Familial hypercholesterolaemia: improving the health of individuals and families

Familial hypercholesterolaemia (FH) is one of the most prevalent, potentially fatal genetic disorders. It causes elevated low-density lipoprotein-cholesterol levels that can lead to premature cardiovascular disease if untreated. Early identification and treatment can substantially improve outcomes. As FH begins at birth and progresses throughout life, GPs are well placed to play an active role in diagnosis and care of all individuals and families with FH.
- Familial hypercholesterolaemia (FH) is a common genetic disorder that leads to premature onset of cardiovascular disease.
- GPs play an important role in identifying people with FH and can access Medicare-rebated genetic testing for the close relative of patients with FH, including their children, before the development of the clinical features of FH.
- The cumulative damaging effect of low-density lipoprotein-cholesterol throughout life justifies the emphasis on early diagnosis and treatment of FH to reduce cardiovascular morbidity and mortality.
- Pathology laboratories need a copy of the genetics report for the first member of a family diagnosed with FH to assist with GP-initiated family cascade screening.
Familial hypercholesterolaemia (FH) is an autosomal dominant genetic disorder caused by pathogenic variants in genes affecting the clearance pathway of low-density lipoprotein (LDL). The condition starts at birth, leading to lifelong elevated levels of LDL-cholesterol, which progress over time. If not treated, FH can accelerate the onset of premature atherosclerotic cardiovascular disease (CVD) by 15 to 30 years.1 FH is recognised as a tier 1 genomic disorder, meaning it is a preventable cause of premature disease and death with significant potential for a positive impact on public health, supported by high-level evidence-based guidelines and recommendations.1
An estimated one in 250 people in Australia have FH. Most remain undetected and unknowingly live with the condition for years, with the risk that the first clinical presentation may be sudden death from cardiac arrest.2 Fewer than 10% of the more than 100,000 high-risk patients in Australia have been diagnosed. A similar number of their relatives could benefit from having the diagnosis excluded.1,3,4 Despite an exponential growth in research and strong clinical practice guidelines on FH, it continues to be underdiagnosed and undertreated and remains a significant global health concern.4
As FH is inherited in an autosomal dominant manner, a child born to a parent with FH has a 50% chance of inheriting the disorder, predisposing them to lifelong elevated LDL-cholesterol levels and a high risk of premature atherosclerotic CVD.5 Entire families can be impacted over generations by the detrimental effects of premature atherosclerotic CVD among so many close relatives. Optimal management includes diagnosing and treating the condition as early in life as possible, as this can significantly reduce cardiac morbidity and mortality.6 However, most individuals who are diagnosed with FH do not meet optimal management and treatment targets.5
As the first point of contact for many individuals seeking health care advice, GPs can play an important role in improving the detection and management of FH. Further, the family orientation of general practice can facilitate the expansion of care from the individual to the nuclear family and beyond.
This article outlines how and why GPs play an important role in the identification, diagnosis and management of people with FH. Key strategies and supports are outlined to help GPs take an active and central role in supporting patients and their families with FH.
Why is the GP’s role important?
GPs are well placed to help identify, diagnose and manage FH in both patients and their families as almost 90% of people visit their GP at least once a year.7 Most (90%) LDL-cholesterol tests are ordered through general practice. This provides a perfect opportunity to identify patients who may have FH and initiate screening and diagnosis.8 After diagnosis, most patients with FH can be managed by their GP; current initiatives aim to facilitate the management of patients with FH by regular GP monitoring in conjunction with specialist support on an ‘as needed’ basis.8,9
It is worth remembering that a predominant increase in LDL-cholesterol level is more specific for FH during childhood. A high LDL-cholesterol level in adulthood is less specific but, when associated with progressive physical signs such as tendon xanthomas or accelerated (before age 45 years) corneal arcus, can suggest a diagnosis of FH. Primary care, supported by improved awareness, guidance and assistance, provides an opportunity to identify more patients with FH.
