Dementia with Lewy bodies: addressing its challenges in primary healthcare

Dementia with Lewy bodies is an aggressive form of dementia with cognitive, motor and neuropsychiatric symptoms. Although research into new biomarkers and disease-modifying therapies may offer hope in the future, early diagnosis and holistic management are currently the most important aspects of care for these patients.
- Dementia with Lewy bodies is the second most common neurodegenerative dementia, accounting for 15% of all dementias in older individuals.
- Understanding of the prodromal stages of the disease is increasing.
- Patients can present with a variety of motor and non-motor symptoms, including cognitive deficits, sleep disorder, parkinsonism and neuropsychiatric features.
- A multidisciplinary management approach using both pharmacological and nonpharmacological strategies is required.
- GPs are critically placed to provide holistic care to these patients.
Dementia with Lewy bodies (DLB) is the second most common neurodegenerative dementia in older people, accounting for 10 to 15% of dementia diagnoses.1 Despite its high prevalence, up to 50% of DLB cases are misdiagnosed during life because of overlapping symptomology and a lack of objective biomarkers.2
DLB is associated with higher mortality and a greater disability than Alzheimer’s disease (AD) because of a combination of cognitive impairment, parkinsonism, neuropsychiatric symptoms and autonomic dysfunction.3,4 Caregiver burden is also greater due to the impact of these often comorbid symptoms.5 GPs are critically placed to provide holistic care to these patients in collaboration with specialists, such as neurologists or geriatricians. This article provides an overview of the pathology, clinical presentation, diagnosis and management of DLB. Helpful online resources to support practitioners in their assessment and management of patients with DLB are listed in Box 1.
Pathology
