Early-stage breast cancer: update on management
Advances in management of early-stage breast cancer include increasing use of neoadjuvant (presurgery) systemic therapy and oncoplastic surgical techniques in selected patients and the availability of new systemic therapies. These include targeted monoclonal antibody therapies, cyclin-dependent kinase 4/6 inhibitors, antibody-drug conjugates and immunotherapies.
- The management of patients with early-stage breast cancer has become increasingly complex and variable, with choices influenced by tumour and patient characteristics.
- Neoadjuvant (presurgery) systemic therapy is increasingly used in patients with high-risk early-stage breast cancer.
- Advances in oncoplastic surgery and breast reconstruction can improve cosmesis and quality of life for patients.
- Developments in systemic therapies, including targeted therapies, antibody-drug conjugates and immunotherapies, have improved patient outcomes.
- There is evidence of benefit from extension of the duration of adjuvant endocrine therapy to 10 years and the augmentation of endocrine therapy with ovarian function suppression in young patients.
Breast cancer is the most common non-skin cancer in Australia, affecting one in seven women by the age of 85 years.1 Management of early-stage breast cancer has become increasingly variable and complex. Current treatments include surgery, chemotherapy, targeted therapies, immunotherapy, radiotherapy and endocrine therapy. Hence, there is a wide scope of therapies, which can greatly affect recurrence rates and overall survival of patients with breast cancer.
This article summarises the current management of patients with early-stage breast cancer in Australia. Early-stage breast cancer is defined as Stage I (T1, N0/N1mi, M0) or Stage II (T1/T2, N1, M0 or T2/T3, N0, M0) disease (where T1 = tumour size less than 2 cm; T2 = size 2 to 5 cm; T3 = size larger than 5 cm; N0 = no axillary nodal involvement; N1mi = presence of micrometastatic disease; N1 = one to three axillary nodes involved; and M0 = no metastases in other parts of the body).2
Presentation and investigations
In Australia, most patients with breast cancer present with symptoms. In 2019, the BreastSurgANZ Quality Audit recorded 3113 breast cancer surgeries, with 1815 of these (58%) for symptomatic presentations.3 These symptoms include a palpable breast lump or thickening, nipple changes (including nipple discharge, new inversion, change in shape), skin dimpling and axillary discomfort or swelling.4 Symptomatic breast cancers have been shown to be more aggressive than breast cancers that have been detected through screening, and typically present at a larger size.5,6
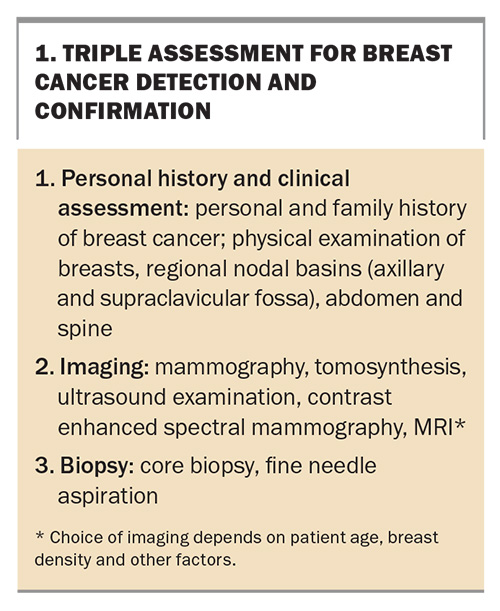
Triple assessment remains the fundamental method of breast cancer detection and confirmation, whether the cancer is symptomatic or asymptomatic (Box 1). In most cases, mammography and ultrasound examination are arranged before specialist referral. Additional staging investigations to evaluate for distant metastases depend on tumour burden, tumour biology and the presence of signs and symptoms suggesting metastatic spread.7 Staging investigations for breast cancer are either a positron emission tomography (PET)-CT scan or the combination of a CT scan of the chest, abdomen and pelvis and bone scintigraphy.
