Crohn’s disease: an overview and update on medical and dietary therapies
Crohn’s disease is an increasingly prevalent condition with substantial impact on patients’ quality of life and economic burden on the healthcare system. There are a growing number of therapies in the treatment armamentarium of Crohn’s disease including medical and dietary therapies. Medical therapies, including corticosteroids, immunomodulators and advanced therapies, have varying mechanisms of action, safety profiles and required monitoring. Dietary therapies, including enteral nutrition, the Crohn’s disease exclusion diet and the Mediterranean diet, are effective in managing Crohn’s disease.
Comment
A Letter to the Editor and Reply from the authors can be read here.
- Crohn’s disease is a progressive alimentary disease with a morbid natural history affecting quality of life with important societal economic implications.
- Timely and appropriate medical therapy can alter the natural history of the disease and significantly improve quality of life.
- Budesonide is an appropriate induction agent for mild to moderate disease and has a superior safety profile to prednisolone.
- Advanced therapies in Crohn’s disease have a variety of different mechanisms of action with vastly different safety profiles and monitoring requirements.
- The Mediterranean diet is the preferred general diet for patients with Crohn’s disease, along with avoiding ultraprocessed foods.
- Exclusive enteral nutrition and the Crohn’s disease exclusion diet are effective dietary therapies for inducing remission in Crohn’s disease.