The underlying pathology in DLB is characterised by the presence of Lewy bodies and Lewy neurites within dying neurones. In 1997, researchers discovered that Lewy bodies arise from a composite buildup of misfolded alpha synuclein protein.6 Indeed, it is now recognised that three neurodegenerative conditions result from an alpha-synuclein proteinopathy, namely DLB, Parkinson’s disease (PD) and multiple system atrophy (MSA). The pathological disparity in these conditions is derived from differences in the molecular structure and the distribution of Lewy bodies.
It is important to note that up to 60 to 80% of patients diagnosed with DLB have concomitant Alzheimer’s pathology in the way of beta-amyloid plaques and some degree of neurofibrillary tangles of tau protein.7 Patients with a greater burden of this Alzheimer’s copathology have a faster cognitive decline compared with those without.8 This is likely to be a key point in the future, given the new era of disease-modifying anti-amyloid treatments.
Clinical presentation
In addition to a shared neuropathological substrate, DLB and PD do have significant clinical overlap. It should be emphasised that patients with DLB typically present at an older age and with a more aggressive progression than patients with PD. Although a significant proportion of PD patients will develop dementia (PDD), this usually occurs over a longer period, with patients typically experiencing several years of a predominant movement disorder before experiencing cognitive decline. However, it is clear that once dementia is established in PD, the clinical management of PDD and DLB is essentially the same. These two conditions are commonly referred to as Lewy body dementias.
Prodromal DLB
The pathological processes underpinning DLB start years to decades before the onset of symptoms. The features associated with this prodromal period have been increasingly recognised and represent a vital strategy for early diagnosis and treatment.
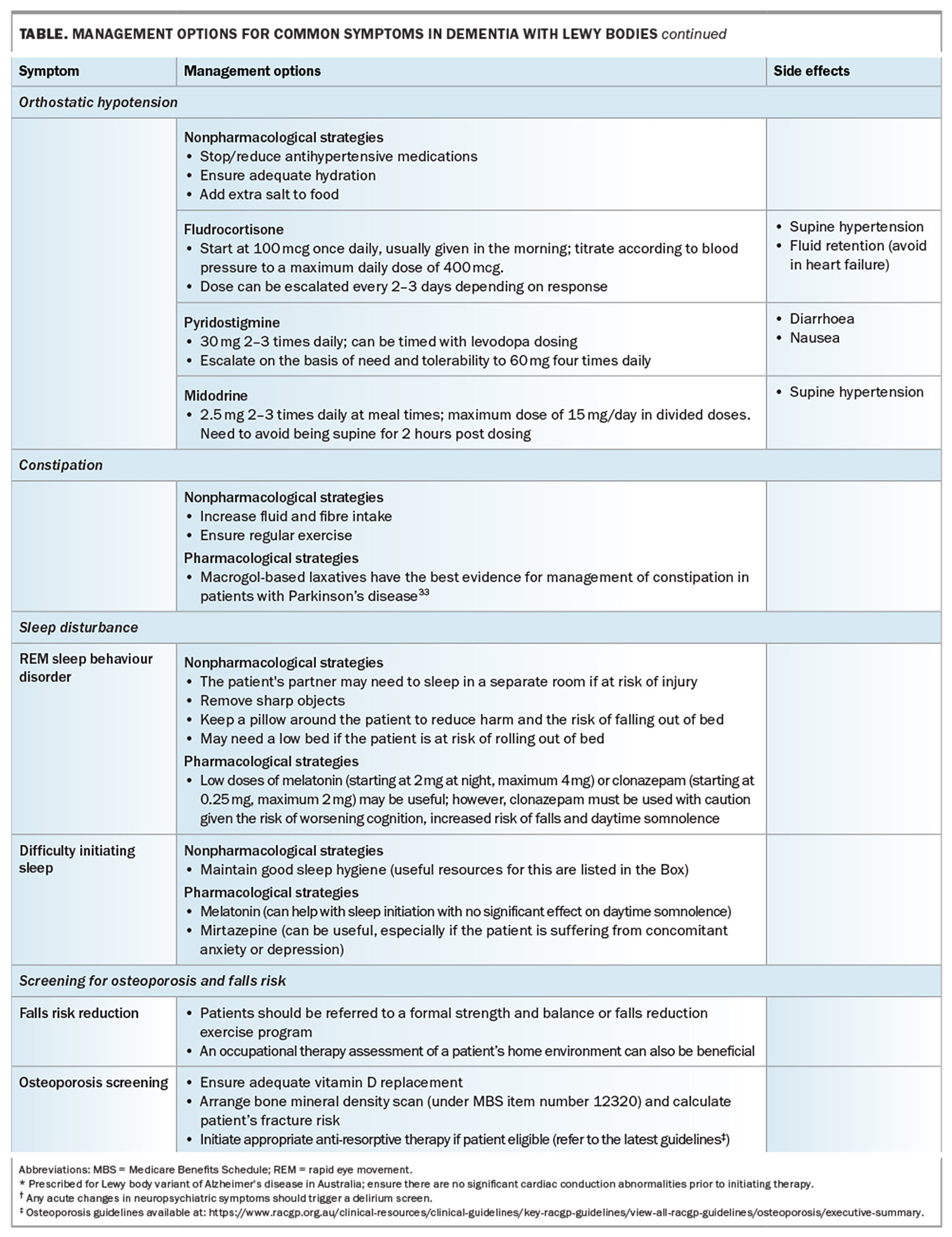