Screening and detection of FH in primary care
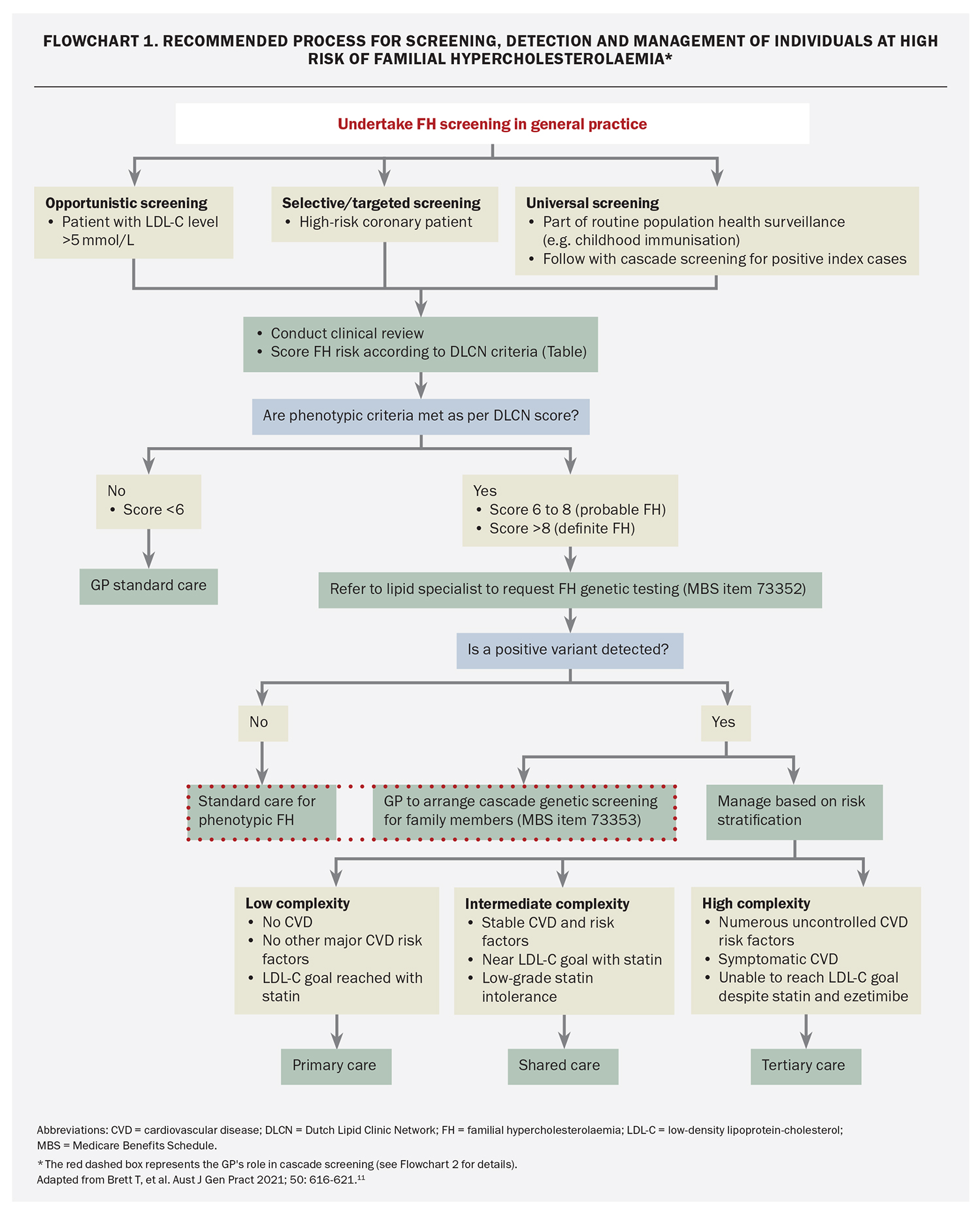
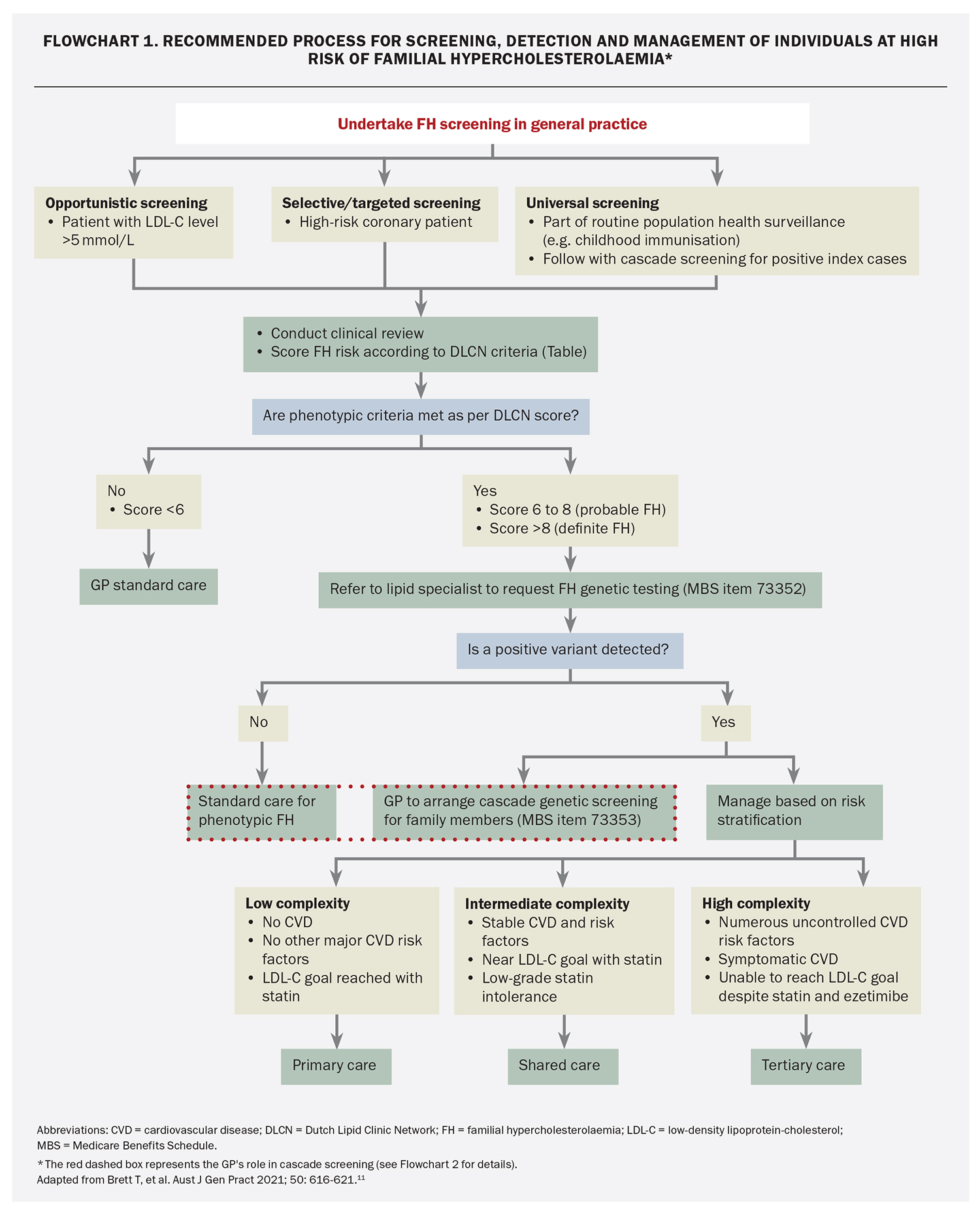
Screening for FH begins by identifying individuals who may be at increased risk. It involves several strategies: opportunistic, selective and universal (followed by cascade family) screening (Flowchart 1).10,11
Opportunistic screening
Opportunistic screening can occur during routine medical appointments after a review of family history and cholesterol levels and a physical examination. In primary care, opportunistic screening should be considered for any adult with a plasma LDL-cholesterol level over 5.0 mmol/L.1 Community laboratories can also support opportunistic screening by flagging elevated cholesterol levels on laboratory reports, alerting the requesting GP of a potential patient with FH. Further, although alerts on laboratory reports can be effective, a direct phone call from the chemical pathologist has been shown to further enhance detection rates.12
Selective screening
Selective screening involves identifying patients with premature atherosclerotic CVD (e.g. at age under 55 years in men and under 60 years in women) or a family history of premature atherosclerotic CVD or hypercholesterolaemia. For example, FH should always be considered if a patient has a total cholesterol level greater than 7.5 mmol/L or an LDL-cholesterol level greater than 6.5 mmol/L, especially if there is a family history of premature CVD.13
