An update on metastatic breast cancer – new targeted therapies

Early breast cancer therapy is associated with a relatively high risk of recurrence; therefore, a low threshold should remain for investigating symptoms suggestive of metastatic spread in women who are diagnosed with early breast cancer. New therapies targeting specific molecular pathways, including oral and intravenous agents and immunotherapies, show promise in prolonging survival in patients with metastatic breast cancer.
- Breast cancer is the most common cancer in women in Australia, with one in seven being diagnosed in their lifetime.
- Metastatic breast cancer occurs in up to 30% of these women and is incurable.
- The aims of treatment for metastatic breast cancer are prolonging life and managing symptoms.
- Targeted therapies have changed the treatment landscape, extending survival to beyond five years in some patient groups.
- New targeted therapies include: inhibitors of cyclin-dependant kinase 4 and 6, the phosphoinositide 3-kinase pathway, poly (ADP-ribose) polymerase and human epidermal growth factor receptor 2; antibody-drug conjugates; and immunotherapies.
Breast cancer is a heterogeneous disease that is classified by the main molecular markers of oestrogen (ER) or progesterone (PR) receptors and human epidermal growth factor receptor 2 (HER2) status. Breast cancers comprise 70% ER- and/or PR-positive tumours, 15 to 20% HER2-amplified tumours (with 50% of these also being ER-positive) and 10 to 15% ER/PR/HER2-negative (triple-negative breast cancer [TNBC]) tumours. Metastatic breast cancer (MBC) refers to breast cancer that has spread to distant organs, such as the lungs, liver, bones or brain. Up to 30% of patients with breast cancer will develop distant metastases over their lifetime, with bones being the most common site.1 Only 6 to 10% of MBC cases are de novo, with 90 to 94% of cases occurring due to relapse of early breast cancer. MBC after therapy for early breast cancer appears to have worse outcomes compared with de novo disease.2
In TNBC and HER2-positive tumours, the risk of recurrence is highest in the first two to three years postdiagnosis and is less likely after five years,3 whereas ER- or PR-positive tumour relapse may occur up to 20 years after initial diagnosis.4 Given the high incidence of early breast cancer and the propensity for hormone receptor-positive breast cancers to metastasise decades later, primary care practitioners must have a high clinical suspicion for MBC if these patients present with any symptoms suggestive of metastatic spread. Common symptoms include back pain, right upper quadrant abdominal pain or breathlessness that are persistent and do not fluctuate. In particular, bone pain from metastatic spread is worse at night. Primary care practitioners have a crucial role in detection and referral of patients with MBC. They also play a major role in co-ordination of care, and in understanding the natural history of the disease, available treatment options and their expected side effects. Resources on metastatic breast cancer for GPs and their patients are listed in Box 1.
Although MBC is incurable, patients with metastatic disease are living longer, with an average overall survival of about 15 months for patients with TNBC and five years for patients with ER- or PR-positive or HER2-positive disease.5 Visceral metastases, brain metastases and multiple metastatic sites all confer a worse prognosis, whereas a better performance status, younger age at diagnosis, bone-only disease and longer interval between initial diagnosis and development of metastatic recurrence all confer improved prognosis.
Hormone treatment and chemotherapeutic agents remain an important part of cancer treatment. These agents have remained relatively unchanged during the past 20 years. Newer targeted therapies have improved the time to disease progression and overall survival. As with all stage 4 cancers, systemic therapy remains the backbone of management and will be the focus of this article. Local therapies, such as surgery and radiation therapy, are palliative in nature and may be considered on a case-by-case basis.
Investigations and diagnosis
Initial investigations for patients presenting with a symptom suggestive of metastatic spread may include a bone scan or CT to investigate specific symptoms. Positron emission tomography-CT, a whole-body molecular imaging modality, is now being used more frequently by specialists if the diagnosis is uncertain. The gold standard for diagnosing MBC is a biopsy of a metastatic lesion to confirm histology and reassess tumour biology, as up to 20% of tumours will have a change in breast cancer subtype compared with the primary tumour.6 The most frequent biomarker changes are from a positive to negative ER/PR or HER2 status; however, there can be conversion from negative to positive.6 Changes in biomarker status are important when determining management options since treatment choice is directly related to the tumour subtype and biology.
