Assessing breast cancer risk: tailored surveillance and risk-reductive interventions
Breast cancer is the most common cancer, affecting one in seven women in Australia. It is important to determine who is at moderate or high risk of breast cancer, as these women require tailored surveillance and consideration of risk-reductive interventions.
Breast cancer is the most common cancer diagnosed in women in Australia.1 It is also the second most common cause of cancer-related death in women.1 The lifetime risk of a woman being diagnosed with breast cancer is one in seven. However, some women have a higher risk, which requires tailored surveillance and consideration of risk-reductive interventions. This article discusses how to determine a patient’s risk of breast cancer, recommendations for surveillance and options for risk-reductive interventions.
Risk definition
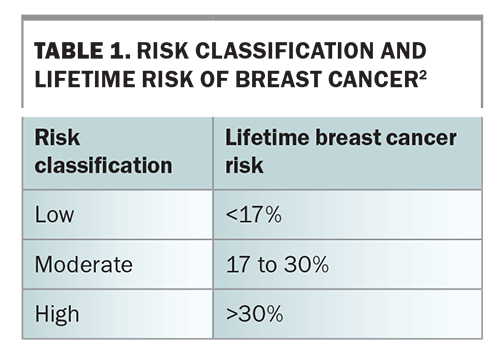
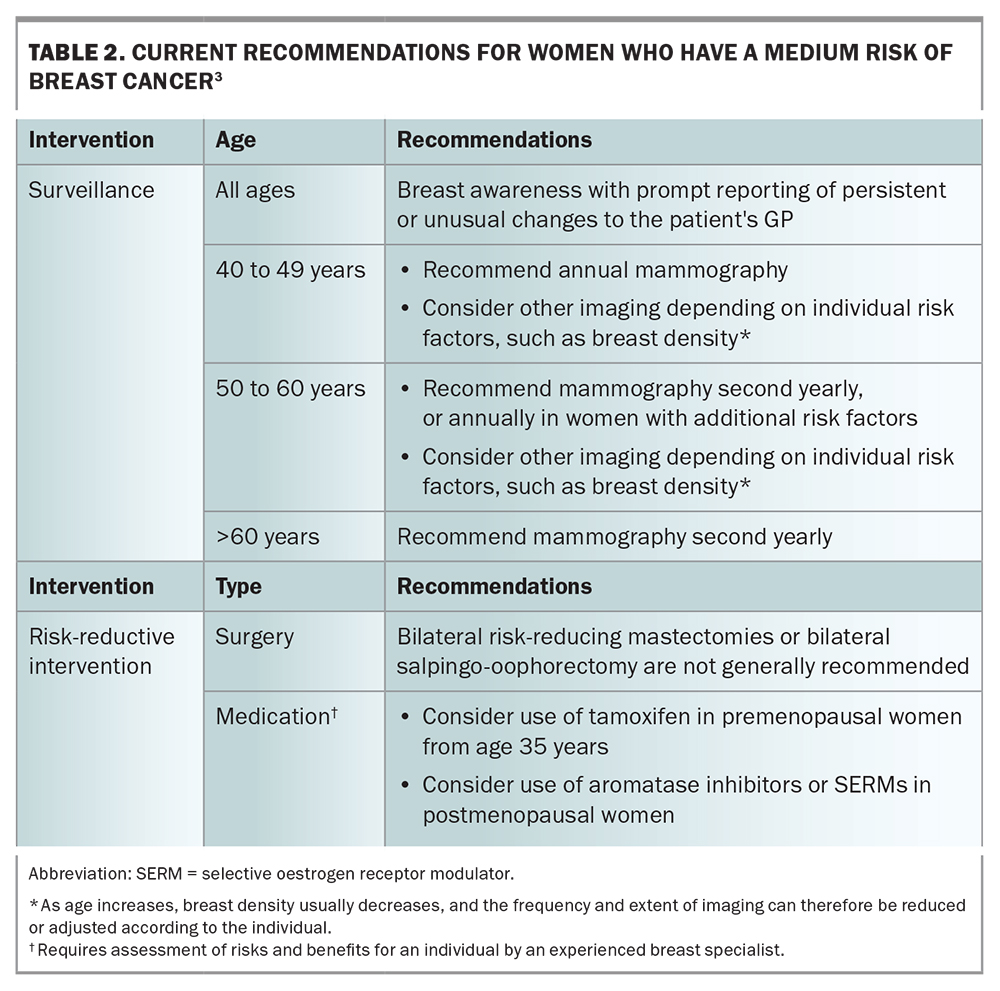
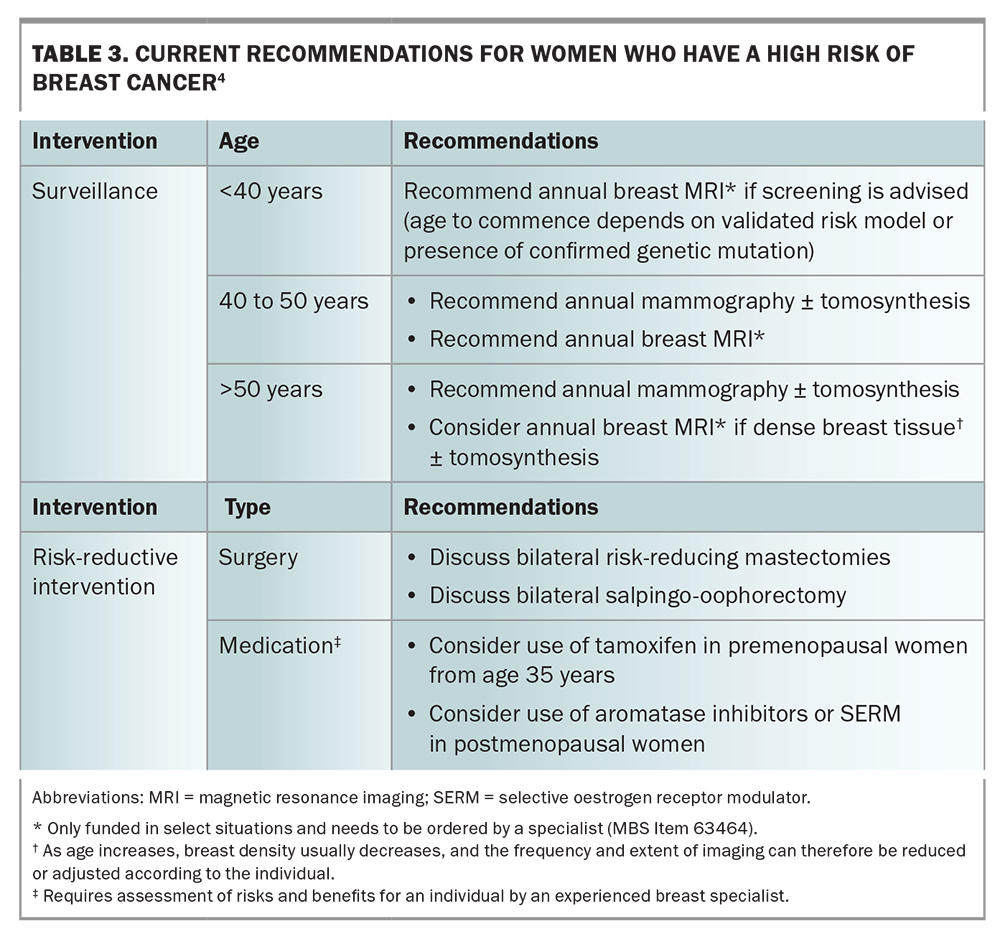
Lifetime breast cancer risk can be statistically stratified as low (baseline population), moderate or high (Table 1) by using risk-assessment tools.2 For women who are classified at moderate- or high-risk for breast cancer, routine biennial screening is insufficient. For these women, tailored screening and consideration of risk-reductive interventions are recommended (Table 2 and Table 3).3,4 The recommendation on when to start surveillance varies widely and depends on the risk-assessment tool used or the particular genetic mutation identified.