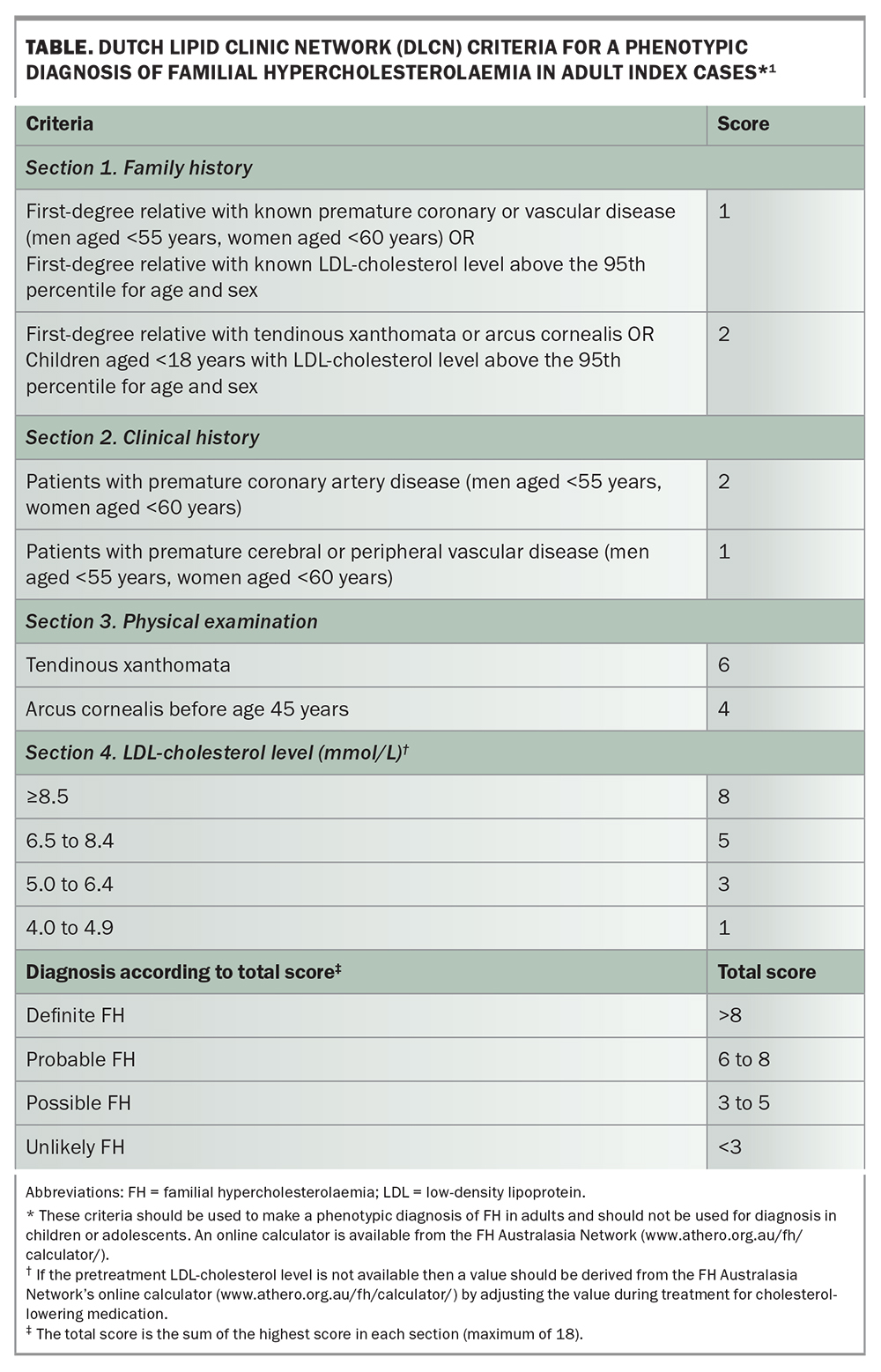
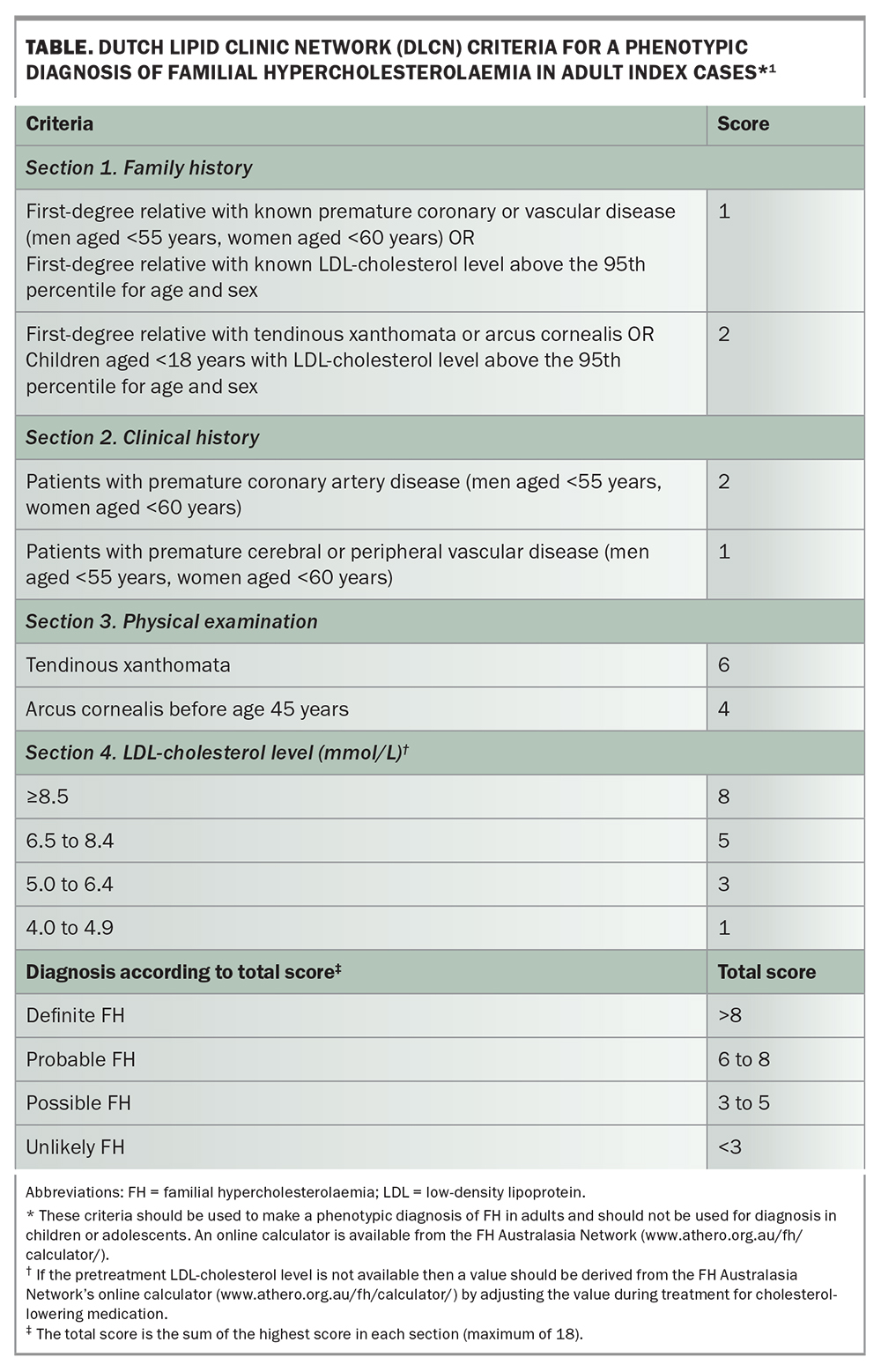
GPs can also conduct selective screening by digitally reviewing electronic health records to detect possible cases using diagnostic criteria such as the Dutch Lipid Clinic Network (DLCN) criteria.5 The DLCN criteria (Table) are one of the most widely used phenotypic methods for diagnosing adult index cases of FH, involving several risk criteria, and are recommended in several guidelines.1,8 Primary Health Networks throughout Australia offer assistance to GPs across a range of areas, including supporting health data management and the collection of information.
Universal screening
Several universal screening approaches to improve the detection and diagnosis of FH in patients and their families are under investigation. In general practice, universal screening coupled with reverse cascade screening (i.e. child to parent) would help identify patients at a young age, facilitating optimal treatment, and has the potential to increase detection numbers by flagging the parents of children who are diagnosed. Universal screening should be considered before puberty.1
Although Australia does not currently conduct universal screening for FH, it might become standard as a cost-effective approach to increasing detection rates.14,15 It is important that GPs are ready to manage children who are diagnosed with