New treatments
Cyclin-dependent kinase 4 and 6 inhibitors
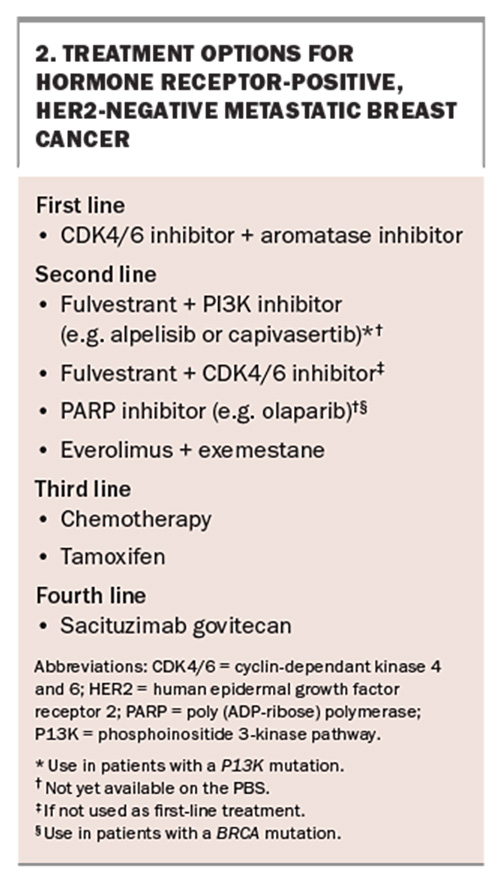
Cyclin-dependant kinase 4 and 6 (CDK4/6) inhibitors, which include ribociclib, palbociclib and abemaciclib, are oral treatments given in combination with endocrine therapy for hormone receptor-positive, HER2-negative MBC (Box 2). They have proven effectiveness and are PBS listed for use in combination with an aromatase inhibitor for patients with de novo metastatic disease and those experiencing locally advanced unresectable, or metastasis of, early breast cancer. CDK4/6 inhibitors can also be used as later-line treatment after endocrine therapy in combination with fulvestrant, a selective oestrogen receptor degrader.
The average time to disease progression for patients treated with a CDK4/6 and an aromatase inhibitor combination has been shown to be 24 months, compared with 12 months for patients treated with endocrine therapy alone.7 CDK4/6 inhibitor side effects differ slightly between the different agents; however, common class effects include myelosuppression and gastrointestinal disturbance.7 Palbociclib and ribociclib more commonly cause neutropenia, whereas abemaciclib more commonly causes diarrhoea.7 Patients receiving CDK4/6 inhibitors are reviewed monthly with blood tests to monitor neutrophil count, platelet count, estimated glomerular filtration rate and liver function. Patients with neutropenia, thrombocytopenia, renal or liver dysfunction may need the treatment dose reduced, or treatment withheld or ultimately discontinued. Additionally, if a patient develops an intercurrent infection during a treatment cycle or requires a surgical procedure, it is recommended CDK4/6 inhibitors are withheld until the patient is well. A rare side effect from ribociclib can be prolonged QTc interval; therefore, an ECG should be performed to monitor the QTc interval before initiating treatment and repeated two weeks into the first treatment cycle and at the beginning of the second cycle, and as clinically indicated.