Crohn’s disease is a chronic inflammatory bowel disease (IBD) which can affect anywhere along the alimentary tract. Crohn’s disease has a previously described annual hospital admission rate of 20%, with half of patients requiring surgery within 10 years of diagnosis.1 Untreated inflammation can lead to significant morbidity, including nutritional abnormalities, fatigue and the development of complications, such as strictures, fistulas and abscesses.
Australia has one of the highest occurrences of Crohn’s disease in the world.2 A study conducted in Tasmania between 2013 and 2014 found an incidence and prevalence rate of 15.4 per 100,000 and 165.5 per 100,000, respectively. Furthermore, IBD has a substantial economic impact in Australia with an estimated healthcare expenditure in 2012 of more than $3 billion.3
This article aims to provide an overview and update of effective medical and dietary therapies in Crohn’s disease, with a particular focus on newer therapies.
Pathophysiology of Crohn’s disease
Crohn’s disease results from a complex interplay between genetics, environmental factors and the gut microbiome, which culminates in immune dysregulation and inappropriate inflammation of the gastrointestinal tract. This results in barrier function defects along the intestinal wall, innate immune function defects and dysregulated adaptive immune function. The combination of these factors promotes inflammation in a transmural fashion.1 Inflammation often leads to scarring, fibrosis, smooth muscle expansion and bowel wall remodelling, which can lead to strictures and fistulas in some patients.4
Medical therapies
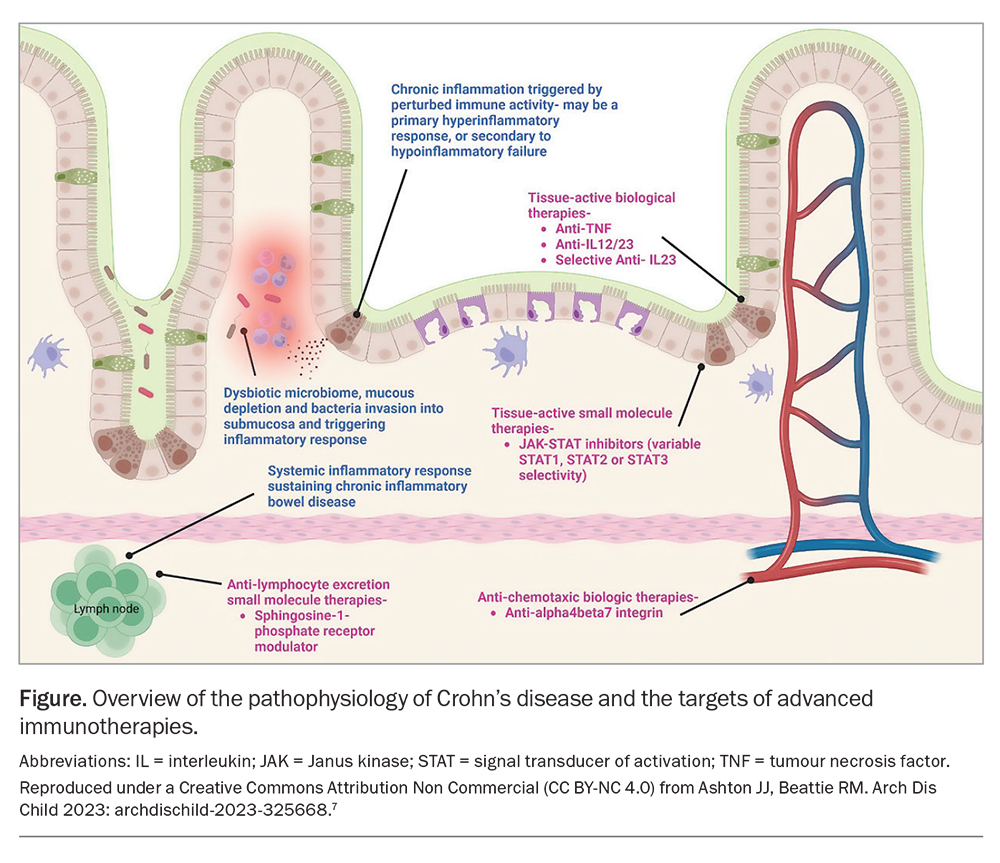
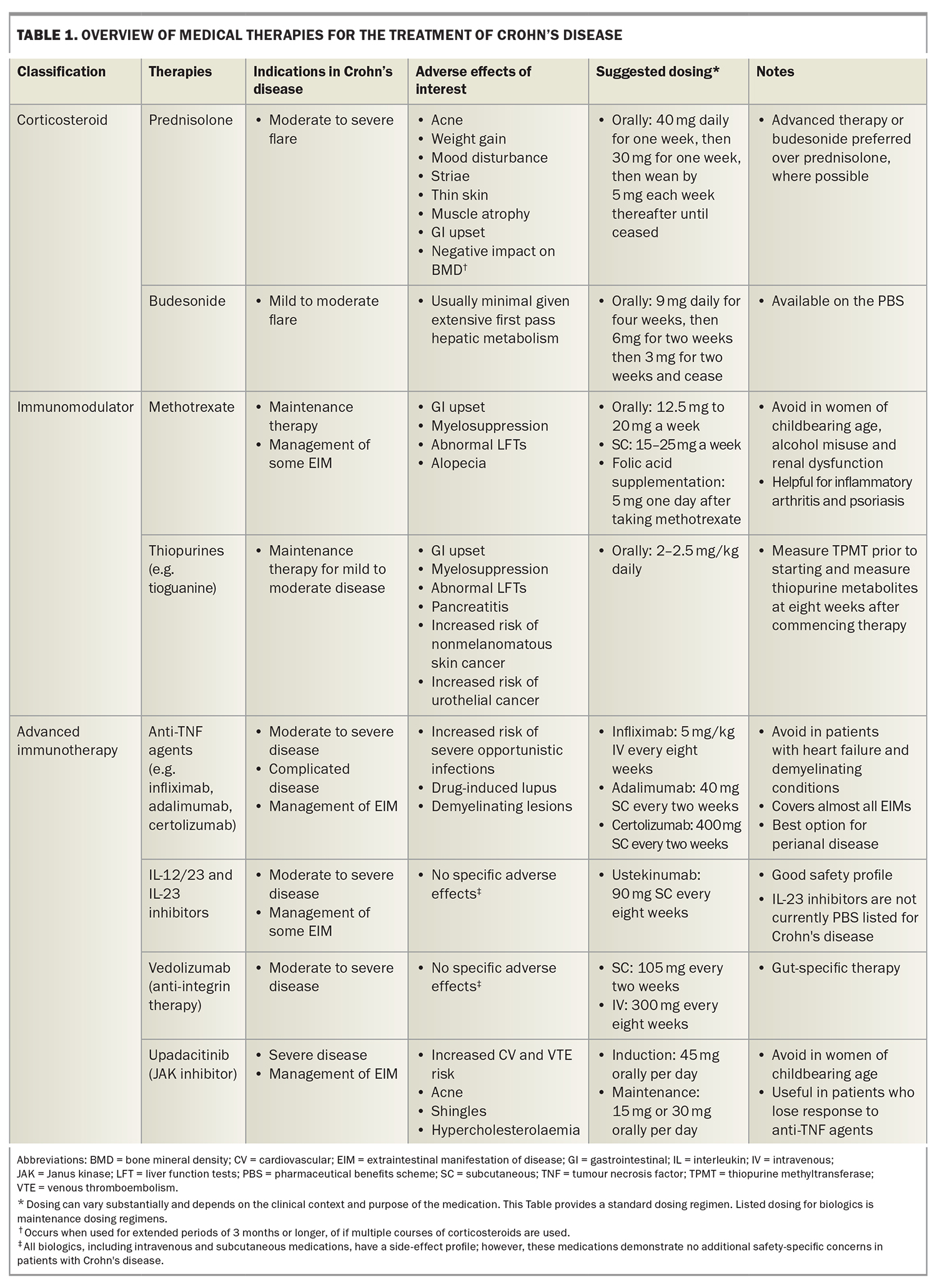