Management
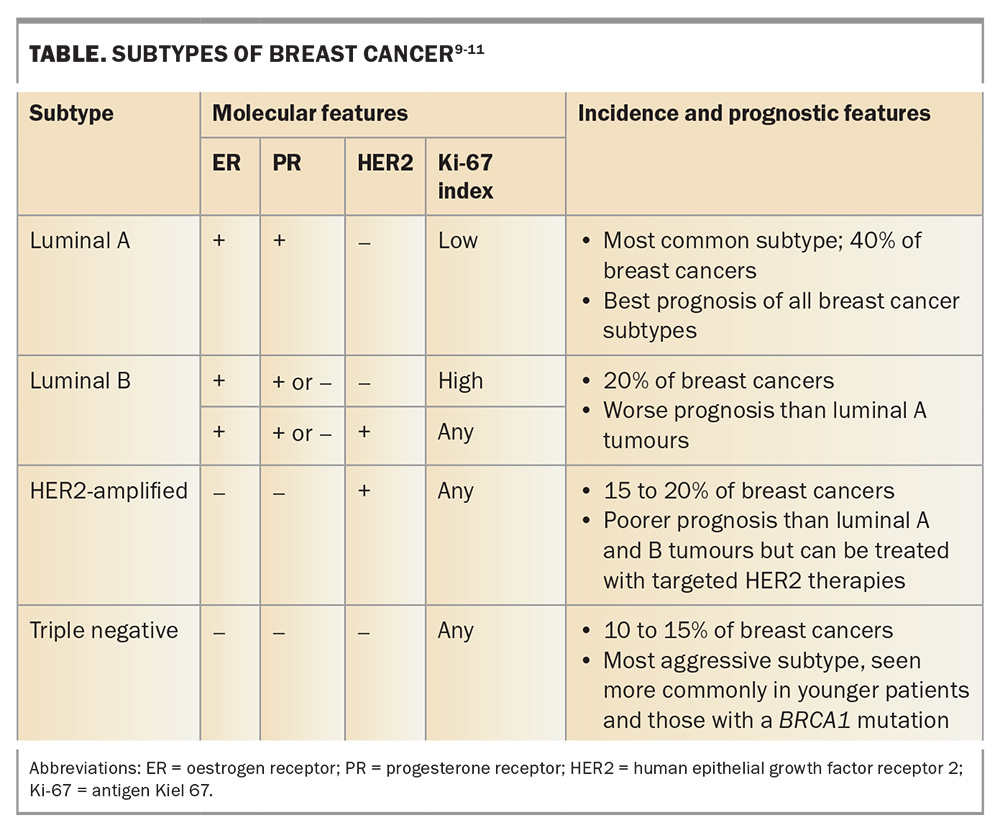
Key considerations in managing patients with early-stage breast cancer include patient factors and tumour characteristics. Breast cancers are differentiated into several different intrinsic subtypes based on molecular testing. These subtypes differ in their behaviour and sensitivity to different systemic therapies. This tumour behaviour is described by:
- oestrogen receptor (ER) and progesterone receptor (PR) status
- human epithelial growth factor receptor 2 (HER2) status
- cancer grade (low, moderate or high, determined by histological features such as tubule formation, nuclear pleomorphism and mitotic activity)8
- antigen Kiel 67 (Ki-67) index (proliferation index).9-11
This information, considered in the wider context of patient factors and preferences, allows clinicians to individualise treatment regimens (Table).
Neoadjuvant therapy
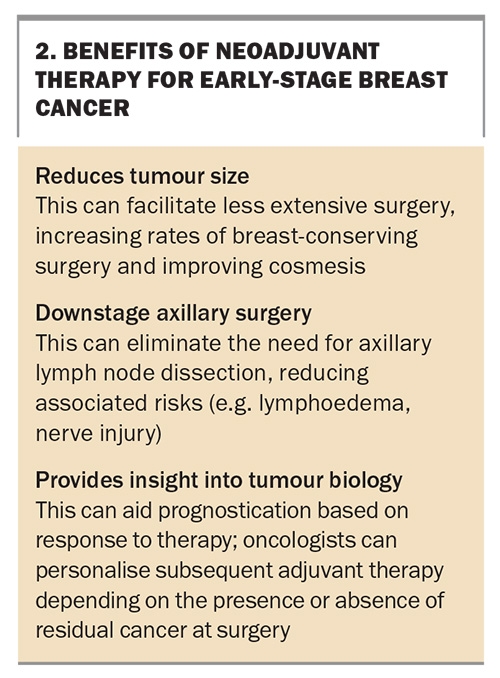
Traditionally, early-stage breast cancers have been treated with surgery, followed by a combination of adjuvant chemotherapy, targeted therapy, radiotherapy and endocrine therapy. However, this practice is evolving. In selected cases, systemic therapy before surgery (neoadjuvant systemic therapy, or NAST) is beneficial (Figure). NAST consists of chemotherapy, combined in selected cases with targeted therapy or immunotherapy. NAST is followed by surgery, with the subsequent treatment options of additional chemotherapy, targeted therapy, radiotherapy and endocrine therapy, depending on tumour biology and response to NAST.