Risk factors
Genetic mutations
About 5 to 10% of all breast cancers are familial.5 However, even in patients with a strong family history, a specific genetic mutation is detected in less than 30% of cases.6 The most common mutations associated with breast cancer are in the genes BRCA1 and BRCA2. Mutations in these genes are thought to cause 3% of all breast cancer diagnoses internationally.7 Due to their aberrancy, these tumour suppressor genes fail to repair double-stranded DNA breaks, resulting in mutation accumulation, eventually leading to breast cancer. The rates of developing breast cancer by age 70 years in women with mutations in the BRCA1 or BRCA2 genes are 55 to 72% and 45 to 69%, respectively.8 This far exceeds the lifetime risk of about 14% for the baseline population.
Other genetic mutations, including PALB2, ATM and CHEK2, have also been identified as increasing breast cancer risk. All gene mutations have variable penetrance and surveillance recommendations are modified by the risk they confer. Surveillance recommendations are made by eviQ, a free online resource developed in NSW providing evidence-based, consensus driven cancer treatment protocols (including guidelines for specific genetic mutations) to help guide patient care (http://eviq.org.au).
Ashkenazi Jewish people
The Ashkenazi Jewish population have a significantly increased risk of carrying BRCA1 or BRCA2 gene mutations. About one in 40 Ashkenazi Jewish women have a BRCA mutation, compared with one in 400 in the general population. There are three specific BRCA1/2 pathogenic variants, referred to as BRCA-Jewish founder mutations, which account for more than 90% of the pathogenic variants in this subpopulation.
JeneScreen is an active project that offers genetic testing (free of charge) for BRCA1/2 mutations in Ashkenazi Jewish women in Sydney or Melbourne, regardless of their family history. This has facilitated the detection of high-risk individuals, many of whom would have otherwise been ineligible for publicly-funded genetic testing.9
Family history
Patients often present to a healthcare professional with concerns of developing breast cancer because of a recent diagnosis in a family member. In cases where there is no identified genetic mutation, assessing familial risk can be difficult. The iPrevent online tool (explained in more detail below) provides tailored risk-management information for these patients. Women who have a higher risk of breast cancer compared with the general population are recommended to undergo genetic testing (Box).10
Personal history
Multiple factors contribute to an increased risk of developing breast cancer, including increasing age, female gender, high mammographic density, prolonged oestrogen exposure, menstrual cycle beginning before 12 years of age, late menopause, nulliparity and first pregnancy at age above 31 years. In addition, any prior history of breast cancer (both in situ and invasive disease) or a history of diagnosed precursor lesions, such as atypical ductal or lobular hyperplasia, or classic lobular carcinoma in situ, statistically increase the risk of developing breast cancer. Less commonly, a history of mantle radiotherapy before 25 years of age is also a risk factor.
Modifiable risk factors for breast cancer include alcohol consumption, obesity, lack of exercise and the use of menopausal hormone therapy (MHT). The use of MHT (for five years or more) increases breast cancer incidence by about one in every 200 users of oestrogen-only preparations, and up to one in every 50 users of oestrogen plus progestogen preparations.11 If possible, it is better to use oestrogen-only MHT but this cannot be prescribed for women who have not had a hysterectomy.
Use of hormonal contraception, especially in high-risk patients, also needs to be considered. The relative risk of breast cancer in all current and recent users of hormonal contraception is 1.20.12 Breast cancer risk increases with duration of hormonal contraception use. This risk increases from 1.09 with less than one year of use to 1.38 with more than 10 years of use.12 This risk applies to current users and continues for up to 10 years post cessation. This risk is also applicable to newer formulations of hormonal contraception, including progesterone-containing implantable devices.
