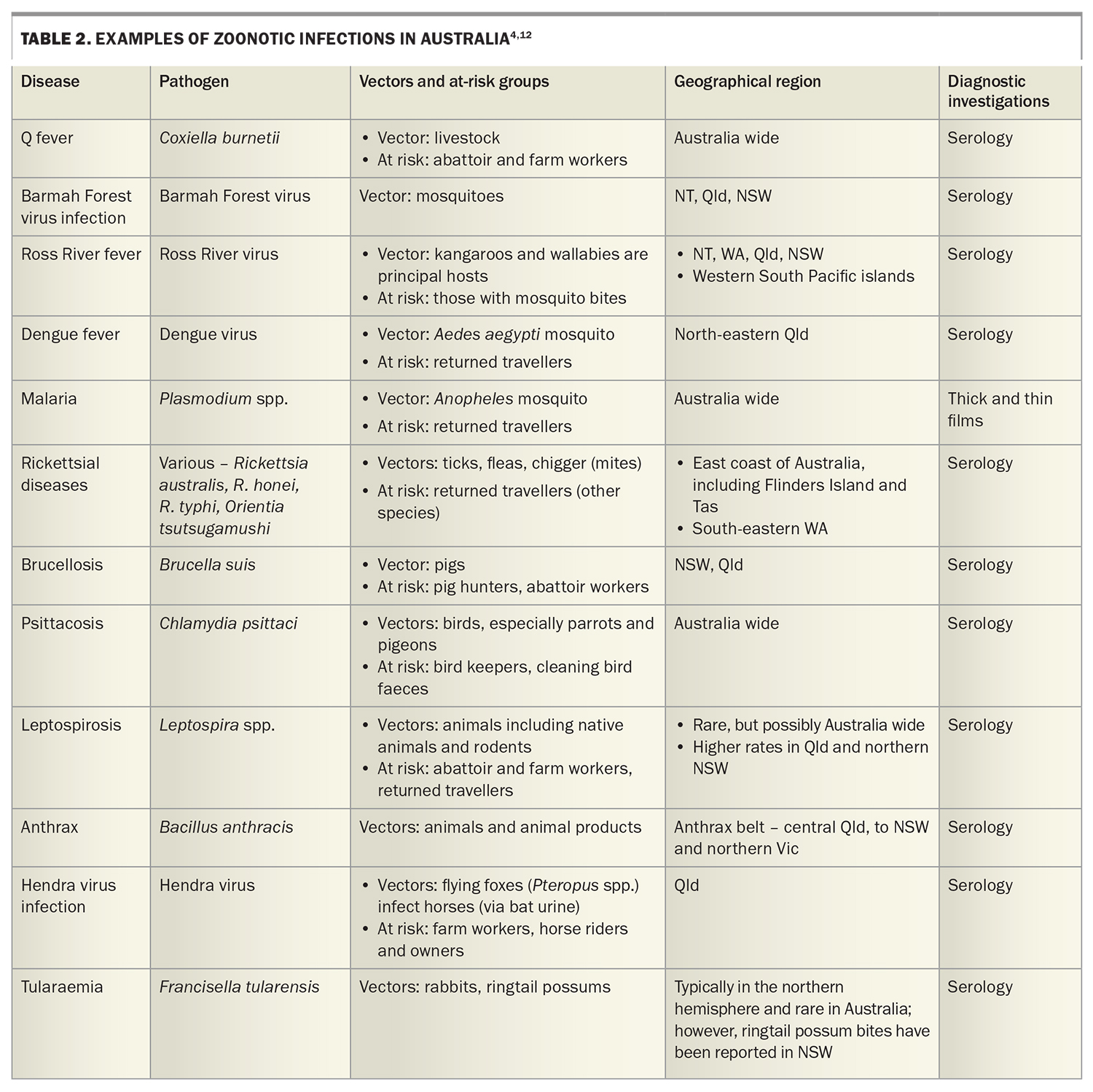
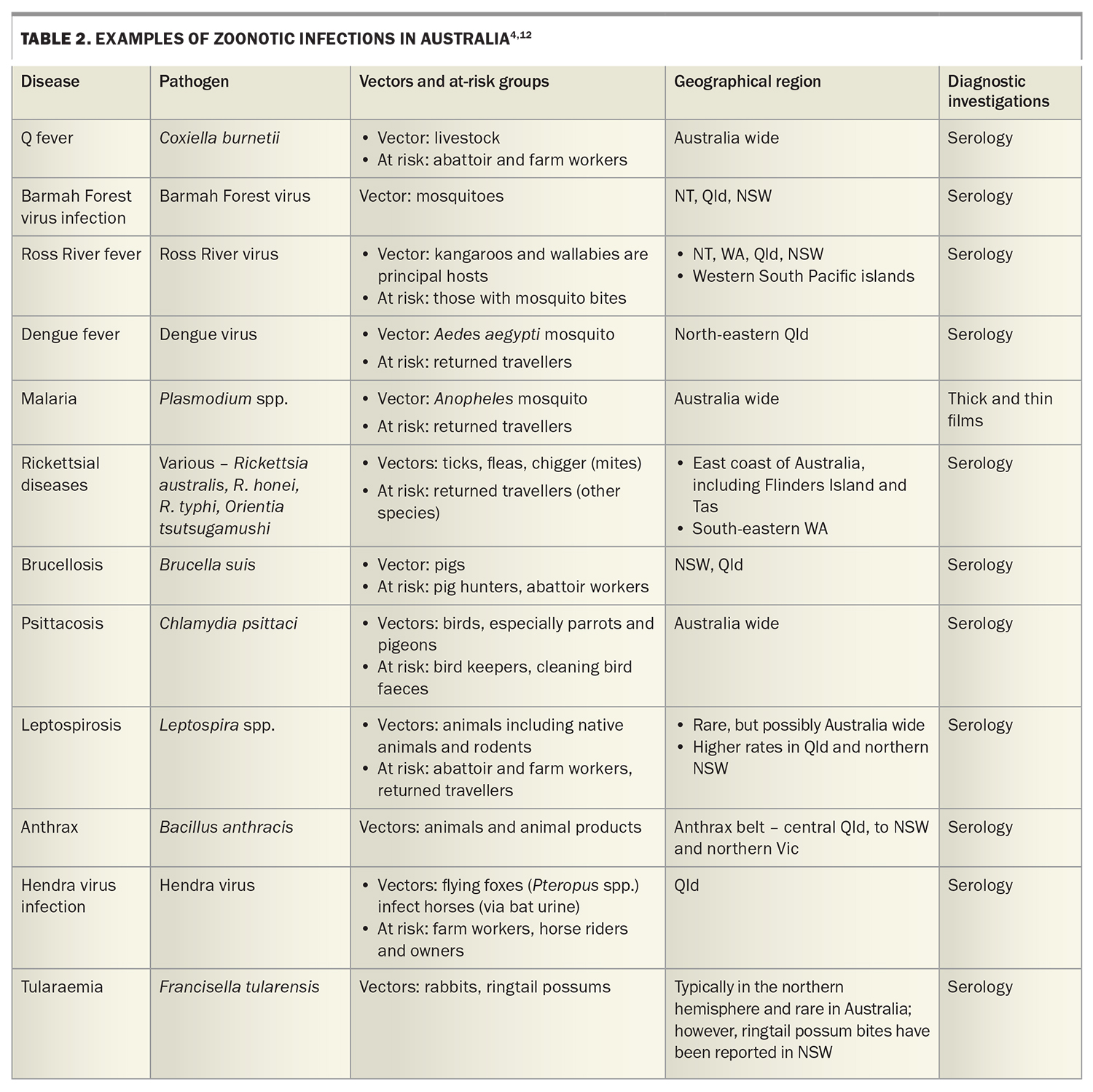
Further diagnostic clues lie in the patient’s social history. Travel should be explored, noting travel within the last year, both overseas and within Australia, and the relevant dates of travel. Enquire about vaccinations, antimalarial use and overseas activities, including food consumed, water activities, animal exposure, overseas sexual history and drug use. Ask about insect and tick bites. Occupation, pet ownership, bird exposure, gardening, domestic outdoor activities and sexual history should be noted. As usual, it is prudent to clarify smoking, alcohol and other drug use. Potential zoonotic and travel exposures and relevant investigations to consider are summarised in Table 2.4,11
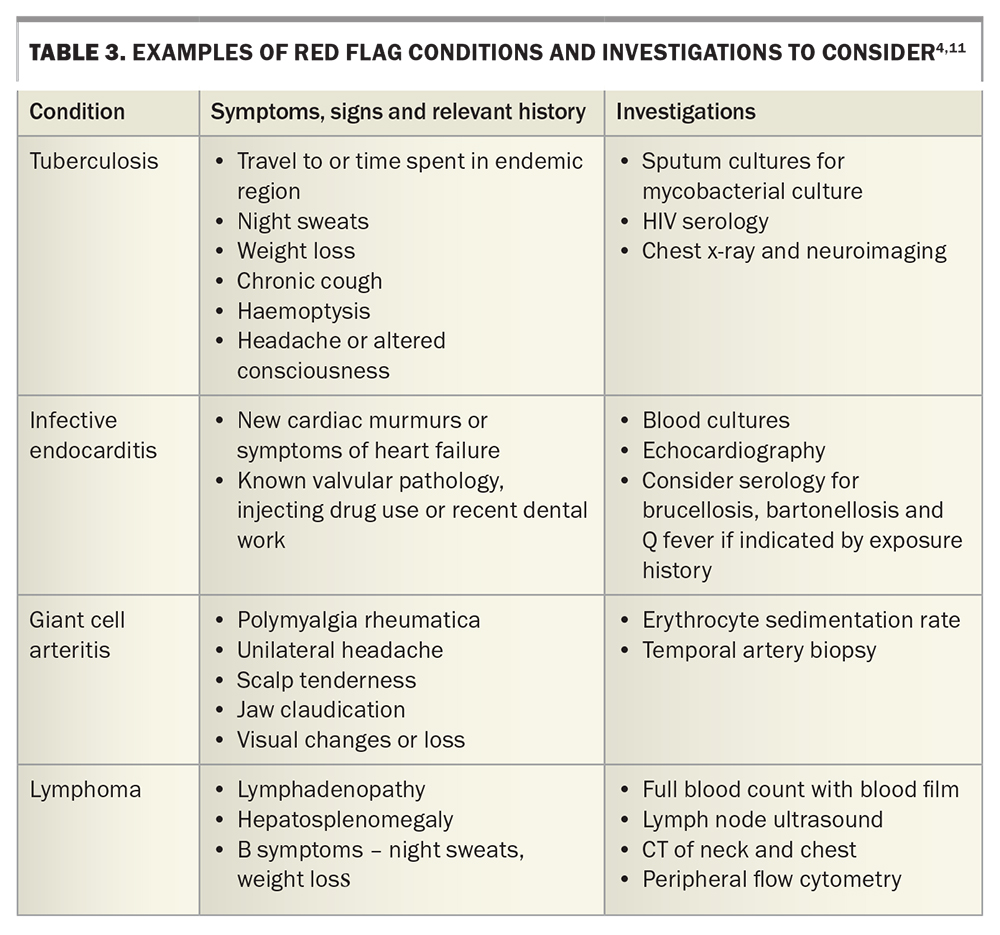
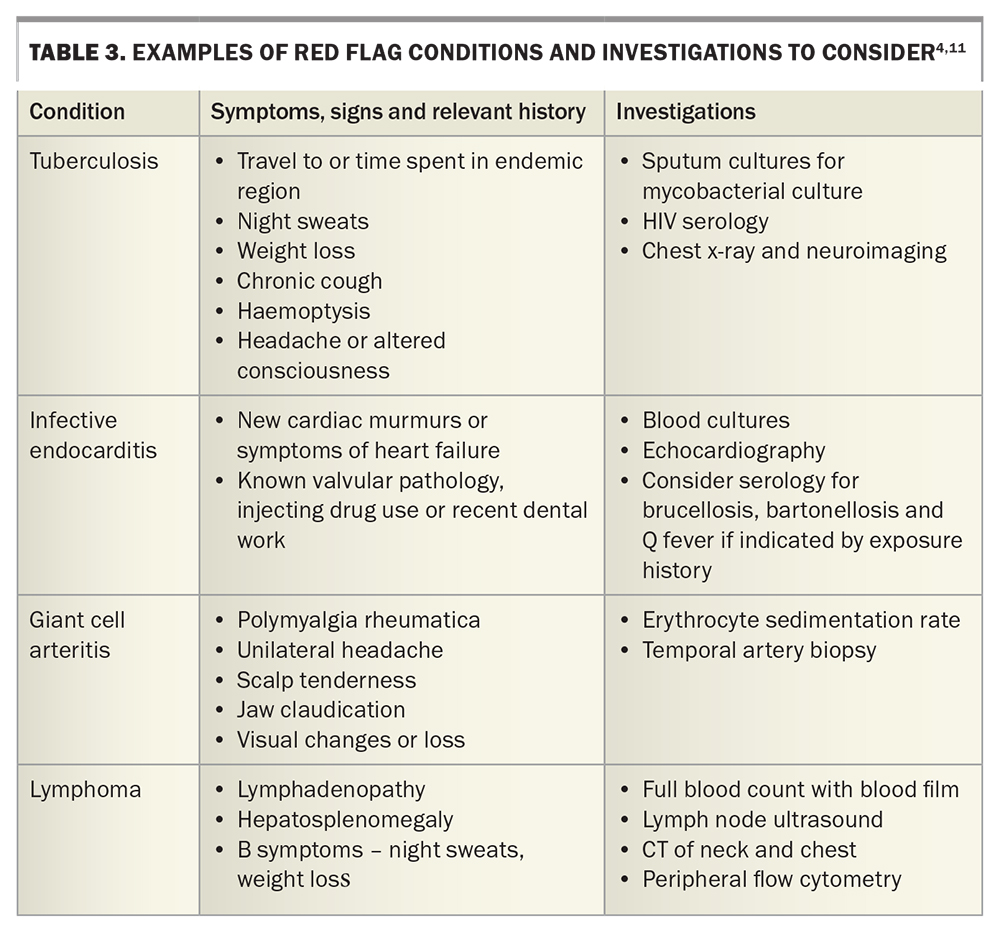