Phosphoinositide 3-kinase pathway inhibitors
Phosphoinositide 3-kinase pathway (PI3K) mutations are the most common mutations in ER-positive breast cancer, occurring in 40% of tumours.8 These mutations are associated with resistance to endocrine therapy and are directly related to mutations in the PIK3CA gene.9,10 The PI3K- alpha-specific inhibitor, alpelisib, has proven effectiveness when used in combination with the anti-oestrogen, fulvestrant, in treating patients with hormone receptor-positive, HER2-negative breast cancer with a PI3K mutation who have previously received endocrine therapy.8 In a phase 3 clinical trial of such patients, the length of time to progression of disease almost doubled to 11 months in patients given alpelisib-fulvestrant, compared with almost six months in those who received placebo-fulvestrant.8
The most common adverse effects of alpelisib-fulvestrant therapy include hyperglycaemia, diarrhoea, gastrointestinal upset, rash and alopecia. Although patients with type 1 and type 2 diabetes were excluded from this clinical trial, about two-thirds of patients experienced hyperglycaemia and required treatment with oral hypoglycaemic agents, usually metformin.8 Monitoring blood glucose levels and titration of oral hypoglycaemic agents in collaboration with primary care practitioners is important for patient safety. Alpelisib must be used in combination with fulvestrant. Alpelisib-fulvestrant combination is not currently PBS listed but may become available in the near future.
Treatments currently available on the PBS to overcome endocrine therapy resistance include the mechanistic target of rapamycin (mTOR) inhibitor everolimus; however, it is rarely used because of its poor tolerability. Another new drug, capivasertib, which aims to overcome endocrine resistance by inhibiting the AKT10 pathway, has recently shown effectiveness when combined with fulvestrant, but is not yet available on the PBS. More therapies will likely be available in the near future that aim to overcome endocrine resistance.
Poly (ADP-ribose) polymerase inhibitors
Poly (ADP-ribose) polymerase (PARP) inhibitors stop damaged cancer cells from repairing themselves, thereby leading to cell death. They have proven efficacy in breast tumours with germline BRCA gene mutations. BRCA1 and BRCA2 are tumour suppressor genes that are involved in repairing damaged double-stranded breaks in DNA. Cells that do not have a functional BRCA1 or BRCA2 gene are unable to repair this damage, which can lead to cancer.11BRCA mutations are inherited as autosomal dominant and increase the risk of breast and ovarian cancer in women. About 5% of women diagnosed with breast cancer have a BRCA mutation.12
The PARP inhibitors olaparib and talazoparib have proven efficacy in the treatment of MBC. Patients who received talazoparib had a longer time to cancer progression compared with those who received physician’s choice chemotherapy. The average time patients received this treatment before disease progression was almost nine months.13 Similarly, in patients who received treatment with olaparib, the average time to progression of breast cancer was seven months, almost double that of those receiving standard chemotherapy.14
The main side effects of PARP inhibitors are cytopenias and gastrointestinal upset. There is a rare increased risk of haematological malignancy with long-term use of these drugs. Olaparib is not yet available on the PBS for MBC but it may be acquired through compassionate access programs. Olaparib is PBS listed as an adjuvant to chemotherapy for HER2-negative early breast cancer.
Tucatinib
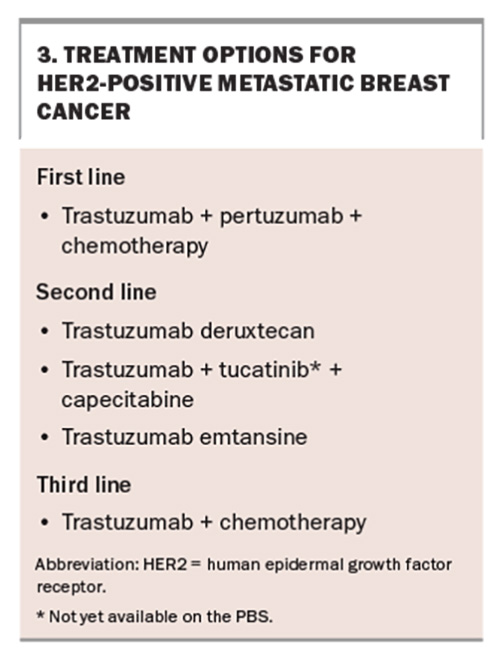
Tucatinib is a highly selective oral inhibitor of HER2 tyrosine kinase. It has been evaluated in combination with trastuzumab and capecitabine in patients with HER2-positive MBC previously treated with trastuzumab combined with pertuzumab and trastuzumab emtansine.15 Patients assigned tucatinib with trastuzumab plus capecitabine had a clinically meaningful lower risk of disease progression and increased overall survival of 4.5 months compared with patients given placebo with trastuzumab and capecitabine.15 The average time patients received this treatment before progression of disease was almost eight months. Importantly, it was effective at slowing disease activity in patients with brain metastases,15 which occur in about 50% of patients with metastatic HER2-positive breast cancer. The most common side effects of tucatinib combined with capecitabine and trastuzumab are hand-foot syndrome, diarrhoea, increased liver enzymes and fatigue. Tucatinib is not currently available on the PBS.