Risk-assessment tools
iPrevent
iPrevent is an online breast cancer risk-management decision support tool (https://www.petermac.org/iprevent). It uses a model choice algorithm by selecting one of two validated breast cancer risk-estimation models (International Breast Cancer Intervention study [IBIS] or Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm [BOADICEA] version 3), based on a woman’s risk factors. It then provides tailored risk-management information for patients. Overall, it has shown good discriminatory accuracy.13,14 iPrevent cannot be used for patients who have a history of breast cancer or precursor lesion, have a confirmed cancer gene mutation (except for BRCA1 and BRCA2) or have had chest radiotherapy. iPrevent can be completed online in about 30 minutes and patients can then download a personalised report to take to their appointment to discuss with their healthcare professional.
CanRisk
CanRisk uses the BOADICEA version 6 model to assist healthcare professionals in calculating an individual’s future risk of breast and ovarian cancer (https://www.CanRisk.org). It can be used in patients who have previously been diagnosed with invasive breast cancer and it also integrates polygenic risk scores. Although both CanRisk and iPrevent excel at family history recording, neither includes the risk associated with a prior history of precursor lesion.
IBIS
The IBIS risk-assessment tool, also known as the Tyrer-Cuzick model breast cancer risk-evaluation tool, is used to estimate the likelihood of a woman developing breast cancer specifically within 10 years of her current age and up to 85 years of age (https://ibis.ikonopedia.com). The IBIS tool can be used to determine breast cancer risk in patients with confirmed BRCA mutations and previously diagnosed breast precursor lesions, and is more useful in the specialist setting.
Risk-reductive strategies
Simple measures to reduce modifiable risk factors for breast cancer include exercise, weight reduction and decreased use of MHT. Interventional strategies for nonmodifiable risk factors include risk-reductive surgery and use of risk-reductive medications.
Breast risk-reductive surgery
Risk-reductive surgery reduces the risk of breast cancer by at least 90% but is currently only recommended for high-risk patients. The two options for risk-reductive surgery are a mastectomy or mastectomy with reconstruction. Patients can either have implant-based reconstruction (prosthesis) or autologous tissue reconstruction (using the patient’s own tissue). If pursuing reconstruction, this can be performed as a skin or nipple-sparing procedure in which there is no increased risk of subsequent breast cancer compared with simple mastectomy.
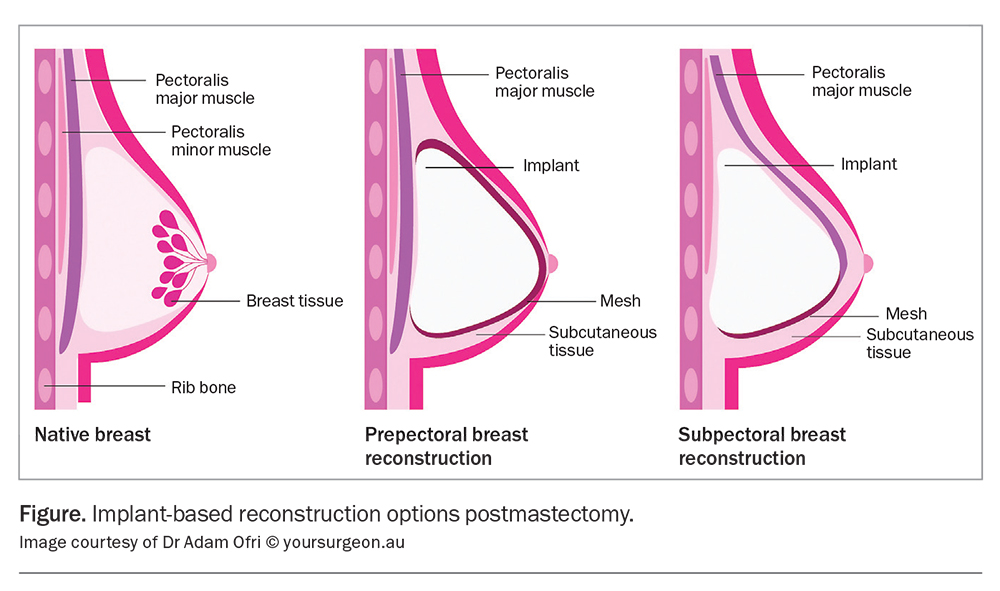
An implant-based reconstruction can be performed as a single staged operation with insertion of a permanent implant at the time of mastectomy, or as a staged procedure with insertion of an expander at the initial mastectomy, with a subsequent planned operation to exchange the expander for the permanent implant later. The implant pocket can either be placed superficial to the muscle (prepectoral approach) or deep to the muscle (subpectoral approach) (Figure). Surgeon preference for implant placement varies across Australia. The prepectoral approach reduces postoperative pain but has a risk of rippling, whereas the subpectoral approach has a lower risk of rippling but a higher risk of animation.15 Postoperatively, there are no significant differences when clinically examining or imaging either technique. It is important for patients to know that these are not lifelong devices (they have a general lifespan of 15 years) and ongoing assessment of the device is required, with potential exchange of the implant in the future.