Prompt referral to hospital may be indicated if there are concerns for immunosuppression (known chemotherapy, neutropenia, immunosuppression), severe disease impacting daily function, disease in the elderly, or the presence of red flags for certain conditions (Table 3).
Examination
Formally measure the patient’s temperature. Red flags must be actively excluded (as above, Table 3). Search for stigmata of infective endocarditis alongside cardiac auscultation; note any lymphadenopathy or hepatosplenomegaly, and palpate the temporal arteries. Perform a thorough skin examination for lesions and rashes, examine the small joints for synovitis and palpate the midline for spinal tenderness.
A systems review should otherwise be conducted, and includes:
- examination of the fundi (stigmata of endocarditis)
- ear, nose and throat examination, including looking for mouth ulcers, thyroid tenderness (subacute thyroiditis) or nodules (thyrotoxicosis)
- potentially, a genital or digital rectal examination (abscess, malignancy, epididymal infection).
Investigations and workflow
Data from a systematic review and meta-analyses suggest no significant difference in diagnostic outcome between a structured (algorithmic) and a nonstructured (clues-led) approach to evaluating PUO.13 Therefore, a staged approach to the classic PUO presentation is recommended. What follows is a suggested set of investigations that, together with any diagnostic clues in the history and examination, should help tailor further investigations to the patient, according to appropriate age-group, demographic and exposure history.
In general, we suggest a doing a full blood count, renal function with electrolytes and liver function tests, and measuring C-reactive protein and erythrocyte sedimentation rate. Urinalysis, three sets of blood cultures (taken at different times and, ideally, from at least two different sites) and a chest x-ray should be performed. Consider also rheumatoid factor, anticitrullinated C-peptide, antinuclear antibodies and thyroid function tests and HIV serology. In younger patients, send a monospot for heterophile antibodies, cytomegalovirus and Epstein–Barr virus serology, especially if there is any liver function derangement. In any patient with significant tuberculosis exposure (living for longer than a month in an endemic area), appropriate investigations for active tuberculosis should be undertaken. An interferon gamma release assay may be falsely negative in patients with acute illness and, when positive, will often remain so lifelong. Further suggested serologies are summarised in Table 2 and Table 3.
In the primary care setting, recommended imaging includes a contrast CT of the chest, abdomen and pelvis to look for an occult abscess, a malignancy or lymphadenopathy. Targeted ultrasonography of the biliary or renal tracts, MRI of the CNS, venous Doppler studies of the lower limbs or transthoracic echocardiography may be of use, depending on results from the initial investigations or history and examination, but would not be considered routine.