Whether chemotherapy is administered as neoadjuvant or adjuvant, studies have shown similar overall survival.12 However, the neoadjuvant strategy offers some significant benefits (Box 2).13 Neoadjuvant therapy can be offered to selected patients based on tumour biology and extent of disease. A key outcome after NAST is the absence of residual tumour cells on histopathological examination of a resected specimen, termed a pathological complete response. This outcome is significant, with improved survival compared with patients who do not have a pathological complete response (and therefore still had viable tumour cells present at the time of resection). The presence of any residual cancer after NAST also affects adjuvant therapy options.14
The tumour phenotypes most likely to respond significantly to NAST, and therefore achieve a pathological complete response, are HER2-amplified and triple- negative breast cancers. Patients with high-grade, hormone receptor-positive tumours can also be considered for NAST. Although their response is quite variable, with typically low rates of pathological complete response, neoadjuvant therapy may have other advantages, such as allowing breast-conserving surgery.
Surgical management
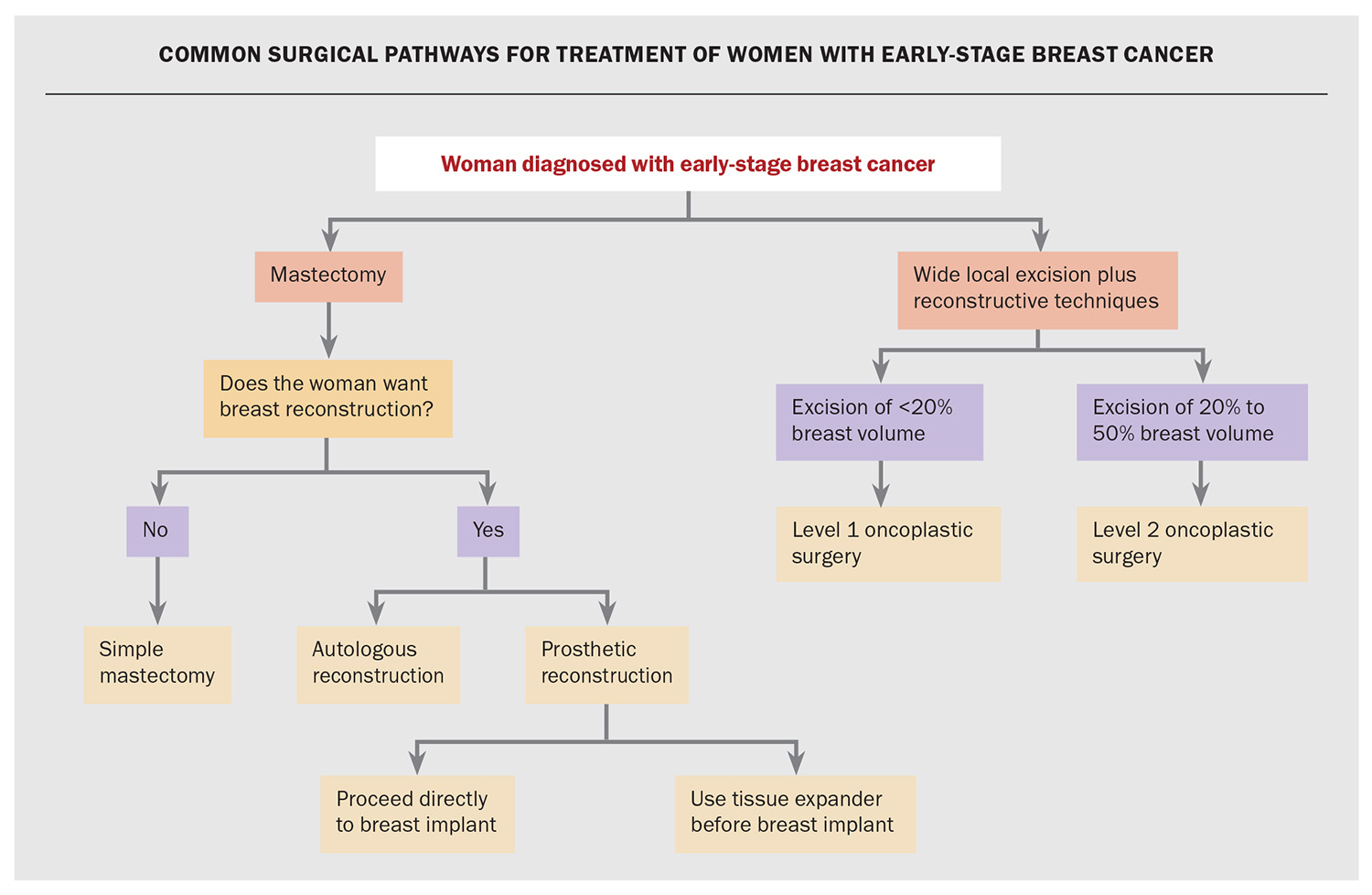
In patients with early-stage breast cancer who are operative candidates, surgery is the mainstay of treatment. There are two key pathways for surgical management: mastectomy and breast-conserving therapy (Flowchart). Breast-conserving therapy is the combination of wide local excision of the cancer (also termed lumpectomy) and adjuvant radiotherapy, with randomised controlled trials showing equivalent outcomes for breast-conserving therapy compared with total mastectomy.15 Breast-conserving therapy has beneficial impacts on patients, with higher positive rates on body image and quality of life.16
Factors that affect the choice between mastectomy and breast-conserving therapy include surgical feasibility, tumour size relative to breast volume, skin involvement, radiotherapy toxicity concerns and patient choice. It is pivotal for patients to understand that it is only with the provision of radiotherapy that the risks of recurrence and survival after breast-conserving therapy are equivalent to those after mastectomy.
Oncoplastic surgery
The development of oncoplastic breast surgery techniques have resulted in significant improvements in breast cosmesis after wide local excision. These techniques generally involve displacing or replacing breast volume and range from mobilising local tissue to fat grafting or creating pedicled flaps. Level 1 oncoplastic techniques involve excision of less than 20% of breast volume, and Level 2 techniques involve excision of 20 to 50% of the breast volume, which requires volume replacement and reshaping of the breast parenchyma.