Medical therapy in Crohn’s disease is directed at downregulating this inflammatory pathway, often by targeting key cytokines or receptors that promote inflammation (Figure).5 The aims of therapy are to improve symptoms, quality of life, reduce complications and, ideally, to induce healing of the gut wall.6 Table 1 gives an overview of the medical therapies used to treat Crohn's disease. At the time of writing, there are no efficacious therapies for reversing fibrosis.7
Corticosteroids
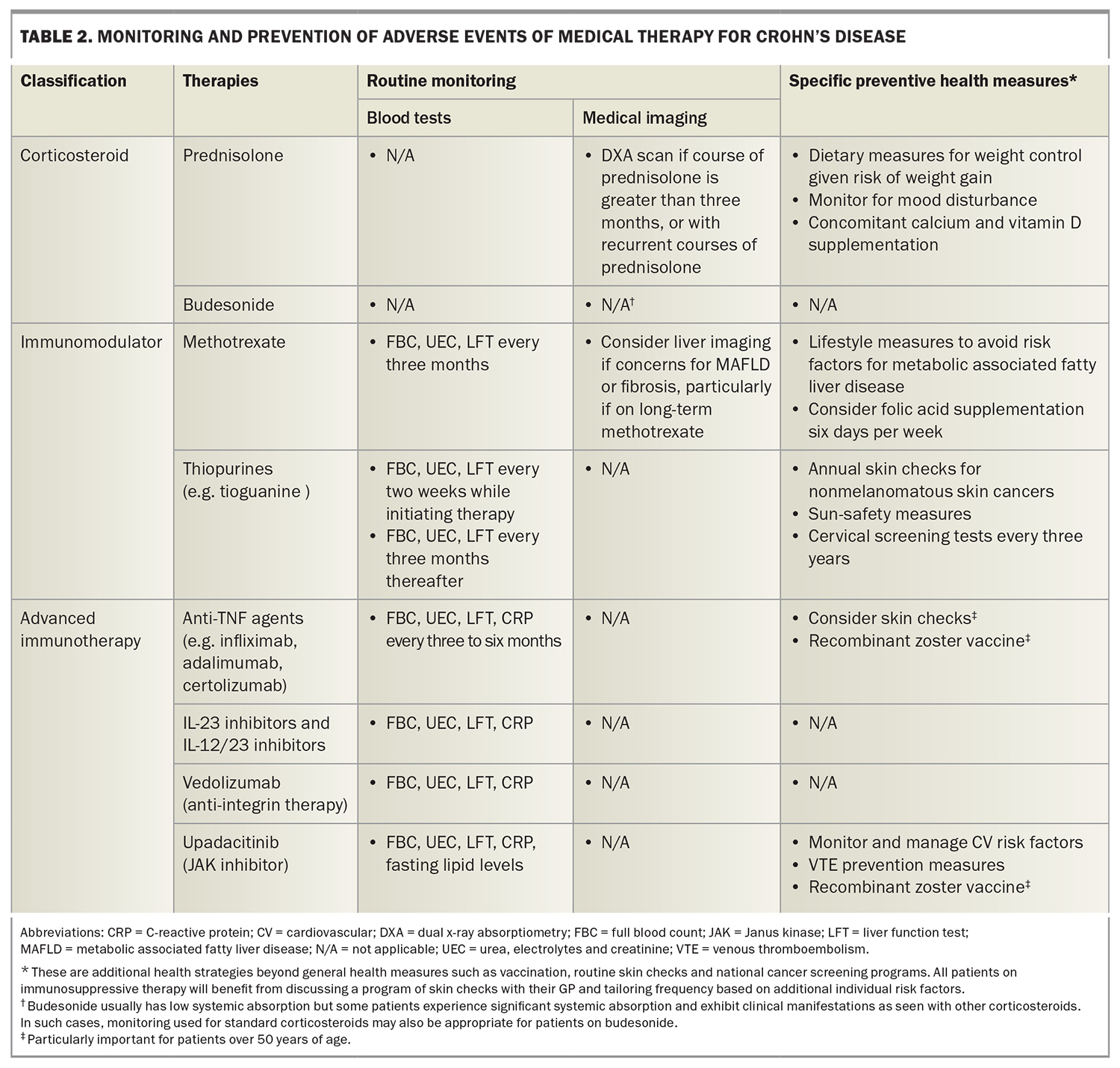
Corticosteroids are frequently used to manage a flare of Crohn’s disease and to induce remission. Prednisolone and methylprednisolone are efficacious in inducing remission.8,9 Prednisolone is usually prescribed at a starting dose of 40 mg daily and weaned over a period of four to eight weeks. Prednisolone’s main disadvantage is its side-effect profile; more than half of patients will experience side effects on high-dose prednisolone, of which 32% will require dose reduction or withdrawal.10
Budesonide is commonly prescribed in patients with mild to moderate Crohn’s disease. Budesonide is a locally-acting corticosteroid which undergoes extensive hepatic first-pass metabolism, thereby minimising the patient’s systemic exposure to corticosteroids.11 A formulation for Crohn’s disease has been developed with slow-release properties, resulting in delivery mainly to the ileum and ascending colon. About one-half to two-thirds of patients with Crohn’s disease achieve remission with budesonide.12 Budesonide is less efficacious in severe disease and extensive colonic disease.