The most well-established feature of prodromal DLB is the emergence of isolated rapid eye movement (REM) sleep behaviour disorder (iRBD), a parasomnia in otherwise healthy individuals who act out their dreams during REM sleep. It is believed that early pathological changes occurring in the brainstem circuitry disrupt the normal muscle atonia that prevents dream enactment. A recent multicentre study assessing over 1200 patients with iRBD demonstrated that 75% of patients went on to develop a synucleinopathy over the course of 12 years, with roughly half developing DLB and the other half developing PD.9 It should also be highlighted that iRBD is seen in around 70% of patients with DLB, making it a useful diagnostic feature, especially when trying to discriminate it from AD.10
In addition to recognising the significance of iRBD, the international DLB Consortium has recently published research criteria identifying three potential subtypes of prodromal DLB, characterised by: a mild cognitive impairment-onset; a delirium-onset; and a psychiatric-onset disease.11 Currently, both delirium and psychiatric-onset DLB have no definitive diagnostic criteria. However, a recent review of the published literature regarding psychiatric-onset DLB has shown that these individuals are likely to be women who present with recurrent, late-onset depression that is associated with psychotic features before the transition to DLB over a 10-year period.12 Although high incidences of delirium have also been observed in patients with DLB one year before transition, a symptom not necessarily observed in AD, the literature on this DLB prodrome is limited and thus requires further study.13
Diagnosis
Early and presenting symptoms of DLB vary widely. Therefore, patients may present to a wide range of specialists at the request of their GP. Presenting symptoms include impaired memory, visual hallucinations and gait problems with falls.14 Importantly, the identification of a history of iRBD can support the diagnosis of DLB and help channel more targeted referrals.15
Apart from the prodromal symptoms mentioned above, the presence of anosmia and autonomic symptoms (e.g. constipation, orthostatic hypotension and urinary urgency or incontinence) should raise suspicion for an underlying synucleinopathy.11
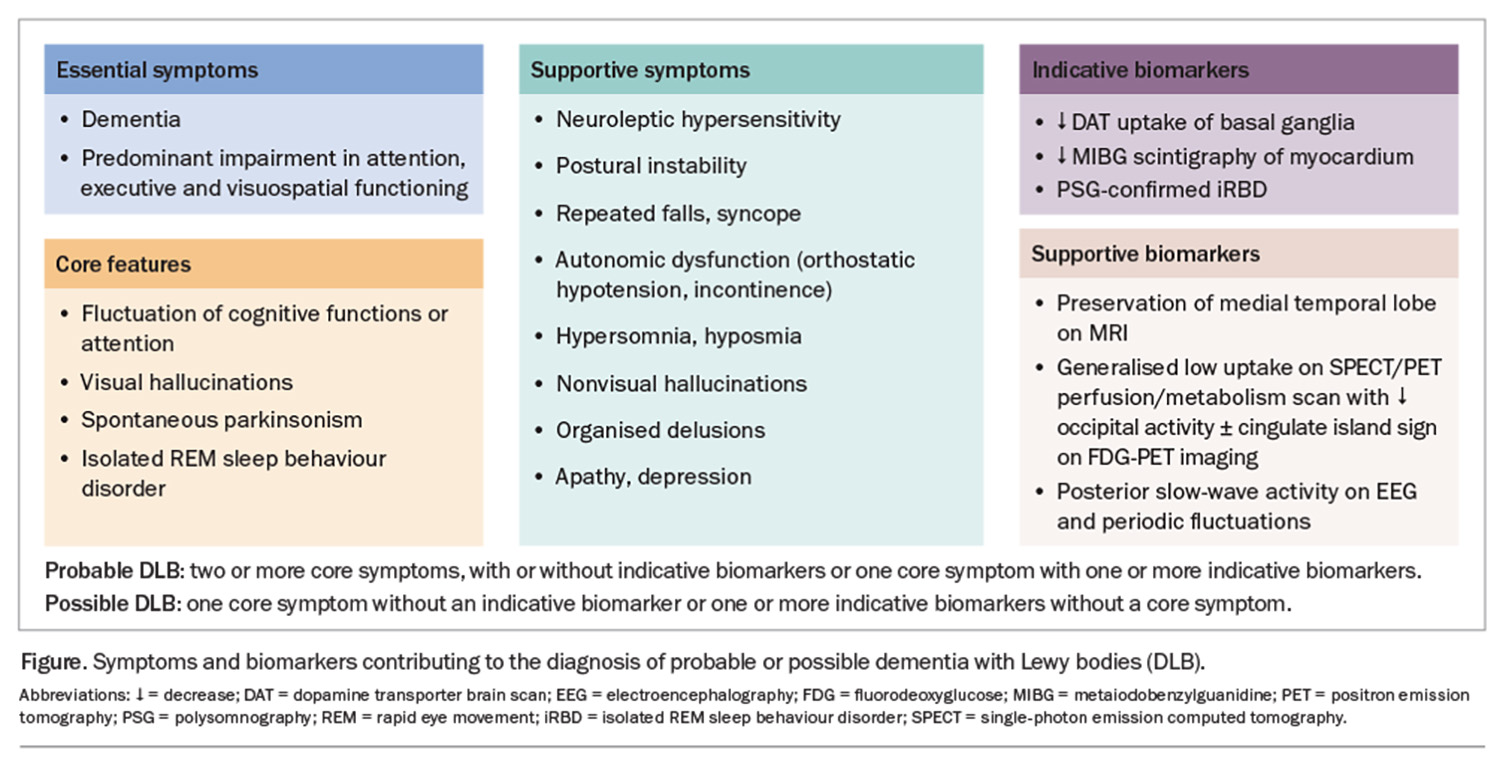
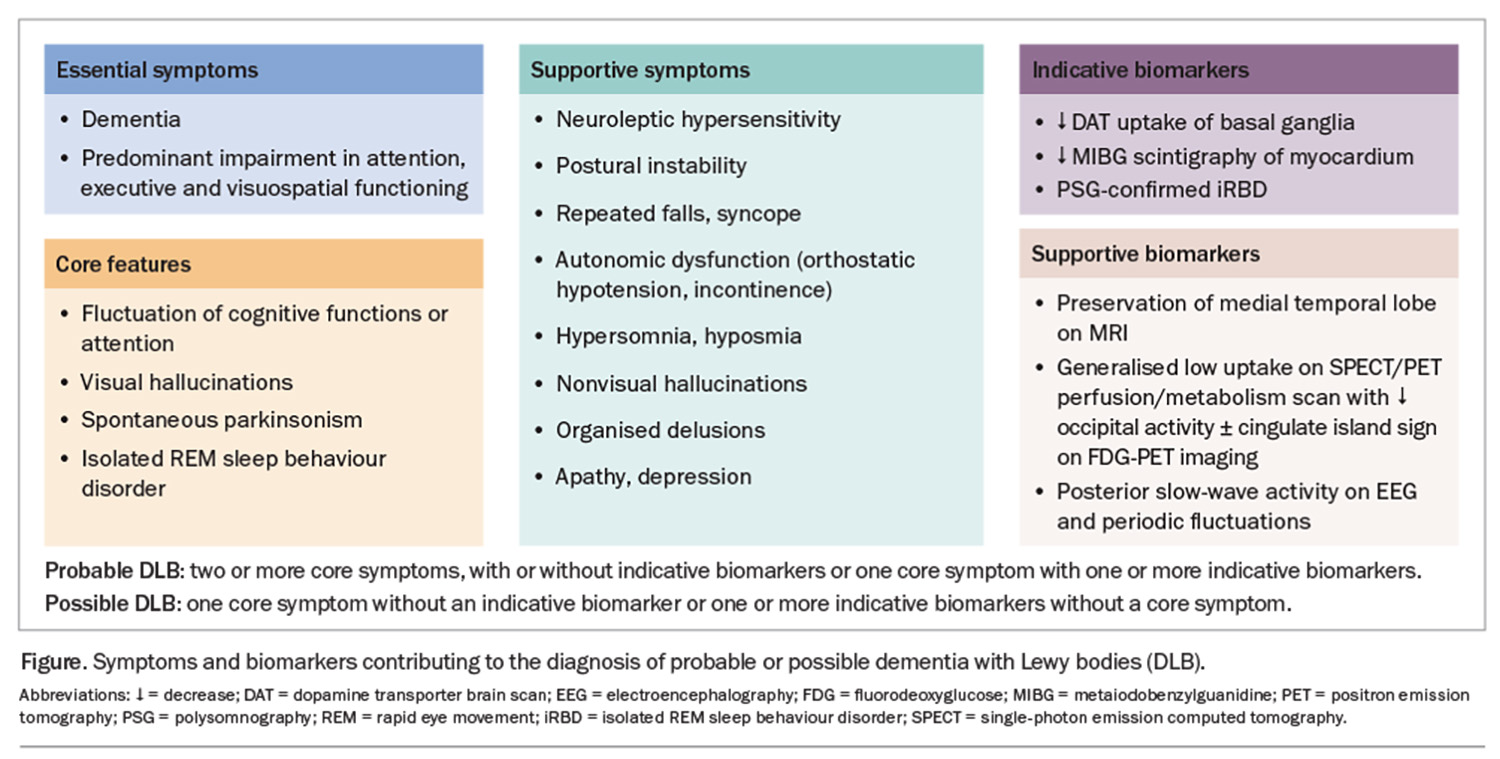
