Prasterone: a new treatment for vulvovaginal atrophy in postmenopausal women
Prasterone is a preparation of dehydroepiandrosterone (DHEA), available as a vaginal pessary for the treatment of postmenopausal vulvovaginal atrophic symptoms. It offers an alternative treatment to vaginal oestrogens, having unique oestrogen and androgen actions within tissues of the genital tract without systemic activity.
- Vulvovaginal atrophy (VVA) is common in postmenopausal women, with up to 70% experiencing symptoms.
- Current treatment for VVA include vaginal oestrogen therapy and moisturisers or lubricants.
- Prasterone is a preparation of dehydroepiandrosterone (DHEA), an inactive precursor steroid, that is available as a pessary for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy in postmenopausal women.
- Prasterone has been shown to improve symptoms of VVA with minimal side effects and is another option to vaginal oestrogens.
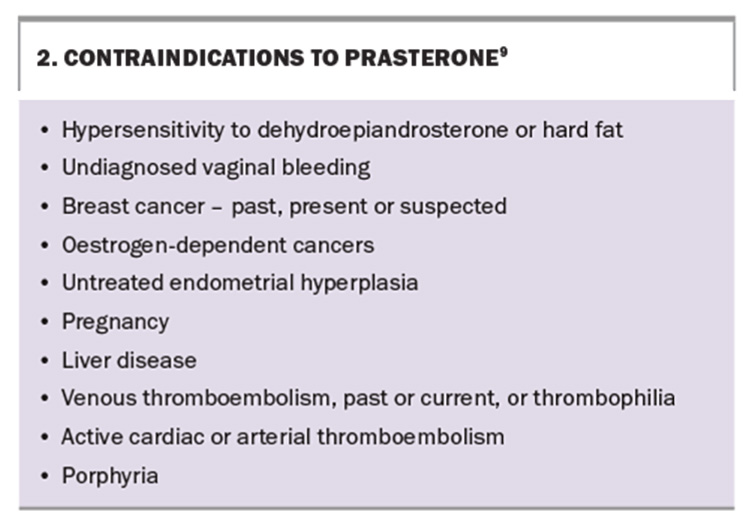
- Contraindications to prasterone include hypersensitivity to DHEA or hard fat, breast or oestrogen-dependent cancers, liver cancer and cardiovascular disorders.
Symptoms of genitourinary syndrome of the menopause, including vulvovaginal atrophy (VVA), are very common in postmenopausal women, under-recognised and undertreated, and have a major impact on a woman’s quality of life.1,2 About 50% of women aged 50 to 60 years and about 70% of women aged over 70 years have symptoms of VVA.3
Many women perceive these symptoms as ‘normal’ and as something they have to endure, are unaware that treatments are available and feel embarrassed to tell their health professionals. In one study, only one in four women reported symptoms and 4% of women were treated.4 It is therefore important that health professionals identify a sensitive and nonthreatening way of initiating a discussion of the symptoms of VVA with their patients.
Common symptoms of VVA are listed in Box 1. Symptoms develop because of the loss of oestrogen, leading to anatomical changes in the genitourinary tissues. Additionally, androgen receptors are present in the genital tract. After the menopause, the expression of both oestrogen and androgen receptors is reduced, resulting in reduced cell growth, mucin and collagen production and blood flow and changes in nerve conduction.5
Treatments for vulvovaginal atrophy
The use of vaginal oestrogen therapy has been the main treatment for local symptoms, as VVA signs and symptoms may persist in about 40% of those prescribed systemic menopause hormone therapy.6 Other therapies available for vaginal dryness include vaginal moisturisers or lubricants, which may be used every three days. Vaginal laser therapy has been used to increase vaginal secretions but studies have shown no improvement in vaginal symptoms after 12 months. Long-term data on safety and efficacy are lacking.7
Prasterone dehydroepiandrosterone (DHEA) provides another treatment option for women with VVA.
Mode of action of prasterone
DHEA is an inactive precursor steroid, which, in vaginal cells, is converted into oestrogens and androgens by intracellular enzymes, allowing the required hormone to be produced depending on the cell’s needs. This intracellular mechanism is called intracrinology. Because prasterone also produces androgens, it is different from vaginal oestrogen products. Prasterone is identical to endogenous human DHEA.