Despite the efficacy of corticosteroids in inducing remission, they are ineffective in maintaining clinical remission, achieving bowel healing and preventing disease complications. Further, long-term use is associated with significant side effects, including bone density loss, an increased risk of infection, mood disturbance, weight gain and metabolic syndrome.13 Advanced therapies (discussed below) are preferred to corticosteroids in inducing remission, where indicated, owing to their superior safety profile and tolerability.
Immunomodulators
Methotrexate
Methotrexate is an antimetabolite, which hinders adenosine metabolism in low doses and leads to numerous anti-inflammatory effects, including decreased T-cell activation and downregulation of B cells.14 Low-dose methotrexate has demonstrated mild to moderate efficacy in maintaining remission in patients with Crohn’s disease.15 Evidence for inducing remission is less robust.16 Methotrexate is a useful therapy for patients who experience extraintestinal manifestations of IBD and in patients with concomitant rheumatic disorders for which methotrexate is also an efficacious treatment (e.g. inflammatory arthritis, psoriasis).15 It can also be used in combination with antitumour necrosis factor (anti-TNF) alpha therapies to reduce immunogenicity against anti-TNF agents. Adverse effects can be problematic (in particular, gastrointestinal upset, myelosuppression and abnormal liver function test results) and may be prevented with the prescription of folic acid (Table 2).17 Methotrexate is teratogenic and rarely prescribed for females of childbearing age; if used, effective contraception is required. Women should be advised not to breastfeed while taking methotrexate. Methotrexate should also be used cautiously in those with chronic alcoholism, advanced liver and kidney disease and haematopoietic disorders.