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) are a relatively new form of systemic therapy for cancer treatment. They target cells expressing specific cancer antigens using a directed antibody-drug delivery system that ultimately releases a cytotoxic agent to cancer cells with minimal exposure to normal cells.16 Over the past 10 years, many new ADCs have been developed, particularly for use in the treatment of breast cancer. Emerging ADCs in the metastatic breast cancer setting include trastuzumab deruxtecan and sacitzumab govitecan.
Trastuzumab deruxtecan
Trastuzumab deruxtecan (T-DXd) is an ADC used for second-line treatment and beyond in HER2-positive MBC. It is used for patients who have had progressive disease after treatment with first-line trastuzumab and pertuzumab combined with taxane chemotherapy (Box 3). A randomised controlled trial in patients with HER2-positive MBC showed a significant benefit with T-DXd use, with a time to disease progression of almost 29 months compared with seven months with trastuzumab emtansine use.17,18 Trials have shown that T-DXd has better central nervous system penetration than conventional first-line HER2-targeted therapies.19 T-DXd is available on the PBS for patients with HER2-positive MBC. Although it has also demonstrated significant efficacy in patients with a lower level of HER2 expression, it is not yet PBS listed for this indication.20
The most common side effects are gastrointestinal and haematological, but the most substantial risk is that of interstitial lung disease which occurs in 10 to 15% of patients.19 Both medical oncologists and primary care physicians must have a high degree of clinical suspicion for their patients receiving this treatment who present with pulmonary symptoms, including dry cough and dyspnoea.20 A high-resolution CT of the chest is recommended if new respiratory symptoms arise.
Sacituzumab govitecan
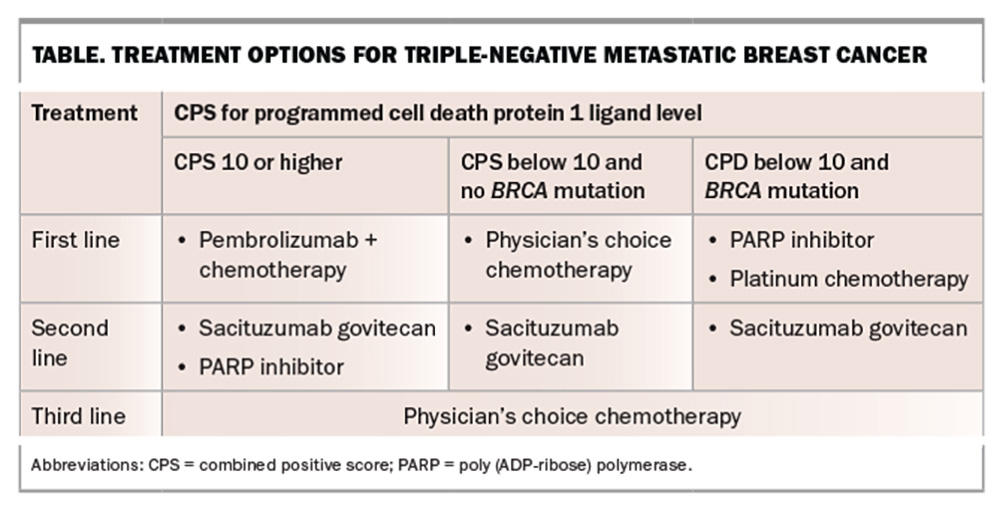
Sacituzumab govitecan is a first-in-class trophoblast cell-surface antigen 2 ADC. It has been shown to improve survival and time to progressive disease in patients with hormone receptor-positive, HER2-negative MBC and those with metastatic TNBC.21,22 In patient with TNBC, those receiving sacituzumab govitecan therapy had a longer time to disease progression of almost six months compared with just under two months for those receiving physician’s choice chemotherapy.22 Survival almost doubled in patients treated with sacituzumab govitecan, extending to 12.1 months compared with 6.7 months for those on chemotherapy.22
Sacituzumab govitecan is approved for use in patients with hormone receptor-positive, HER2-negative breast cancer who have received endocrine-based therapy and at least two additional systemic therapies, and for those with metastatic TNBC who have received at least two previous systemic therapies. It is PBS listed for use in patients with TNBC. The most common side effects include neutropenia, diarrhoea, nausea and alopecia.23 These may be more pronounced in patients known to have Gilbert’s syndrome.
Immunotherapy
Pembrolizumab targets and blocks the programmed cell death protein 1 (PD-1) on the surface of T cells and is used to treat many cancer types. In patients with TNBC, pembrolizumab is combined with chemotherapy as first-line therapy for those with high levels of expression of the PD1 ligand, defined as a combined positive score (CPS) of at least 10, and no contraindication to immunotherapy (Table). CPSs are used as indicators of likely response to immunotherapy.