FH and to reverse cascade test their adult relatives. With an integrated, shared-care approach that includes the patient, GP and other specialists, universal screening combined with cascade screening could be successfully implemented and lead to a substantial increase in the early detection and diagnosis of FH.
Cascade screening of family members
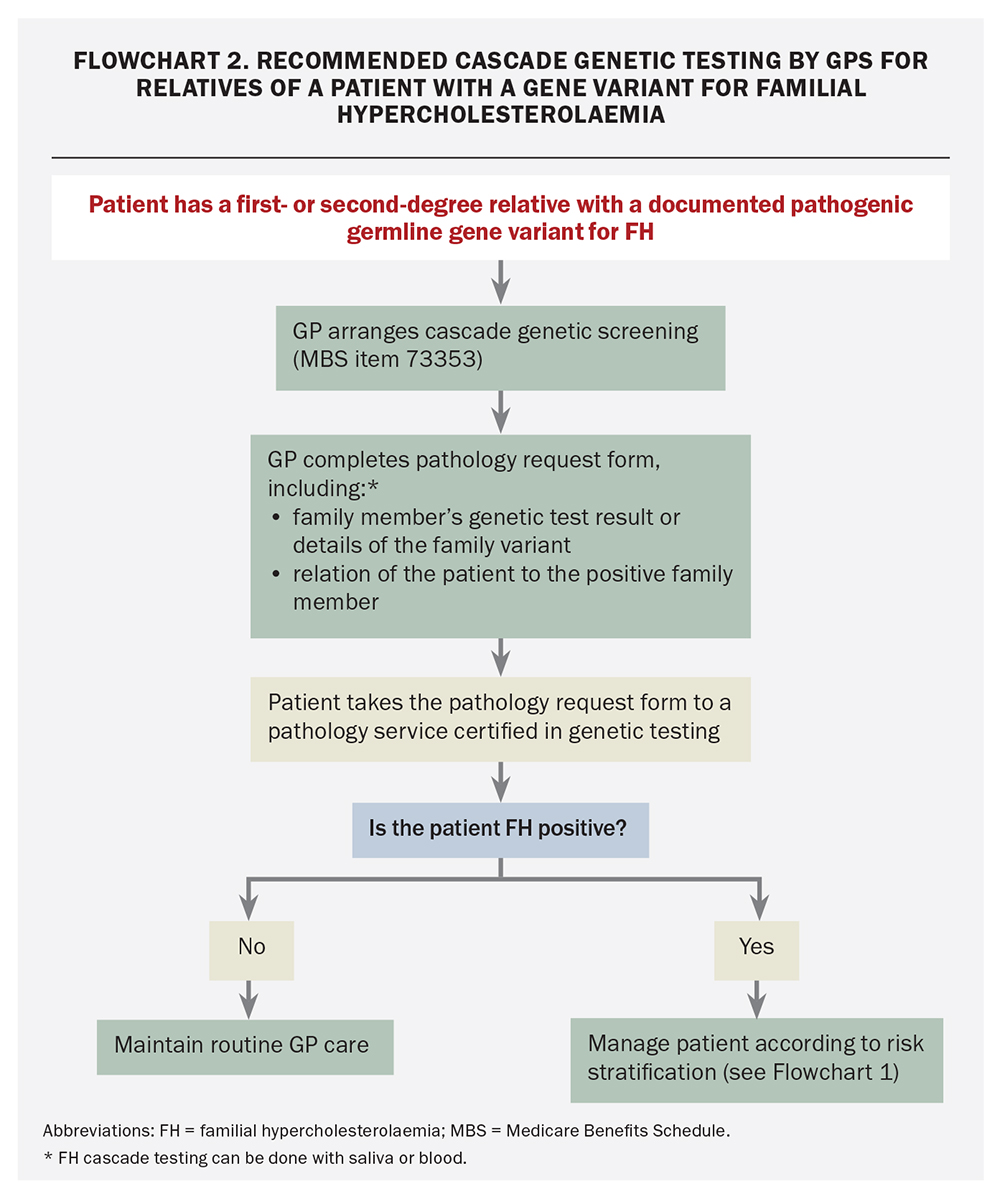
Cascade screening refers to the screening of close family members of index cases who have a known genetic variant. Cascade screening is a highly cost-effective approach. It is one of the key strategies where GPs can have the most impact on increasing detection and diagnostic rates.16 When an index case is identified, GPs can offer Medicare- rebated genetic cascade testing to first- and second-degree relatives, provide genetic counselling and work with lipid specialists regarding treatment and management.17
Before cascade testing of a relative, GPs should provide a brief summary of FH and discuss the person’s risk of inheriting the family variant and the potential benefits and implications of genetic testing, including possible insurance considerations. Those who test positive for the variant are encouraged to communicate their result to at-risk relatives to allow further cascade testing and should continue to closely monitor their cholesterol levels and manage accordingly. Those who test negative for the variant are advised to follow population screening guidelines.