The most common autologous tissue reconstruction is a deep inferior epigastric perforator flap using abdominal fat for reconstruction. Enough abdominal fat is required to recreate both breasts. This procedure provides reliable long-term results for patients, with excellent long-term satisfaction and good cosmesis.
It is important that patients are aware that there is still a risk of developing breast cancer in the future after having risk-reductive surgery, albeit small. This risk is far less than the baseline population risk.
Ovarian risk-reductive surgery
Patients with BRCA1/2 mutations have a higher risk of both breast and ovarian cancers. By age 70 years, the risk of developing ovarian cancer is about 39 to 44% for patients with the BRCA1 mutation and about 11 to 17% for BRCA2 mutation.8 It is currently recommended that bilateral salpingo-oophorectomy should be performed between the ages of 35 and 40 years for patients with BRCA1 mutation and 40 and 45 years for those with BRCA2 mutations to reduce ovarian cancer risk, although the optimal timing should be individualised. Patients can take MHT after bilateral salpingo-oophorectomy, without increasing their risk of breast cancer, for a period of about five years to minimise side effects. Previously, risk-reducing bilateral salpingo- oophorectomy was recommended for premenopausal patients with BRCA mutations as a means to reduce breast cancer risk; however, most recent evidence has failed to corroborate this.16
Risk-reductive medications
The two main classes of risk-reductive medication for breast cancer are selective oestrogen receptor modulators (SERMs e.g. tamoxifen and raloxifene) and aromatase inhibitors (e.g. letrozole and anastrozole, which are both used off-label for breast cancer prevention). SERMs have an antiestrogenic effect on breast tissue and can be used in both pre- and postmenopausal women.17 However, they have predominantly oestrogenic effects on the uterus and liver. Key side effects are venous thromboembolism, endometrial hyperplasia and hepatotoxicity. Aromatase inhibitors reduce oestrogen synthesis by blocking the aromatase enzyme, which converts androgens into oestrogens. They are only effective in postmenopausal women. Side effects include arthralgia, fatigue and osteoporosis. Decision making regarding which class of medication to use depends on a patient’s menopausal state, as well as any relevant contraindications. Currently, only tamoxifen is PBS listed for reduction of breast cancer risk in patients at moderate to high risk, and costs about AU$25 per month. These drugs can reduce breast cancer risk in high-risk women by about 30 to 60%.18 Discussions about risk-reductive medications should take place in consultation with a breast specialist.
The BRCA-P trial is an international breast cancer prevention clinical trial investigating whether the use of denosumab, a monoclonal antibody that inhibits nuclear factor kappa-B ligand, is a safe and effective option for preventing breast cancer in women with a confirmed BRCA1 gene mutation who have not had breast risk-reductive surgery (https://www.breastcancertrials.org.au/trials/brca-p/). Eligible patients will be randomised to either placebo or denosumab for five years.
Conclusion
Breast cancer is the most common cancer affecting women in Australia. Biennial screening mammography for women aged 50 to 74 years has been shown to be appropriate for those at population level risk; however, it is insufficient in moderate- and high-risk groups. It is important to understand who is, or might be, at high risk of breast cancer to ensure they have appropriate tailored breast cancer surveillance. Risk-reductive strategies are recommended for patients at moderate- or high-risk of beast cancer and should be discussed in consultation with a breast specialist. MT
COMPETING INTERESTS: None.