When to refer for tertiary investigations
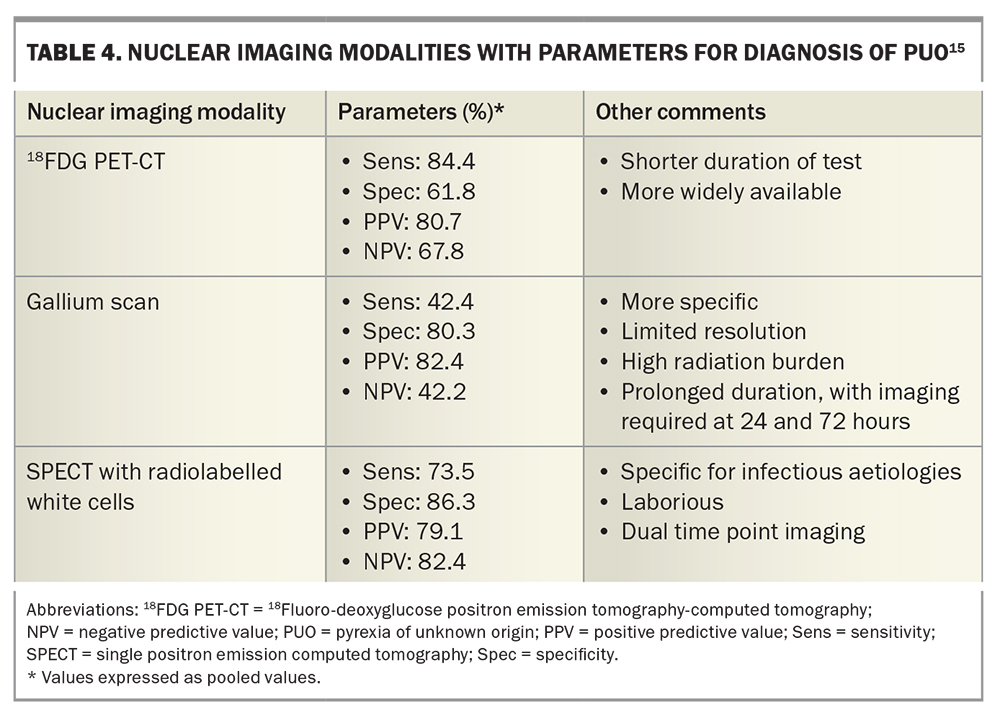
There is a role for further nuclear imaging (positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated with computed tomography [18FDG PET-CT], gallium scan, white blood cell scan), biopsies of relevant tissues (bone marrow aspirate and trephine, biopsies of lymph nodes or skin lesions) and molecular testing for infectious aetiologies, which should be performed with specialist input.
With regards to nuclear imaging, 18FDG PET-CT has the most evidence to support its use. As a widely available, shorter duration and more sensitive test, it has the potential to survey the whole body for malignant, infectious or inflammatory sites of increased 18fluoro-deoxyglucose uptake. Evidence as to which patients are best suited to 18FDG PET-CT is limited; however, some studies recommend 18FDG PET-CT especially for febrile, older (>50 years) nonobese patients with elevated inflammatory markers.14 Gallium scans are limited by availability, high radiation burden and prolonged procedural duration (up to 72 hours), although many clinicians find it a useful test. Single positron emission CT with radiolabelled white cells has greater specificity for infectious aetiologies, but is also laborious and, overall, less sensitive for causes of PUO. A comparison of sensitivities and specificities for nuclear imaging modalities is included in Table 4.15
Depending on the degree of suspicion for an infectious aetiology, further molecular testing of otherwise sterile sites may be possible. Polymerase chain reaction assays for bacteria (16s ribosomal RNA gene) and fungi (28s rRNA gene) and next-generation sequencing are available, and could be considered with input from an infectious disease specialist.16
Principles of management and take-home messages
It can be frustrating for both patients and clinicians to have a prolonged period of febrile symptoms and potentially negative investigations. Despite this, it is prudent to delay empirical treatment with antimicrobials or immunosuppression in an otherwise stable non-neutropenic and nonimmunocompromised patient in whom a diagnosis is not apparent and who is not thought to be an acute risk of deterioration without therapy. Given the tendency for invasive bacterial or fungal disease in neutropenic and immunocompromised patients, it is wise to commence treatment in this population, including antipseudomonal therapy after adequate microbiological sampling and referral to hospital.
In the setting of classic PUO, empirical trials of corticosteroids, aspirin, antimicrobials and antineoplastics have been studied. However, they are rarely indicated as they may cause adverse drug effects and delay diagnosis and appropriate therapy.7 Trials of empirical naproxen to distinguish between malignant and nonmalignant causes of fever have had mixed results.17 If red flags are present for infective endocarditis, tuberculosis or vasculitis, a case may be made for empirical treatment after appropriate sampling and with specialist input.11
The prognosis of PUO is ultimately determined by the underlying cause and, overall, the time taken to establish the diagnosis does not appear to affect prognosis. Mortality is less than 10%, and is largely due to malignancy in older people.18 In a small study of long-term follow up of patients with PUO, resolution of fever after one month without a clear diagnosis was associated with a favourable outcome and no significant sequelae of the febrile illness.19
Conclusion
PUO encompasses a heterogenous group of febrile conditions and continues to present a conundrum to the modern-day clinician. Identifying the cause of PUO should start with considering the most likely diagnosis first. As such, a thorough patient history should be taken and examination performed before moving on to other investigations. Practice points on the paradigm of workup of a patient with PUO are presented in Box 2. The overarching recommendation is to withhold treatment, provided there is no risk of acute deterioration, until the cause is determined so treatment can be tailored appropriately. MT
COMPETING INTERESTS: None.