Prasterone also has little systemic effect on other tissues as minimal hormones diffuse into the bloodstream, with oestrogen and testosterone levels remaining within the postmenopausal range. This differs from oestrogen vaginal products, where active hormones are absorbed directly into the bloodstream, with direct effects on other tissues, such as the bladder and pelvic floor. Prasterone shows no endometrial stimulation because the endometrium does not contain enzymes to convert DHEA to oestrogens.8
How to use, and indications for, prasterone
Prasterone is an intravaginal pessary approved by the Therapeutics Goods Administration for the treatment of vulvar and vaginal atrophy in postmenopausal women having moderate to severe symptoms.9 Each pessary contains 6.5 mg of prasterone (DHEA) in hard fat. It is administered intravaginally with the use of the provided applicator or with fingers. One pessary is administered once a day at bedtime. The trade pack contains 28 pessaries and six reusable applicators. Prasterone would be the preferred choice for the treatment of VVA for women who experience adverse reactions or sensitivities to oestrogen vaginal preparations.
Efficacy and side effects of prasterone
Placebo-controlled phase 3 trials of prasterone use in postmenopausal women with VVA symptoms showed the following positive outcomes:10,11
- improvement in vaginal superficial cell numbers and a reduction in parabasal cells
- an increase in vaginal wall thickness
- an increase in vaginal secretions
- a reduction in vaginal pH to more acidic
- improved sexual function with reduction in dyspareunia and increase in sexual modalities and satisfaction.
The most common side effects observed were vaginal discharge, urinary tract infections (UTIs) and headache.10,11 The discharge was due to the melting of the hard fat, which is the vehicle used containing the DHEA. It is the only excipient used in the production of prasterone. The rate of UTIs was small and lower than that for postmenopausal women.12 Other side effects were not attributed to the product. Abnormal cervical smears were noted in 2% of participants at the end of the 52-week study. The reason for the increased abnormal smear is not thought be due to use of prasterone, as DHEA has an antiproliferative effect and induces apoptosis of human papilloma virus-positive and -negative cancer cells.13
At present, the 52-week study is the longest published.10,11 Prasterone has not been studied in women with breast cancer, hormone-dependent cancers and other conditions, which are listed as contraindications. Many of the contraindications, special precautions and conditions for prasterone are similar to those for systemic and topical estrogen-based therapies.9 Additional contraindications to prasterone use, including an allergy to hard fat, are listed in Box 2.
Conclusion
Prasterone is a newly registered vaginal pessary for moderate to severe postmenopausal VVA symptoms. It is a nightly pessary that has the side effect of some increase in vaginal discharge. The hormonal actions of androgens and oestrogens take place intracellularly (intracrinology) and, therefore, prasterone has no systemic effects. It is another option to vaginal oestrogens for treating symptoms of VVA. MT
COMPETING INTERESTS: Dr Farrell is on the Advisory Boards for Theramex and Astellas; and a speaker for Theramex on prasterone.
This article is for general information purposes only, and the full Product Information should be consulted before prescribing any of the mentioned medications.
References
1. Davis SR, Lambrinoudaki I, Lumsden M, et al. Menopause Nat Rev Dis Primers 2015; 1: 15004
2. Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014; 21(10): 1063-1068
3. Rossin-Amar B. Vaginal dryness in the menopausal woman (physiological and psychological aspects). Gynecol Obstet Fertil 2000; 28: 245-249.
4. Palacios S, Nappi RE, Bruyniks N, Particco M, Panay N; EVES Study Investigators. The European Vulvovaginal Epidemiological Survey (EVES): prevalence, symptoms and impact of vulvovaginal atrophy of menopause. Climacteric 2018; 21: 286-291.
5. Traish AM, Vignozzi L, Simon JA, Goldstein I, Kim NN. Role of androgens in female genitourinary tissue structure and function: implications in the genitourinary syndrome of menopause. Sex Med Rev 2018; 6: 558-571.
6. Notelovitz M. Urogenital aging: solutions in clinical practice. Int J Gynecol Obstet 1997; 59 Suppl 1: S535-S539.
7. Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA 2021; 326: 1381-1389.
8. Labrie F, Labrie C. DHEA and intracrinology at menopause, a positive choice for evolution of the human species. Climacteric 2013; 16: 205-213.
9. Therapeutic Goods Administration (TGA). Australian prescription medicine decision summaries: Intrarosa. TGA; Canberra, June 2023. Available online at: https://www.tga.gov.au/resources/auspmd/intrarosa (accessed August 2024).
10. Labrie F, Archer D, Koltun W et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause 2018; 25: 1339-1353.
11. Labrie F, Archer D, Martel C et al. Combined data of intravaginal prasterone against vulvovaginal atrophy of menopause. Menopause 2017; 24: 1246-1256.
12. Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol 2019; 11: 1756287219832172.
13. Girón RA, Montaño LF, Escobar ML, López-Marure R. Dehydroepiandrosterone inhibits the proliferation and induces the death of HPV-positive and HPV-negative cervical cancer cells through an androgen- and estrogen-receptor independent mechanism. FEBS J2009; 276: 5598-5609.