References
1. Australian Institute of Health and Welfare (AIHW). BreastScreen Australia monitoring report 2022. 2022, AIHW: Canberra.
2. Australian Institute of Health and Welfare (AIHW). Cancer in Australia 2021; Canberra: AIHW. Available online at: https://www.aihw.gov.au/getmedia/ 0ea708eb-dd6e-4499-9080-1cc7b5990e64/aihw-can-144.pdf?v=20230605165731&inline=true (accessed December 2023).
3. NSW government Cancer Institute. Breast cancer (moderately increased risk) risk management (female). eviQ 2023. Available online at: https://www.eviq.org.au/cancer-genetics/adult/risk-management/1424-breast-cancer-moderately-increased-risk-r (accessed December 2023).
4. NSW government Cancer Institute. Breast cancer (high risk with no family history of ovarian cancer) risk management (female) eviQ. 2023. Available online at: https://www.eviq.org.au/cancer-genetics/adult/risk-management/743-breast-cancer-high-risk-with-no-family-histor (accessed December 2023).
5. Olopade OI, Grushko TA, Nanda R, Huo D. Advances in breast cancer: pathways to personalized medicine. Clin Cancer Res 2008; 14: 7988-7999.
6. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol 2015; 26: 1291-1299.
7. Centers for Disease Control and Prevention. The BRCA1 and BRCA2 Genes. US Department of Health and Health and Human Services. Available online at: https://www.cdc.gov/genomics/disease/breast_ovarian_cancer/genes_hboc.htm#:~:text=
The%20genes%20most%20commonly%20affected,the%20BRCA1%20and%20BRCA2%20genes (accessed December 2023).
8. Petrucelli N, Daly MB, Pal T, et al. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. 1998 Sep 4 [Updated 2023 Sep 21]. In: Adam MP, Feldman J, Mirzaa GM, et al. (eds). GeneReviews [Internet]. Seattle: University of Washington, Seattle; 1993-2023.
9. Tiller JM, Cousens, NE, Kaur R, et al. Population-based BRCA1/2 testing programmes are highly acceptable in the Jewish community: results of the JeneScreen Study. J Med Genet 2023; 60: 265-273.
10. NSW government Cancer Institute. Breast cancer - referring to genetics eviQ. Available from: https://www.eviq.org.au/cancer-genetics/referral-guidelines/1620-breast-cancer-referring-to-genetics (accessed December 2023).
11. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 2019; 394: 1159-1168.
12. Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med 2017; 377: 2228-2239.
13. Phillips KA, Liao Y, Milne RL, et al. Accuracy of risk estimates from the iPrevent breast cancer risk assessment and management tool. JNCI Cancer Spectr 2019 3: pkz066.
14. Collins IM, Bickerstaffe A, Ranaweera T, et al. iPrevent(R): a tailored, web-based, decision support tool for breast cancer risk assessment and management. Breast Cancer Res Treat 2016; 156: 171-182.
15. Yang, JY, Kim CW, Lee JW, Kim SK, Lee SA, Hwang E. Considerations for patient selection: prepectoral versus subpectoral implant-based breast reconstruction. Arch Plast Surg 2019; 46: 550-557.
16. Conduit C, Milne RL, Friedland ML, Phillips KA. Bilateral salpingo-oophorectomy and breast cancer risk for BRCA1 and BRCA2 mutation carriers: assessing the evidence. Cancer Prev Res 2021; 14: 983-994.
17. Australian government Cancer Australia. Risk reducing medication for women at increased risk of breast cancer due to family history. Cancer Australia; 2012. Available online at: https://www.canceraustralia.gov.au/sites/default/files/publications/risk-reducing-medication-women-increased-risk-breast-cancer-due-family-history/pdf/2018_rrm_frequently_asked_questions_int.pdf (accessed December 2023).
18. Mocellin SA, Goodwin, Pasquali S. Risk-reducing medications for primary breast cancer: a network meta-analysis. Cochrane Database Syst Rev 2019; 4(4): CD012191.