By supporting the timely treatment of people with FH, cascade screening by GPs has the potential to be a highly effective method for the primary prevention of CVD among families with FH. The greatest opportunity for improved detection and management of FH in current circumstances is expansion of family cascade testing by GPs.
Diagnosis
Irrespective of the approach taken, it is crucial to emphasise that all models of care for FH start with early detection. The sooner a diagnosis is made and treatment commences, the better are the outcomes for patients and their families.
After a patient has been screened and identified as possibly having FH, GPs can apply the DLCN criteria to establish a phenotypic diagnosis of probable FH (score 6 to 8) or definite FH (score over 8) in adult patients (Table). If FH is suspected, the GP is recommended to refer the patient to a lipid specialist, cardiologist or endocrinologist for further evaluation, including consideration of Medicare-rebated genetic testing.
The Medicare-funded genetic test for index cases can be ordered based on any one of the following criteria:
- DLCN score of 6 or more
- LDL-cholesterol level of 6.5 mmol/L or more in the absence of any secondary cause
- LDL-cholesterol level of 5.0 to 6.5 mmol/L with signs of premature or accelerated atherogenesis.
Although genetic testing can be used to confirm a diagnosis, a negative result does not exclude FH. The diagnosis can still be made based on phenotypic criteria (Table).5
The DLCN criteria are not suitable for use in children or adolescents because there has not been sufficient time for the development of associated clinical signs. Children who are at risk should be tested between the ages of 5 and 10 years. A probable diagnosis of FH is considered in children if:
- LDL-cholesterol level is over 5.0 mmol/L
- LDL-cholesterol level is 4.0 to 5.0 mmol/L and there is a parental history of hypercholesterolaemia or premature atherosclerotic CVD
- LDL-cholesterol level is over 3.5 mmol/L and a parent has a pathogenic or likely pathogenic gene variant.18
Most patients can be successfully managed in an ongoing manner by their GP, with specialist input and support as needed.16
Genetic testing for FH
The Medicare Benefits Schedule (MBS) includes genetic testing items that can open the door for enhanced identification:
- item 73352 for index cases (which requires a non-GP specialist)
- item 73353 for cascade testing (which can be ordered by any doctor, including GPs).
Primary care physicians are empowered under Medicare to conduct ‘predictive’ genetic testing of family members of patients with a genetic diagnosis of FH. This has been available since 2020, but testing numbers suggest cascade testing is underutilised. As a result, family members at risk of FH are missing out on early diagnosis.