Although oncoplastic breast surgery takes longer to perform and is more complex, the aesthetic outcome can be far superior to simple mastectomy or wide local excision. For example, studies have shown the statistical superiority of Level 2 oncoplastic surgery to simple mastectomy in both patient psychosocial wellbeing and satisfaction with their breasts.17 However, given the complex nature of these surgeries, not all patients are appropriate candidates, because of patient factors, tumour size and location, or surgical expertise as specialised training is often required.18
Mastectomy with or without reconstruction
Mastectomy is an important surgical option in breast cancer management. A key consideration is whether the patient wishes to undergo breast reconstruction, which should be discussed with the patient. For patients who do not want reconstruction, a simple mastectomy with a flat closure is a very acceptable option, with good patient satisfaction.19 Reconstruction, if desired, can be immediate (at the time of mastectomy) or delayed (requiring a later operation). Currently, 29% of mastectomy patients in Australia undergo immediate reconstruction.20
Reconstruction options after mastectomy are autologous (using the patient’s own tissue) or prosthetic (with breast implants). Autologous reconstruction is applicable in selected candidates and is performed by a plastic surgeons in both the public and private health sectors. This surgery is often a longer procedure, with associated morbidity at the tissue donor site (generally the abdomen). However, the cosmetic outcomes are typically excellent, with a far more natural ‘feel’ compared with prostheses.21
Prosthetic reconstruction after mastectomy can be performed by either a breast surgeon or a plastic surgeon. Immediate prosthetic breast reconstruction results in a single anaesthetic for the patient. However, there are associated risks, including reduced perfusion of the skin flaps increasing the risk of necrosis and, if postmastectomy radiotherapy is required, an increased risk of capsular contraction, thinning of the skin envelope or prosthetic infection. A technique to reduce these risks involves the insertion of tissue expanders, which are devices that are slowly inflated postoperatively until they reach a size appropriate for the patient. The slow increase in size reduces the risk of skin necrosis. Although expanders can reduce the risk of complication, they require a subsequent operation for exchange to a formal prosthesis.