Thiopurines
Thiopurines are small molecules that inhibit lymphocyte proliferation and have immunomodulatory effects on intestinal inflammation and on trafficking effector cells to the gastrointestinal tract.18 Thiopurines are efficacious as maintenance therapy in mild to moderate Crohn’s disease. Their main role in moderate to severe disease is acting as an immunomodulator to prevent the development of antidrug antibodies with anti-TNF agents.19
Thiopurines have a significant toxicity profile. Clinical manifestations of toxicity include nausea, vomiting, haematopoietic dysfunction, deranged liver function test results, pancreatitis, opportunistic infections and increased risk of nonmelanomatous skin cancers. Although the absolute risk is low, thiopurines can also increase the risk of urothelial malignancies and lymphoma (in particular, in young males who have not been exposed to Epstein-Barr virus).18 The risk of adverse effects is affected by the patient’s genotype and phenotype of thiopurine methyltransferase activity, which plays an important role in drug metabolism. Thiopurine methyltransferase activity testing should be requested before initiating therapy, and routine blood monitoring ordered when thiopurines are initiated or uptitrated.
Tioguanine is becoming an increasingly popular choice of thiopurine among clinicians who manage patients with IBD. Azathioprine and mercaptopurine are prodrugs that must be metabolised into the active form, 6-thioguanine. However, tioguanine is not a prodrug and thus lacks some of the potential toxic effects of metabolism (e.g. pancreatitis).20 Previously, its clinical use had been limited by the perceived risk of significant liver disease due to nodular regenerative hyperplasia; however, subsequent studies have alleviated this concern.21 Due to its superior tolerability and theoretical safety profile, tioguanine is an appealing option for treating Crohn’s disease and can be prescribed for patients who have experienced intolerances to azathioprine or mercaptopurine.
Advanced therapies
Antitumour necrosis factor alpha agents
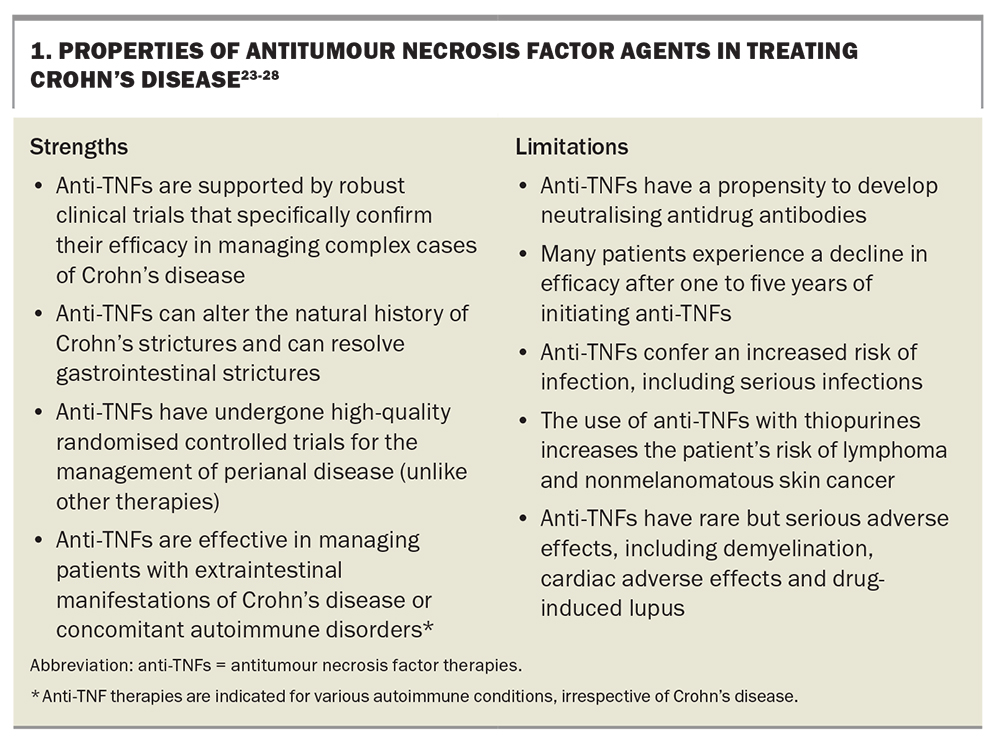
Tumour necrosis factor (TNF) alpha is one of the key proinflammatory cytokines in Crohn’s disease. TNF-alpha is involved in the pathogenesis and continuing inflammatory cascade in Crohn’s disease through the recruitment and activation of various immune cells and the upregulation of other proinflammatory cytokines.22 Anti-TNF agents are monoclonal antibodies against TNF-alpha. They were the first class of biological therapy developed for the management of Crohn’s disease and have been used for more than 20 years. As such, clinicians are most familiar with this class of advanced therapies including their short- and long-term risks and benefits (Box 1).23-28 In contrast, all the other advanced therapies have emerged within the past decade, with numerous agents still lacking real-world or long-term data.