The use of pembrolizumab combined with chemotherapy in patients with advanced TNBC has shown increased survival to an average of almost two years, compared with 16 months for treatment with chemotherapy alone.24 Additionally, the average time before cancer progression was almost doubled to 10 months on pembrolizumab combined with chemotherapy compared with chemotherapy alone.24 About 25% of patients experienced an immune-mediated adverse event (iRAE) from pembrolizumab combined with chemotherapy.24 Most iRAEs were low grade and, when promptly treated, resulted in good safety outcomes. High-grade iRAEs were infrequent.25 The most common organ systems affected by PD-1 inhibitors include dermatological (rash and pruritis), gastrointestinal (diarrhoea), endocrine (hypothyroidism, hyperthyroidism, hypophysitis and adrenal insufficiency), hepatic (hepatitis) and pulmonary (interstitial lung disease).
Pembrolizumab is PBS listed for use in combination with chemotherapy as first-line therapy for patients with TNBC who have a CPS of at least 10, and no contraindication to immunotherapy. Treatment of iRAEs includes withholding immunotherapy and administering steroid therapy. In severe cases, additional immunosuppression, such as with infliximab, may be required in addition to permanent cessation of immunotherapy. Patients with a personal history of an autoimmune condition are more likely to experience iRAEs. This is not a contraindication, and the decision to administer immunotherapy is made on a case-by-case basis.
Role of next-generation sequencing in treatment decisions
Routine multigene next-generation sequencing testing has limited value in determining treatment pathways in MBC. A targeted panel to identify PIK3CA and ER mutations is useful to identify patients who may benefit from a PI3K-alpha- specific inhibitor and selective ER degraders. Prospective trials of patients selected for therapies based on specific mutations are ongoing but next-generation sequencing testing is not recommended outside the clinical trial setting.
Conclusion
MBC is relatively common and overall survival is improving with an evolving treatment landscape. Many women live for at least five years with metastatic disease and can remain on a single treatment modality for many months to years. Primary care practitioners have a crucial role in the diagnosis and ongoing management of patients with MBC. They must have high clinical suspicion for metastatic disease in patients with previously treated breast cancer who have been discharged from their oncology service and in those with concerning symptoms that warrant further investigation. Additionally, primary care practitioners play an important role in assisting with the management of treatment-related toxicities, reinforcing the importance of exercise for improving quality of life and the provision of psychological and wellbeing support through GP management plans. MT
COMPETING INTERESTS: Dr Smith, Dr Hui, Associate Professor McNeil, Associate Professor: None. Dr Kumar has received consulting fees from Novartis and Roche; and speaker fees and/or travel support from AstraZeneca, Eli Lilly, Gilead, MSD, Novartis and Pfizer.