Evaluation of axillary nodes
With all breast cancer surgeries, axillary surgery is required as a means of prognostication. For patients with clinically node-negative breast cancer, a sentinel lymph node biopsy is indicated. This involves removal of one or more lymphatic nodes that drain the breast cancer region. Regional spread to the lymph nodes may require further axillary intervention with either an axillary clearance or axillary radiotherapy. In selected patients undergoing breast-conserving therapy who have one to two positive nodes only, without any further spread, no further axillary surgery may be required.22
Targeted axillary dissection is a new procedure for axillary staging that can be performed in clinically node-positive patients who have undergone neoadjuvant systemic therapy. This procedure involves performing a sentinel node biopsy as well as resecting the specific lymph node(s) that were confirmed to be involved with tumour before neoadjuvant therapy.
Chemotherapy
Neoadjuvant or adjuvant chemotherapy is recommended for patients with early-stage breast cancer who have particular tumour biology and larger size or nodal involvement. Tumour subtypes that respond well to chemotherapy include triple-negative, HER2-enriched and luminal B breast cancers. Luminal A tumours do not respond well to chemotherapy. Recent advances in molecular profile testing can help quantify the expected benefits an individual patient may receive from adjuvant chemotherapy. This is especially helpful for luminal tumours, where chemotherapy benefits can be variable and testing results can help guide therapy choices. These tests are not currently PBS-funded but may be discussed with the patient after discussion in a multidisciplinary team meeting.
Chemotherapy is typically given for three to six months, depending on whether there is nodal involvement. Adverse effects from chemotherapy depend on the specific drugs being administered. For the more commonly used combinations, adverse effects include febrile neutropenia, peripheral neuropathy, hair loss, nail changes, myalgia, arthralgia and fluid retention.16 Longer term risks that are of concern include cardiac toxicity, secondary haematological malignancy and infertility.
Targeted therapy
Trastuzumab
In HER2-amplified breast cancers, the HER2 protein is overexpressed on the cell surface, enabling the cells to behave aggressively. Trastuzumab is a monoclonal antibody that acts by binding to the HER2 receptor in cancer cells, preventing their proliferation and survival. Trastuzumab is administered concurrently with chemotherapy and continued after chemotherapy is completed, for a total duration of one year. Significant improvement in recurrence risk and overall survival has been seen with this therapy.23 The most significant adverse effect of trastuzumab is cardiac toxicity, especially when it is administered in sequence following anthracycline chemotherapy.
Pertuzumab is another monoclonal antibody that has also been shown to improve disease-free survival when used in combination with trastuzumab.24 It is currently not PBS funded for early-stage breast cancer.
Trastuzumab emtansine
Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate consisting of the monoclonal antibody trastuzumab linked to the cytotoxic agent DM1. It is used to treat patients with HER2-amplified breast cancer, where a pathological complete response has not been achieved after neoadjuvant systemic therapy with trastuzumab and chemotherapy. Adjuvant trastuzumab emtansine therapy in this setting has been shown to increase disease-free and overall survival when compared with adjuvant trastuzumab therapy.25
Cyclin-dependent kinase 4/6 inhibitors
Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors are a new class of medication that target proteins involved in cell proliferation. They include ribociclib, palbociclib and abemaciclib. CDK4/6 inhibitors are currently used to treat node-positive, hormone receptor-positive metastatic breast cancer. New data have confirmed that two to three years of adjuvant abemaciclib or ribociclibib in addition to endocrine therapy significantly improves invasive disease-free survival in patients with hormone receptor-positive breast cancer at high risk of recurrence when compared with endocrine therapy alone.26 These medications are not currently PBS funded for early-stage breast cancer.