Anti-TNF agents are effective for treating patients with Crohn’s disease (especially complex Crohn’s disease); however, their safety profile and possible need for concomitant immunomodulator therapy may make them less appealing for the management of certain patients. Multiple anti-TNF therapies are efficacious in treating moderate to severe Crohn’s disease, including infliximab, adalimumab and certolizumab.23 These can all be administered subcutaneously; however, infliximab has the option of intravenous administration, which offers benefits in some clinical scenarios due to the difference in pharmacokinetics with intravenous dosing. When considering real-world practice, most patients tolerate anti-TNF agents well without any major side effects; however, anti-TNF agents are not the preferred treatment for older patients with IBD or patients with multiple comorbidities.
Interleukin inhibitors
Interleukin (IL)-23 is one of the chief proinflammatory cytokines in the pathogenesis of Crohn’s disease.29 IL-23 causes expansion of pathogenic T-helper cells and induces a cascade of other proinflammatory molecules including TNF. The first TGA approved IL-23 therapy for Crohn’s disease was ustekinumab, which is a monoclonal antibody to the p40 subunit of IL-12 and IL-23.30 Specific IL-23 inhibitors have since been developed which target the p19 subunit of IL-23, and have demonstrated efficacy in phase 2 and some phase 3 trials.31 These include risankizumab, mirikizumab and gusulkumab. This class of agents is usually administered intravenously or subcutaneously for induction and maintenance therapy, respectively.
There are a number of advantages to using IL-23 inhibitors for the treatment of Crohn's disease. They are efficacious in both induction and maintenance therapy of moderate to severe Crohn’s disease.31 Despite being an immunosuppressant, this class has an excellent safety profile and has not been associated with an increased risk of infection or malignancy.28 They are generally well tolerated. This class can also be used to treat certain extraintestinal manifestations and concomitant autoimmune conditions, such as psoriasis and peripheral small-joint arthritis.27
Ustekinumab has been associated with worsening axial spondyloarthropathy in clinical trials; therefore, consideration of potential worsening of concomitant axial spondyloathropathies is warranted in applicable patients. However, this is not a contraindication. In patients who fail to respond to anti-TNF treatment, ustekinumab is less effective (like most biologic therapies) and often requires dose escalation. IL therapies may not be suitable for patients who have phobias of self-injecting.
There is emerging evidence that the p19 subunit IL-23 inhibitors may have superior efficacy to ustekinumab.32 This is an exciting development, given the theoretically superior safety profile associated with targeting only one interleukin.