References
1. Wright WF, Auwaerter PG. Fever and fever of unknown origin: review, recent advances, and lingering dogma. Open Forum Infect Dis 2020; 7(5): ofaa132.
2. Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore) 1961; 40: 1-30.
3. Durack DT, Street AC. Fever of unknown origin – re-examined and redefined. Curr Clin Top Infect Dis 1991; 11: 35-51.
4. Beresford RW, Gosbell IB. Pyrexia of unknown origin: causes, investigation and management. Intern Med J 2016; 46: 1011-1016.
5. Haidar G, Singh N. Fever of unknown origin. N Engl J Med 2022; 386: 463-477.
6. Gottlieb T, Hoy J, McDonald M. Fever: mechanisms and symptomatic treatment. In: Yung A, Spelman D, Street A, McCormack J, Sorrell T, Johnson P, eds. Infectious Diseases: A clinical approach. Third Edition. Melbourne: IP Communications; 2010. p. 18-26.
7. Wright WF. Fever of unknown origin. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s Principles and Practices of Infectious Diseases. Ninth edition. Canada: Elsevier; 2019. p. 790-800.
8. Betrains A, Wright WF, Moreel L, Staels F, Blockmans D, Vanderschueren S. Etiological spectrum and outcome of fever and inflammation of unknown origin. Does symptom duration matter? Eur J Intern Med 2022 Dec; 106; 103-110.
9. Betrains A, Moreel L, De Langhe E, Blockmans D, Vanderschueren S. Rheumatic disorders among patients with fever of unknown origin: a systematic review and meta-analysis. Semin Arthritis Rheum 2022; 56: 152066.
10. Sogaard KK, Farkas DK, Leisner MZ, Schmidt SAJ, Lash TL, Sorensen HT. Fever of unknown origin and incidence of cancer. Clin Infect Dis 2022; 75: 968-974.
11. Sorrell T, Yung A, Stanley P. Pyrexia of unknown origin. In: Yung A, Spelman D, Street A, McCormack J, Sorrell T, Johnson P, eds. Infectious Diseases: A clinical approach. Third Edition. Melbourne: IP Communications; 2010. p. 78-93.
12. Robson J, Bursle E. Zoonoses in Australia. Established and emerging risks. Med Today 2021; 22: 38-44.
13. Wright WF, Betz JF, Auwaerter PG. Prospective studies comparing structured vs nonstructured diagnostic protocol evaluations among patients with fever of unknown origin. A systematic review and meta-analysis. JAMA Netw Open 2022; 5(6): e2215000.
14. Holubar J, Broner J, Arnaud E, et al. Diagnostic performance of 18F-FDG-PET/CT in inflammation of unknown origin: a clinical series of 317 patients. J Intern Med 2022; 291: 856-863.
15. Van Rijsewijk ND, Ijpma FF, Wouthuyzen-Bakker MW, Glaudemans AW. Molecular imaging of fever of unknown origin: an update. Semin Nucl Med 2023 Jan; 53: 4-17.
16. Wright WF, Simner PJ, Carroll KC, Auwaerter PG. Progress report: next-generation sequencing, multiplex polymerase chain reaction, and broad-range molecular assays as diagnostic tools for fever of unknown origin investigations in adults. Clin Infect Dis 2022; 74; 924-932.
17. Foggo V, Cavenagh J. Malignant causes of fever of unknown origin. Clin Med 2015; 15; 292-294.
18. Vanderschueren S, Ecykmans T, De Munter P, Knockaert D. Mortality in patients presenting with fever of unknown origin. Acta Clin Belg 2014; 69: 12-16.
19. Knockaert DC, Dujardin KS, Bobbaers HJ. Long-term follow-up of patients with undiagnosed fever of unknown origin. Arch Intern Med 1996; 156: 618-620.