Implementation projects are currently seeking ways to provide the counselling and other support measures that will assist family cascade screening to be used for more patients and thereby allow far more people, in particular young children and adolescents, to be diagnosed. As the burden of elevated LDL-cholesterol accumulates over time, younger individuals have the most to gain from an early diagnosis of FH and prevention of CVD.19
GPs can lead the way in enhancing detection rates with the new Medicare-funded cascade genetic testing, which should be offered to FH family members regardless of cholesterol levels. The transition from index case detection to widespread cascade screening has been the missing link in FH diagnosis. The role of the family GP is seen as the fundamental solution. A recommended process for GPs to arrange FH cascade screening is shown in Flowchart 2.
Why can FH be managed successfully in general practice?
Several features of primary care make this the ideal setting for managing FH. These features include regular patient visits, an established therapeutic relationship, shared decision-making, the potential for seeing multiple family members, continuity of care and the capacity to implement preventive measures and provide comprehensive health care.
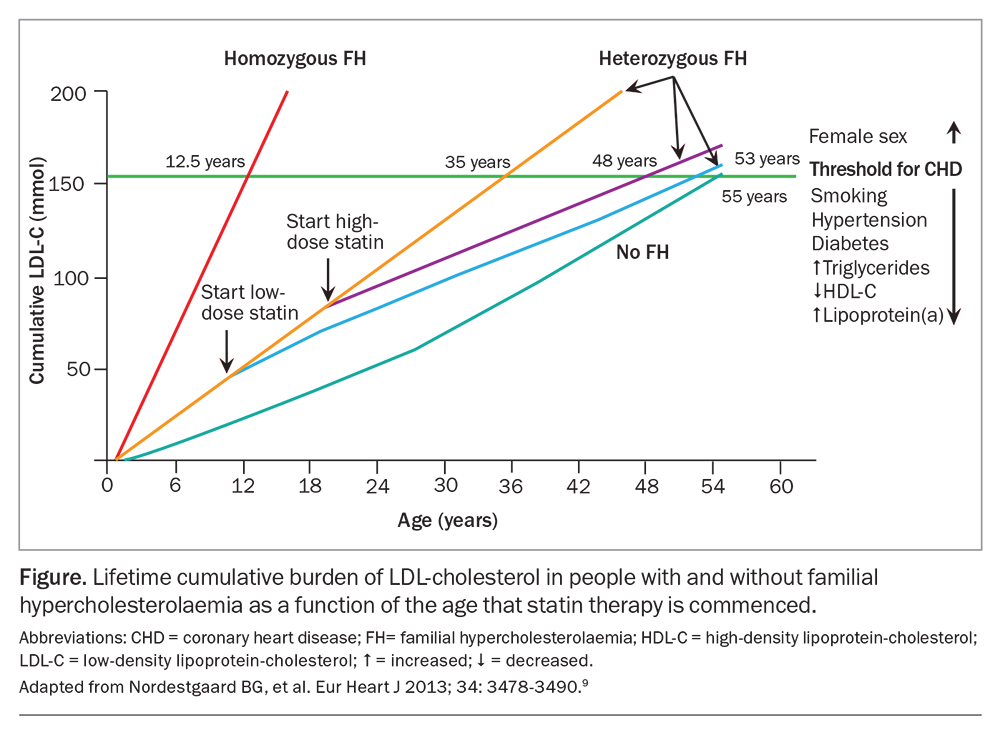
The incentive to intervene early and substantially reduce lifetime risk is highlighted in the Figure through the concept of ‘cholesterol years’. The Figure illustrates how the cumulative burden of LDL-cholesterol typically reaches 160 mmol by about the age of 55 years in the general population, by which stage clinical coronary heart disease may develop. An untreated individual with heterozygous FH will reach this threshold by the age of about 35 years. Starting a low-dose statin early will slow the progression of disease and delay the threshold for coronary heart disease to the age of 53 years, just behind the population age of 55 years.9 For individuals with FH, improved outcomes are achieved in those who are identified and treated early.
Support for GPs
The optimal care of FH requires patient-centred management that includes a multidisciplinary team.5 Risk stratification will help identify the best pathway for management and help distribute care across teams depending on risk level (Flowchart 1). The best management approach involves the use of cholesterol-lowering medications, but also includes adherence to a heart-healthy lifestyle and avoiding non-cholesterol risk factors (e.g. smoking, hypertension, obesity and diabetes).1
Resources to assist GPs in the identification and management of FH are listed in the Box. GPs are also encouraged to access Health Pathways through their local Primary Health Network (www.health.gov.au/our-work/phn/your-local-PHN) for specific guidance on the diagnosis, treatment, and management of FH.