Anti-integrin therapies
Anti-integrin therapies work in Crohn’s disease by blocking lymphocyte trafficking into the gut and, therefore, reducing inflammation.23
Natalizumab is a commonly used anti-integrin therapy in multiple sclerosis, and is also an effective treatment for Crohn’s disease. However, natalizumab is not indicated specifically for Crohn’s disease because of the risk of John Cunningham virus reactivation.33 Etrolizumab, an α4β7 and αEβ7 integrin blocker, was developed for the treatment of Crohn’s disease and ulcerative colitis. However, etrozliumab has not transitioned to use in clinical practice due to underwhelming trial results, where it was found to be modestly superior to placebo as a maintenance therapy in Crohn’s disease but not superior as induction therapy.34
Vedolizumab, an α4β7 integrin blocker, is a gut-selective therapy and currently the only TGA approved anti-integrin therapy with a PBS listing for Crohn’s disease. Systematic review and meta-analysis suggest that vedolizumab has an excellent safety profile comparable with placebo.35 It is well tolerated, with side effects similar to those experienced with most biologic medications. Vedolizumab is typically administered eight-weekly as intravenous therapy or every two weeks as subcutaneous therapy.
Vedolizumab has an excellent real-world safety profile, which makes it an attractive option for patients in whom systematic immunosuppression could be particularly problematic. Vedolizumab does not work as quickly as the other biologic agents; therefore, unwell patients often need bridging therapy with corticosteroids or tacrolimus.36 Vedolizumab does not work as well in patients who have not responded to anti-TNF therapy.35
Janus kinase inhibitors
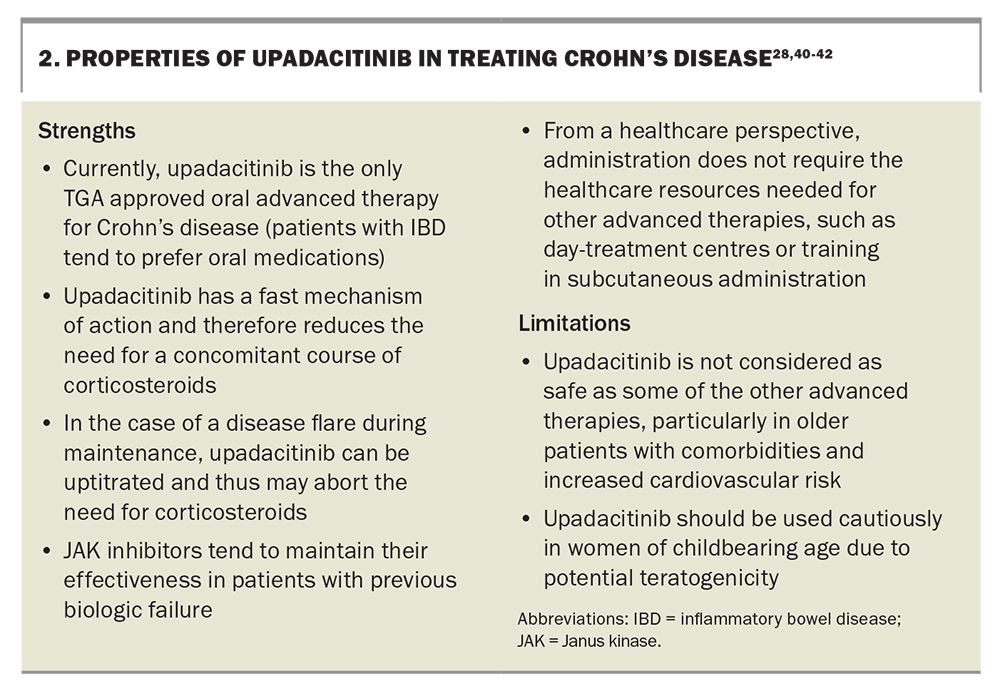
Upadacitinib is the newest agent available for the treatment of Crohn’s disease. It is a small molecule that selectively inhibits Janus kinase (JAK) 1 enzymes.37 The JAK signal transducer of activation (STAT) pathway is important in Crohn’s disease: interleukins activate the JAK-STAT receptors of T cells, which leads to the secretion of proinflammatory cytokines.38 Upadacitinib blocks this pathway (in particular, by blocking JAK1), which in turn downregulates inflammation. Upadacitinib was originally indicated for rheumatoid arthritis and has recently been PBS listed for specialist use in the treatment of severe Crohn’s disease based on data from phase 3 clinical trials.37
The Study of the Efficacy and Safety of Upadacitinib in Participants With Moderately to Severely Active Crohn’s Disease Who Have Inadequately Responded to or Are Intolerant to Conventional and/or Biologic Therapies (U-EXCEL) and the Study of the Efficacy and Safety of Upadacitinib (ABT-494) in Participants With Moderately to Severely Active Crohn’s Disease Who Have Inadequately Responded to or Are Intolerant to Biologic Therapy (U-EXCEED) induction trials of upadacitinib demonstrated clinical remission rates of 49.5% and 38.9%, respectively. The U-EXCEED arm consisted exclusively of a cohort who did not respond to at least one biological therapy and, because of the usual diminishing return of subsequent therapies, this makes the results even more impressive. The Maintenance and Long-Term Extension Study of the Efficacy and Safety of Upadacitinib (ABT-494) in Participants With Crohn’s Disease Who Completed the Studies M14-431 or M14-433 (U-ENDURE) maintenance trial demonstrated clinical remission rates at 12 months of 37.3% for 15 mg daily and 47.6% for 30 mg daily maintenance dosing, both of which were superior to placebo.