The DLB diagnostic criteria were last updated in 2017. In patients presenting with parkinsonism, a progression to dementia within a 12-month period is required for the diagnosis of DLB. Furthermore, the diagnosis is based on four core features: iRBD, cognitive fluctuations, spontaneous visual hallucinations and parkinsonism. Patients can be diagnosed with probable or possible DLB based on the number of core features and biomarkers they have (Figure).
Dementia is defined as a progressive decline in performance in one or more cognitive domains with subsequent impairment of activities of daily living in the absence of other physical or psychiatric conditions that could account for the presentation. Cognitive impairment in DLB is characterised by multidomain deficits with disproportionate involvement of attention, executive function and visuoperceptual domains, with relative sparing of memory.16
A variety of cognitive screening assessments are used in Australia and there are no DLB-specific screening tests. The Montreal Cognitive Assessment (MOCA) has been found to better differentiate DLB from AD compared with the Addenbrooke’s Cognitive Examination and the Mini-Mental State Examination (MMSE), although it may be more time-consuming than the latter.17 In a busy general practice environment, it has been suggested that clinicians could use the clock drawing test or intersecting pentagons as a quick tool to assess a patient’s executive and visuospatial domains. An enquiry about the patient’s driving skills is also strongly recommended.18,19
Cognitive fluctuations are seen as spontaneous alterations in consciousness and attention. Cognitive fluctuations are present in up to 40% of patients at diagnosis.14 Carers or loved ones may report patients ‘zoning out’ during conversations, incoherent speech or episodes of behavioural inconsistency.16 These episodes can lead to hospitalisation and the incorrect diagnosis of transient ischaemic attacks or nonconvulsive seizures. It is important to note that cognitive fluctuations can occur in other dementias, usually in the advanced stages of the disease.