Conclusion
Considering the highly treatable and preventable nature of FH and its consequences, it is crucial to address gaps and challenges in the early identification and treatment of this common genetic disorder. GPs are well placed to play an important role in the prevention of early onset of CVD through early diagnosis and optimal management. The substantial benefits to individuals and families highlight the importance of addressing these challenges. A multidisciplinary, shared care, patient-centred management approach that includes GPs is important. MT
Acknowledgement
The authors thank Associate Professor Charlotte Hespe, Head of General Practice, Sydney School of Medicine, University of Notre Dame Australia, for her contribution to this article.
COMPETING INTERESTS. Dr Sundercombe is an employee of Douglas Hanly Moir Pathology, which provides genetic testing for familial hypercholesterolaemia. Associate Professor Sullivan has received grants from Regeneron, Amgen, Arrowhead, Ionis and Novartis via Sydney Local Health District; consulting fees and speaker fees from Amgen and Novartis; and drug samples for a treatment adherence program from Sanofi. Dr Birkenhead, Dr Sarkies, Ms Trumble, Professor Vagholkar: None. This work was funded by the NSW Health Translational Research Grant Scheme, which had no other role in the decision to publish or preparation of the manuscript.
References
1. Watts GF, Sullivan DR, Hare DL, et al. Integrated guidance for enhancing the care of familial hypercholesterolaemia in Australia. Heart Lung Circ 2021; 30: 324-349.
2. Hertz CL, Christiansen SL, Ottesen GL, Frank-Hansen R, Bundgaard H, Morling N. Post-mortem investigation of young deceased individuals with ischemic heart disease-outcome of supplementary genetic testing for dyslipidemia. Int J Legal Med 2016; 130: 947-948.
3. Watts GF, Shaw JE, Pang J, Magliano DJ, Jennings GL, Carrington MJ. Prevalence and treatment of familial hypercholesterolaemia in Australian communities. Int J Cardiol 2015; 185: 69-71.
4. Wilemon KA, Patel J, Aguilar-Salinas C, et al. Reducing the clinical and public health burden of familial hypercholesterolemia: a global call to action. JAMA Cardiol 2020; 5: 217-229.
5. Watts GF, Gidding SS, Mata P, et al. Familial hypercholesterolaemia: evolving knowledge for designing adaptive models of care. Nat Rev Cardiol 2020; 17: 360-377.
6. de Ferranti SD, Steinberger J, Ameduri R, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association. Circulation 2019; 139: e603-e634.
7. Qureshi N, Da Silva MLR, Abdul-Hamid H, Weng SF, Kai J, Leonardi-Bee J. Strategies for screening for familial hypercholesterolaemia in primary care and other community settings. Cochrane Database Syst Rev 2021; 10(10): CD012985.
8. Vickery AW, Bell D, Garton-Smith J, Kirke AB, Pang J, Watts GF. Optimising the detection and management of familial hypercholesterolaemia: central role of primary care and its integration with specialist services. Heart Lung Circ 2014; 23: 1158-1164.
9. Nordestgaard BG, Chapman MJ, Humphries SE, et al; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013; 34: 3478-3490a. Erratum in: Eur Heart J 2020; 41: 4517.
10. Pang J, Sullivan DR, Brett T, Kostner KM, Hare DL, Watts GF. Familial hypercholesterolaemia in 2020: a leading tier 1 genomic application. Heart Lung Circ 2020; 29: 619-633.
11. Brett T, Radford J, Heal C, et al. Implications of new clinical practice guidance on familial hypercholesterolaemia for Australian general practitioners. Aust J Gen Pract 2021; 50: 616-621.
12. Bell DA, Hooper AJ, Edwards G, et al. Detecting familial hypercholesterolaemia in the community: impact of a telephone call from a chemical pathologist to the requesting general practitioner. Atherosclerosis 2014; 234: 469-472.
13. Brett T, Arnold-Reed D. Familial hypercholesterolaemia: a guide for general practice. Aust J Gen Pract 2019; 48: 650-652.
14. Marquina C, Lacaze P, Tiller J, et al. Population genomic screening of young adults for familial hypercholesterolaemia: a cost-effectiveness analysis. Eur Heart J 2021; 43: 3243-3254.
15. Australian Health Ministers’ Advisory Council. Newborn bloodspot screening - National Policy Framework. Canberra: Australian Government Department of Health; 2018.
16. Brett T, Qureshi N, Gidding S, Watts GF. Screening for familial hypercholesterolaemia in primary care: time for general practice to play its part. Atherosclerosis 2018; 277: 399-406.
17. Brett T, Watts GF, Garton-Smith J, et al. Familial hypercholesterolaemia: challenges in primary care. Med Today 2015; 16(8): 20-26.
18. Watts GF, Sullivan DR, Hare DL, et al. Essentials of a new clinical practice guidance on familial hypercholesterolaemia for physicians. Intern Med J 2021; 51: 769-779.
19. Wiegman A, Gidding SS, Watts GF, et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J 2015; 36: 2425-2437.