Immunotherapy
Immunotherapy harnesses the patient’s immune system in the treatment of breast cancer. Pembrolizumab is a monoclonal antibody that targets programmed cell death protein 1 (PD-1) and acts as an immune checkpoint inhibitor. Pembrolizumab is combined with neoadjuvant chemotherapy to treat patients with high-risk early-stage triple-negative breast cancer, and then continued as adjuvant treatment after surgery. Studies have shown a significantly higher rate of complete pathological response among patients with early-stage triple-negative breast cancer who received pembrolizumab with neoadjuvant chemotherapy when compared with neoadjuvant chemotherapy alone.27 However, use of pembrolizumab carries the risk of additional immune-related adverse events.
Radiotherapy
Adjuvant radiotherapy is another key therapy in breast cancer management. Radiotherapy is almost always given after wide local excision and is performed to sterilise any remaining microscopic disease. A standard radiotherapy regimen involves daily whole-breast irradiation, administered over three to six weeks. A ‘boost’, with extra doses of radiotherapy targeted to the tumour bed, is often also given. The effectiveness of adjuvant radiotherapy has been shown in multiple landmark trials and meta-analyses with prolonged follow up.28
In most patients with early-stage breast cancer treated with mastectomy, radiotherapy can be omitted. However, postmastectomy radiotherapy is indicated in certain situations, such as larger tumour size (5 cm or more), presence of more extensive nodal disease, younger patients and presence of high-risk tumour features.
Hypofractionation is a radiobiologically equivalent dose of radiation given over an abbreviated timeframe of three to four weeks. The START-A and START-B trials have validated this technique and confirmed its safety and effectiveness.29 Recent studies have found a one-week duration of radiotherapy is noninferior to standard practice for local control of small, node-negative breast cancers.30
Accelerated partial breast irradiation (APBI) is based on the premise that the highest risk of recurrence is in and around the tumour bed. APBI involves irradiation of the tumour bed and a margin of local breast tissue, with a reduced dose to surrounding normal structures. APBI may be suitable in patients aged over 50 years with a clearly demarcated tumour bed and a unifocal, ductal carcinoma less than 20 mm in size with no high-risk features (lymphovascular invasion or nodal involvement).31 APBI can be performed intraoperatively, with a single dose of radiotherapy to the breast tumour cavity while the patient is under anaesthesia for the breast cancer surgery. Intraoperative radiotherapy is not commonly available.
Adverse effects of breast radiotherapy are typically reported as acute, subacute or late. Acute effects include skin changes and patient fatigue. A concerning subacute adverse effect is pneumonitis. Late changes include skin pigmentation, fibrosis and telangiectasia. Rarer late adverse effects include lung fibrosis, cardiac toxicity (such as major coronary events) and secondary malignancies such as angiosarcoma.
Endocrine therapy
Endocrine therapy is indicated in the treatment of hormone receptor-positive or ‘luminal’ breast cancers. The recommended duration of adjuvant endocrine therapies has traditionally been five years; however, there is evidence of an advantage when extended to 10 years.32 This duration is typically used for higher-risk patients.
Selective oestrogen receptor modulators (SERMs) such as tamoxifen have long been available for adjuvant endocrine treatment primarily in premenopausal women. SERMs are also used in lower-risk postmenopausal women and more infrequently in men with breast cancer. The most common adverse effects of tamoxifen are hot flushes, vaginal dryness and endometrial hyperplasia, and there is a low but real risk of venous thromboembolism.
Aromatase inhibitors are used as endocrine therapy in postmenopausal women. In premenopausal women without ovarian suppression, these medications stimulate ovarian oestradiol production via the pituitary gland. However, in high-risk premenopausal women with early-stage breast cancer, aromatase inhibitors have shown advantages over tamoxifen when given alongside ovarian suppression; the key indication is the return of menses after chemotherapy in a woman aged under 40 years.33 The most common adverse effects of aromatase inhibitors are hot flushes, vaginal dryness, arthralgia and bone density loss.