Visual hallucinations occur in up to 80% of patients and can be the first symptom patients or carers report to their GPs.16 These can range from minor visual hallucinatory phenomena (e.g. sense of presence, sense of passage, visual illusions or misperceptions) to well-formed visual hallucinations. Complex visual hallucinations occur in the absence of visual stimuli and often involve people, children or animals. Patients may under-report these symptoms; therefore, careful questioning of both carers and patients is required.20,21
Spontaneous parkinsonism is present in a quarter of patients with DLB at presentation.14,16 It is important to note that the parkinsonism in patients with DLB does not have to meet the diagnostic criteria for PD (bradykinesia in combination with tremor and/or rigidity). Indeed, rigidity and tremor are the most commonly described parkinsonian symptoms in patients with DLB. Of note, tremor in DLB can be complex, with both postural and action tremors occurring rather than being restricted to the classic rest tremor observed in PD.22
The authors recommend performing postural blood pressure readings to screen for orthostatic hypotension that may be otherwise underdiagnosed. Patients may not always report presyncope but report feeling unsteady or tired after changing position. Similar symptoms occurring after meals can be related to postprandial hypotension. Ambulatory blood pressure monitoring may be helpful to assess a patient’s blood pressure trajectory accurately.
Biomarkers
Several indicative biomarker tests or tests for biomarkers specific for DLB are available in Australia; however, most are only available in tertiary care centres and are only accessible to specialists. Polysomnography conducted with additional electromyography channels for recording the mentalis, flexor digitorum superficialis and extensor digitorum brevis muscles can help detect REM sleep without atonia, the neurophysiological correlate of iRBD.
Unlike with AD, patients with DLB demonstrate a relative preservation of the medial temporal lobes on MRI, which can be used as a supportive biomarker.16 Furthermore, fluorodeoxyglucose (FDG)-positron emission tomography (PET) may demonstrate occipital lobe hypometabolism with relative preservation of posterior or midcingulate metabolism (cingulate island sign) (Figure).16 Dopamine transporter (DAT) imaging using single-photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging can be helpful in differentiating AD from DLB.16
There is emerging evidence that alpha-synuclein seed amplification assays can detect pathological alpha-synuclein species in skin, cerebrospinal fluid and, more recently, serum. This assay has been shown to have a high sensitivity and specificity in classifying patients with PD.23 The utility of this assay in diagnosing patients with DLB is yet to be fully determined, but it is certainly a promising biomarker.
Management
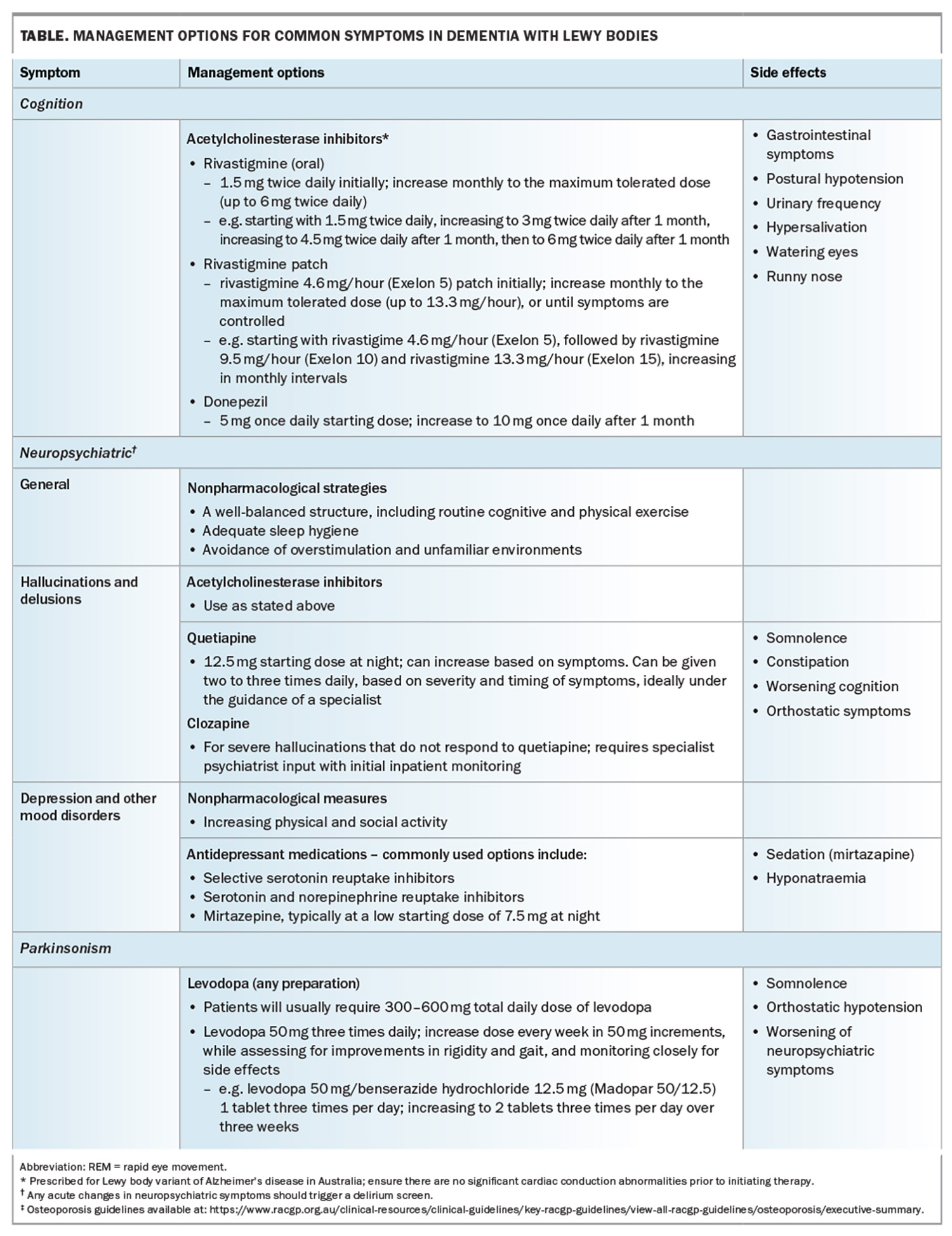
Management of DLB can be complex, given the wide range of cognitive, parkinsonian, neuropsychiatric and autonomic symptoms that patients with DLB present with. A comprehensive checklist guideline developed by the Diamond Lewy group is an excellent resource for managing these complex patients (Box 1).24
Motor symptoms
Rigidity and bradykinesia can impact negatively on patients’ mobility and functioning, leading to falls and increased frailty. Levodopa therapy can be effective in managing parkinsonism in DLB patients but requires careful monitoring and titration.25 Patients with DLB may require higher doses of levodopa, and up to two-thirds of patients may have only a minimal response. A slow titration of levodopa therapy while carefully monitoring for adverse effects (e.g. orthostatic hypotension and worsening of neuropsychiatric symptoms) is recommended. Dopamine agonists should be avoided as these can potentially worsen neuropsychiatric symptoms and somnolence.