References
1. Pulido C, Vendrell, I, Ferreira A, et al. Bone metastasis risk factors in breast cancer. Ecancer Medical Sciences 2017; 11: 715.
2. Gennari A, Andre F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 2021; 32: 1475-1495.
3. Eldridge L. Late recurrence vs early relapse of breast cancer. New York, NY: Dotdash Meredith; 2022. Available online at: https://www.verywellhealth.com/late-recurrence-of-breast-cancer-4766608 (accessed July 2024).
4. Pfeiler G, Hlauschek D, Mayer EL, et al; PALLAS Groups and Investigators. Impact of BMI in patients with early hormone receptor-positive breast cancer receiving endocrine therapy with or without palbociclib in the PALLAS Trial. J Clin Oncol 2023; 41: 5118-5130.
5. Courtinard C, Gourgou S, Jacot W, et al. Association between progression-free survival and overall survival in women receiving first-line treatment for metastatic breast cancer: evidence from the ESME real-world database. BMC Medicine 2023; 21: 87.
6. Woo J, Chung YR, Ahn S, et al. Changes in biomarker status in metastatic breast cancer and their prognostic value. J Breast Cancer 2019; 22: 439-452.
7. Desnoyer A, Nadler MB, Kumar V, Saleh R, Amir E. Comparison of treatment-related adverse events of different cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: a network meta-analysis. Cancer Treat Rev 2020; 90: 102086.
8. Andre F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med 2019; 380: 1929-1940.
9. Alpelisib for breast cancer. Aust Prescr 2022; 45: 95-96.
10. Turner N, Oliveira M, Howell SJ, et al. Capivasertib in hormone receptor–positive advanced breast cancer. N Engl J Med 2023; 388: 2058-2070.
11. Lau C, Suthers G. BRCA testing for familial breast cancer. Aust Prescr 2011; 34: 49-51.
12. Litton J, Burstein HJ, Turner NC. Molecular testing in breast cancer. Am Soc Clin Oncol Educ Book 2019; 39: e1-7.
13. Litton J, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018; 379: 753-763.
14. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017; 377: 523-533.
15. Murthy R, Loi S, Okines A, et al. Tucatinib, trastuzumab and capecitabine for HER2-positive metastatic breast cancer. Erratum in: N Engl J Med 2020; 382: 586.
16. Xiao T, Ali S, Mata DGMM, Lohmann AE, Blanchette PS. Antibody-drug conjugates in breast cancer: ascent to destiny and beyond-a 2023 review. Curr Oncol 2023; 30: 6447-6461.
17. Hurvitz S, Hegg R, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 2023; 401: 105-117.
18. Cortes J, Kim SB, Chung WP, et al. Trastuzumab derxtecan versus trasuzumab emtansine for breast cancer. N Engl J Med 2022; 386: 1143-1154.
19. Bartsch R, Berghoff AS, Furtner J, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nature Med 2022; 28: 1840-1847.
20. Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 2022; 387: 9-20.
21. Rugo H, Bardia D, Marme F, et al. Sacituzumab govitecan in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2022; 40: 3365-3376.
22. Bardia A, Hurvitz SA, Tolaney SM, et al; ASCENT Clinical Trial Investigators. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med 2021; 384: 1529-1541.
23. Spring L, Nakajima E, Hutchinson J, et al. Sacituzumab govitecan for metastatic triple-negative breast cancer: clinical overview and management of potential toxicities. Oncologist 2021; 26: 827-834.
24. Cortes J, Rugo HS, Cescon DW, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med 2022; 387: 217-226.
25. Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther 2017; 6: 57-71.