Minimal use of topical oestrogen therapy may be considered to treat vaginal dryness in women receiving endocrine therapy, in discussion with the treating oncologist.34-36
A baseline bone mineral density test is recommended for women commencing aromatase inhibitor therapy, with follow-up testing every one to two years while aromatase inhibitor therapy continues. Weightbearing exercise and vitamin D and dietary calcium supplementation should be routinely recommended for patients receiving aromatase inhibitor therapy. The antiresorptive agents zoledronic acid and denosumab may be recommended for patients with osteoporosis. Zoledronic acid is associated with renal toxicity and fragility fractures; denosumab is associated with a higher risk of hypocalcaemia. Both are associated with a cumulative risk of osteonecrosis of the jaw.
Adjuvant bisphosphonates are recommended for postmenopausal women with early-stage breast cancer to reduce recurrence and mortality. Studies have shown the benefits of adjuvant bisphosphonates in postmenopausal women in reducing the rate of bone metastases and improving survival.37-39 Although zoledronic acid is not PBS funded as adjuvant treatment for breast cancer, international guidelines suggest treating postmenopausal women with early-stage breast cancer with intravenous zoledronic acid twice yearly for three years, particularly those considered at high risk of recurrence.40 This strategy is also often incorporated in the adjuvant treatment of premenopausal women receiving adjuvant ovarian function suppression.
Conclusion
There has been considerable progress in the management of patients with early-stage breast cancer. Surgical techniques have advanced with oncoplastic techniques and breast reconstruction resulting in improved cosmesis and patient quality of life. Major advances in the availability of systemic therapies, including targeted therapy, antibody-drug conjugates and immunotherapy, have improved patient outcomes. Extension of the duration of adjuvant endocrine therapy to 10 years, the augmentation of endocrine therapy with ovarian function suppression in young patients, and use of CDK4/6 inhibitors in high-risk patients have further reduced recurrence rates. Neoadjuvant systemic therapy is also more widely used for management of high-risk breast cancer. Understanding these advances in management of early-stage breast cancer and their toxicities and complications will help GPs to have greater involvement as fundamental members of the multidisciplinary team managing patients with early-stage breast cancer. MT
COMPETING INTERESTS: Dr Kumar has received consulting fees, speaker fees or support for attending meetings from Astra Zeneca, Eli Lilly, Gilead, MSD, Novartis, Pfizer and Roche. Professor Warrier has received speaker fees from Astra Zeneca, Merit Medical, Smith and Nephew, and Stryker. Dr Ofri, Dr Cui, Dr Elstner: None.
References
1. ANZ Breast Cancer Trials Group. Breast cancer statistics. Available online at: https://www.breastcancertrials.org.au/breast-cancer-resources/breast-cancer-statistics/ (accessed April 2024).
2. Cancer Australia. Stages of breast cancer. Canberra: Commonwealth of Australia; 2023. Available online at: https://www.canceraustralia.gov.au/cancer-types/breast-cancer/symptoms-and-diagnosis/stages-breast-cancer (accessed April 2024).
3. Breast Surgeons of Australia and New Zealand (BreastSurgANZ). BreastSurgANZ Quality Audit. Available online at: https://www.bqa.org.au/ (accessed April 2024).
4. Cancer Council Australia. Understanding breast cancer. A guide for people with cancer, their families and friends. Sydney: The Council; 2022. Available online at: https://www.cancer.org.au/cancer-information/types-of-cancer/breast-cancer (accessed April 2024).
5. Australian Institute of Health and Welfare (AIHW). BreastScreen Australia monitoring report 2023. Canberra: Australian Government AIHW; 2023.
6. Ofri A, Bhimani N, Warrier S. Older breast cancer in Australia: tumour characteristics of screened versus symptomatic breast cancers. J Oncol Res Ther 2022; 7: 10126.
7. Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 1674.
8. DePolo J. Breast cancer grades (Nottingham grade). Available online at: https://www.breastcancer.org/pathology-report/breast-cancer-grades (accessed April 2024).
9. Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 2013; 24: 2206-2023.
10. Untch M, Gerber B, Harbeck N, et al. 13th St. Gallen International Breast Cancer Conference 2013: primary therapy of early breast cancer evidence, controversies, consensus - opinion of a German team of experts (Zurich 2013). Breast Care (Basel) 2013; 8: 221-229.