Although direct evidence for DLB is lacking, patients are likely to benefit from regular exercise. The exercise should ideally be directed at improving aerobic capacity, core strength and balance, given the beneficial effects seen in patients with PD and its effects on reducing the risk of falls.
Cognitive and neuropsychiatric symptoms
Both the cognitive decline and neuropsychiatric symptoms in DLB have a significant impact on an individual’s functional ability and carer stress, which is associated with a lower quality of life.5
Acetylcholinesterase inhibitors (ACHEI) play an overarching role in the management of both cognition and visual hallucinations. Several studies have demonstrated improvement in cognition, completion of daily activities and reduction in carer burden.26 Furthermore, ACHEI have been shown to reduce composite neuropsychiatric scores.26 Both donepezil and rivastigmine are available in Australia for patients who do not have any contraindications (e.g. heart block). In our experience, rivastigmine patches are easier to use and titrate than oral preparations of ACHEI. Nausea and local skin irritation are the commonly reported symptoms but occur in less than 5% of patients. The use of
N-methyl-D-aspartate-(NMDA)-receptor antagonist memantine has been shown to be safe in patients with DLB; however, the evidence regarding its efficacy remains mixed.27-29
It is important to ensure no underlying drivers of neuropsychiatric symptoms are present, such as infection or constipation. Furthermore, all attempts should be made to ensure nonpharmacological methods are optimised before considering antipsychotic medications. Although the empirical evidence for the specific use of nonpharmacological management in DLB remains weak and needs further investigation, these therapies or interventions remain a crucial part of management.30 In the authors’ experience, strategies such as establishing a routine, which involves both physical and cognitive stimulation, can be beneficial. Furthermore, not all neuropsychiatric symptoms require treatment. For example, patients may have nonthreatening visual hallucinations that they have insight into and sometimes even find pleasant.31
Although commonly used in practice, it must be noted that the evidence for antipsychotic use is scarce.32 Therefore, the use of these agents needs to be reserved for situations where there is a major risk to the person or others and balanced against the plethora of potential side effects that are associated with their use. Furthermore, antipsychotics used to control behaviour in people lacking consent are considered restrictive practices and their use is regulated according to the relevant state or territory guardianship laws. Practitioners should first and foremost obtain patient consent and, when this is lacking, follow the guidelines relevant to their jurisdiction.
Quetiapine is the most studied and best-tolerated antipsychotic. It can be prescribed at a starting dose of 12.5 mg once daily, and titrated based on timing and symptom burden. It is important to gauge the timing of neuropsychiatric symptoms and their impact prior to embarking on therapy. For example, patients may report more distressing symptoms in the evenings. In the authors’ experience, timing of antipsychotics one to two hours prior to the onset of symptoms can help reduce their burden.