11. Orrantia-Borunda E, Anchondo-Nunez P, Acuna-Aguilar LE, Gomez-Valles FO, Ramirez-Valdespino CA. Subtypes of breast cancer. In: Mayrovitz HN, editor. Breast cancer. Brisbane: Exon Publications; 2022.
12. Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 2007; (2): Cd005002.
13. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001; (30): 96-102.
14. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164-172.
15. Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg 2014; 149: 267-274.
16. Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Holzel D. Quality of life following breast-conserving therapy or mastectomy: results of a 5-year prospective study. Breast J 2004; 10: 223-231.
17. Bazzarelli A, Baker L, Petrcich W, Zhang J, Arnaout A. Patient satisfaction following Level II oncoplastic breast surgery: a comparison with mastectomy utililizing the Breast-Q questionnaire will be published in Surgical Oncology. Surg Oncol 2020; 35: 556-559.
18. Campbell EJ, Romics L. Oncological safety and cosmetic outcomes in oncoplastic breast conservation surgery, a review of the best level of evidence literature. Breast Cancer (Dove Med Press) 2017; 9: 521-530.
19. Baker JL, Dizon DS, Wenziger CM, et al. "Going flat" after mastectomy: patient-reported outcomes by online survey. Ann Surg Oncol 2021; 28: 2493-2505.
20. Dayaratna N, Nguyen CL, Spillane A, Mak C, Warrier SK, Dusseldorp JR. Trends and variations in post-mastectomy breast reconstruction rates in Australia over 10 years. ANZ J Surg 2023; 93: 242-250.
21. Santosa KB, Qi J, Kim HM, Hamill JB, Wilkins EG, Pusic AL. Long-term patient-reported outcomes in postmastectomy breast reconstruction. JAMA Surg 2018; 153: 891-899.
22. Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA 2017; 318: 918-926.
23. Early Breast Cancer Trialists’ Collaborative group (EBCTCG). Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13864 women in seven randomised trials. Lancet Oncol 2021; 22: 1139-1150.
24. Jagosky M, Tan AR. Combination of pertuzumab and trastuzumab in the treatment of HER2-positive early breast cancer: a review of the emerging clinical Data. Breast Cancer (Dove Med Press) 2021; 13: 393-407.
25. von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019; 380: 617-628.
26. Francis PA, Fleming GF, Láng I, et al. Adjuvant endocrine therapy in premenopausal breast cancer: 12-year results from SOFT. J Clin Oncol 2023; 41: 1370-1375.
27. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020; 382: 810-821.
28. Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011; 378: 1707-1716.
29. Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013; 14: 1086-1094.
30. Murray Brunt A, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020; 395: 1613-1626.
31. Cancer Institute NSW. Breast invasive cancer/ductal carcinoma in situ adjuvant partial breast EBRT. Available online at: https://www.eviq.org.au/radiation-oncology/breast/3658-breast-invasive-cancer-ductal-carcinoma-in-si# (accessed April 2024).
32. Merck and Co. Australian product information – Keytruda (pembrolizumab (rch)). Canberra: Therapeutic Goods Administration; 2024. Available online at: https://www.tga.gov.au/resources/artg/263932 (accessed April 2024 ).
33. Carson E, Dear R. Advanced breast cancer: an update to systemic therapy. Aust J Gen Pract 2019; 48: 278-283.
34. Sussman TA, Kruse ML, Thacker HL, Abraham J. Managing genitourinary syndrome of menopause in breast cancer survivors receiving endocrine therapy. J Oncol Pract 2019; 15: 363-370.
35. McVicker L, Labeit AM, Coupland CAC, et al. Vaginal estrogen therapy use and survival in females with breast cancer. JAMA Oncol 2024; 10: 103-108.
36. Cancer Institute NSW. Side effect and toxicity management. Vaginal dryness. Available online at: https://www.eviq.org.au/clinical-resources/radiation-oncology/side-effect-and-toxicity-management/1781-vaginal-dryness#management (accessed April 2024).
37. Coleman R, Cameron D, Dodwell D, et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol 2014; 15: 997-1006.
38. Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol 2011; 12: 631-641.
39. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 2015; 386: 1353-1361.
40. Coleman R, Hadji P, Body JJ, et al; ESMO Guidelines Committee. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann Oncol 2020; 31: 1650-1663.