It must be emphasised that patients with DLB can be prone to neuroleptic sensitivity; therefore, atypical antipsychotics should be used with caution and older generation agents, such as haloperidol, should be avoided completely.
Clozapine has been shown to be effective in PD psychosis; however, no trials have been done specifically in patients with DLB.29 Nonetheless, it is still used when there are ongoing severe neuropsychiatric symptoms and can be effective in low doses. Initiation of clozapine may require the involvement of a psychiatrist and cardiologist and inpatient admission for monitoring.
A summary of common symptoms and their management is summarised in the Table and Table cont.33
What can patients tell their family?
Both patients and their family should be made aware that DLB is a rapidly progressive dementia, with many patients requiring residential care within two to three years of onset. The burden of neuropsychiatric symptoms and multidomain cognitive decline can be associated with increasing care burden and carer burnout. Key issues that we discuss with patients and their families are listed in Box 2.
Research and future therapies
As mentioned previously, alpha-synuclein seed amplification assays from both cerebrospinal fluid and plasma have been suggested as plausible diagnostic biomarkers that may be able to detect synuclein filament subtypes based on disease phenotype (i.e. DLB, PD and MSA).34,35 The use of this biomarker in prodromal disease is yet to be fully investigated. Nonetheless, it is likely that such methods may be used to detect DLB at a prodromal stage, hence allowing such patients to be included in trials of disease-modifying therapies. Several disease-modifying therapies have already been trialled in patients with PD.36 Currently, a trial in Australia is investigating the efficacy of ambroxol (a cough medicine) and doxycycline in reducing the accumulation of alpha-synuclein in patients with DLB (ACTRN12623000004662).37,38 Furthermore, the use of antiamyloid therapy in this population of patients (in whom up to 50% have a significant amyloid burden) is yet to be fully elucidated.7
Conclusion
DLB is an aggressive form of dementia that is underdiagnosed and has a significant impact on patients and their families. Early, accurate diagnosis and evidence-based treatment can improve quality of life and reduce carer stress. We believe that GPs play a crucial role in the early diagnosis and management of these patients. MT
COMPETING INTERESTS: Professor Lewis has received research grants from the Michael J Fox Foundation, Parkinson’s UK, NHMRC, MRFF and NHMRC/EU; payment or honoraria from the International Parkinson’s and Movement Disorders Society; and consulting fees from Acceler8 and Pharmaxis. Dr Gunawardana: None.
References
1. Vann Jones SA, O’Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med 2014; 44: 673-683.
2. Palmqvis S, Hansson O, Minthon L, Londos E. Practical suggestions on how to differentiate dementia with Lewy bodies from Alzheimer’s disease with common cognitive tests. Int J Geriatr Psychiatry 2009; 24: 1405-1412.
3. Hanyu H, Sato T, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Differences in clinical course between dementia with Lewy bodies and Alzheimer’s disease. Eur J Neurol 2009; 16: 212-217.
4. McKeith IG, Rowan E, Askew K, et al. More severe functional impairment in dementia with Lewy bodies than Alzheimer disease is related to extrapyramidal motor dysfunction. Am J Geriatr Psychiatry 2006; 14: 582-588.
5. Ricci M, Guidoni SV, Sepe-Monti M, et al. Clinical findings, functional abilities and caregiver distress in the early stage of dementia with Lewy bodies (DLB) and Alzheimer’s disease (AD). Arch Gerontol Geriatr 2009; 49: e101-104.
6. Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature 1997; 388: 839–840.
7. Bencze J, Seo W, Hye A, Aarsland D, Hortobágyi T. Dementia with Lewy bodies – a clinicopathological update. Free Neuropathol 2020; 1: 7.
8. Biundo R, Weis L, Fiorenzato E, et al. The contribution of beta-amyloid to dementia in Lewy body diseases: a 1-year follow-up study. Brain Commun 2021; 3: fcab180.
9. Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain J Neurol 2019; 142: 744–759.
10. Högl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration – an update. Nat Rev Neurol 2018; 14: 40–55.
11. Mckeith IG, Ferman TJ, Thomas AJ, et al; prodromal DLB Diagnostic Study Group. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 2020; 94: 743-755.
12. Gunawardana CW, Matar E, Lewis SJG. The clinical phenotype of psychiatric-onset prodromal dementia with Lewy bodies: a scoping review. J Neurol 2024; 271: 606-617.
13. Hansen N, Timäus C, Bouter C, Lange C, Packroß K. Delirium-onset of prodromal dementia with Lewy bodies—putative brainstem-related pathomechanism and clinical relevance. Front Aging Neurosci 2022; 14: 829098.
14. Auning E, Rongve A, Fladby T, et al. Early and presenting symptoms of dementia with Lewy bodies. Dement Geriatr Cogn Disord 2011; 32: 202–208.
15. Matar E, Lewis SJ. REM sleep behaviour disorder: not just a bad dream. Med J Aust 2017; 207: 262-268.
16. Mckeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies. Neurology 2017; 89: 88-100.
17. Yamamoto E, Mourany L, Colleran R, Whitman C, Tousi B. Utility of Montreal Cognitive Assessment in differentiating dementia with Lewy bodies from Alzheimer’s dementia. Am J Alzheimers Dis Dementias 2017; 32: 468-471.
18. Ala T, Hughes L, Kyrouac G, Ghobrial M, Elble R. Pentagon copying is more impaired in dementia with Lewy bodies than in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2001; 70: 483-488.
19. Cagnin A, Bussè C, Jelcic N, Gnoato F, Mitolo M, Caffarra P. High specificity of MMSE pentagon scoring for diagnosis of prodromal dementia with Lewy bodies. Parkinsonism Relat Disord 2015; 21: 303–305.
20. Shine JM, Mills JMZ, Qiu J, et al. Validation of the psychosis and hallucinations questionnaire in non-demented patients with Parkinson’s disease. Mov Disord Clin Pract 2015; 2: 175-181.
21. Muller AJ, Mills JMZ, O’Callaghan C, et al. Informant- and self-appraisals on the Psychosis and Hallucinations Questionnaire (PsycH-Q) enhances detection of visual hallucinations in Parkinson’s disease. Mov Disord Clin Pract 2018; 5: 607–613.
22. Onofrj M, Varanese S, Bonanni L, et al. Cohort study of prevalence and phenomenology of tremor in dementia with Lewy bodies. J Neurol 2013; 260: 1731-1742.
23. Siderowf A, Concha-Marambio L, Lafontant DE, et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol 2023; 22: 407-417.
24. DIAMOND-Lewy - Newcastle University. DIAMOND-Lewy: Management toolkit. Newcastle University, UK; 2017. Available from: https://research.ncl.ac.uk/diamondlewy/managementtoolkit/ (accessed July 2024)
25. Molloy S, McKeith IG, O’Brien JT, Burn DJ. The role of levodopa in the management of dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2005; 76: 1200-1203.
26. Wang HF, Yu JT, Tang SW, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry 2015; 86: 135-143.
27. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol 2009; 8: 613-618.
28. Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9: 969-977.
29. Taylor JP, McKeith IG, Burn DJ, et al. New evidence on the management of Lewy body dementia. Lancet Neurol 2020; 19: 157-169.
30. Morrin H, Fang T, Servant D, Aarsland D, Rajkumar AP. Systematic review of the efficacy of non-pharmacological interventions in people with Lewy body dementia. Int Psychogeriatr 2018; 30: 395-407.
31. Powell A, Ireland C, Lewis SJG. Visual hallucinations and the role of medications in Parkinson’s disease: triggers, pathophysiology, and management. J Neuropsychiatry Clin Neurosci 2020; 32: 334-343.
32. Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry 2015; 172: 731-742.
33. Pedrosa Carrasco AJ, Timmermann L, Pedrosa DJ. Management of constipation in patients with Parkinson’s disease. Npj Park Dis 2018; 4: 1-10.
34. Grossauer A, Hemicker G, Krismer F, et al. α‐Synuclein seed amplification assays in the diagnosis of synucleinopathies using cerebrospinal fluid—a systematic review and meta‐analysis. Mov Disord Clin Pract 2023; 10: 737-747.
35. Okuzumi A, Hatano T, Matsumoto G, et al. Propagative α-synuclein seeds as serum biomarkers for synucleinopathies. Nat Med 2023; 29: 1448-1455.
36. McFarthing K, Buff S, Rafaloff G, et al. Parkinson’s disease drug therapies in the clinical trial pipeline: 2023 update. J Park Dis 2023; 13: 427-439.
37. Chwiszczuk LJ, Breitve MH, Kirsebom BEB, et al. The ANeED study – ambroxol in new and early dementia with Lewy bodies (DLB): protocol for a phase IIa multicentre, randomised, double-blinded and placebo-controlled trial. Front Aging Neurosci 2023; 15: 1163184. eCollection 2023.
38. Gibson LL, Abdelnour C, Chong J, Ballard C, Aarsland D. Clinical trials in dementia with Lewy bodies: the evolving concept of co-pathologies, patient selection and biomarkers. Curr Opin Neurol 2023; 36: 264-275.