Upadacitinib is an oral tablet taken once daily and is generally well-tolerated. Although it exhibits a clear positive dose-efficacy response relationship, upadacitinib also increasingly inhibits other JAK receptors (JAK2, JAK3, and TYK2) at higher doses.38 This results in an increased risk of side effects. The safety profile of upadacitinib is generally acceptable and likely safer than pan-JAK inhibitors, such as tofacitinib. Key safety considerations for JAK inhibitors include higher cholesterol levels and increased risks of major adverse cardiovascular events, malignancy (mostly in older people with a history of smoking), reactivation of herpes zoster virus (increasing risk with age) and venous thromboembolism (associated more with rheumatoid arthritis than IBD).39 These risks may be overestimated in the IBD population, as most of this safety data comes from patients with rheumatoid arthritis who tend to be older and experience more comorbidities than patients with IBD.
Regarding risk management, recombinant zoster vaccination is suggested for those with previous varicella exposure, particularly in patients older than 50 years of age. Monitoring cholesterol levels and active management of cardiovascular risk factors is prudent. Upadacitinib should be used cautiously in women of childbearing age due to potential teratogenicity, and effective contraception should be in place. The strengths and limitations of upadacitinib are listed in Box 2.28,40-42
The future of medical therapies
Despite the emergence of multiple medical therapies, the treatment options available for Crohn’s disease remain limited.43 In clinical trials, advanced therapies typically achieve remission for only 30 to 60% of patients with moderate to severe disease. This results in a significant treatment gap among patients with Crohn’s disease. Additionally, patients often experience a loss of response to these treatments over time and subsequent therapies offer diminishing returns, thus presenting the challenge of a ‘therapeutic ceiling’. To address this issue, novel strategies for management are being developed, including faecal microbial transplants, dual biologic treatments and agents that target immunometabolism pathways instead of individual cytokines.44-48
Diet and Crohn’s disease
Dietary considerations have frequently taken a back seat to medical and surgical treatments in the context of Crohn’s disease. Nevertheless, patients are increasingly interested in how their diet can influence their condition. Notably, the body of research on dietary therapies for Crohn's disease is not as extensive as for medical therapies, and is often characterised by limitations in sample sizes, study design, or a lack of result replication.49 However, with the significant progress that has been made, dietary advice is expected to occupy a more central role in the comprehensive management of Crohn’s disease.
Specific dietary therapies
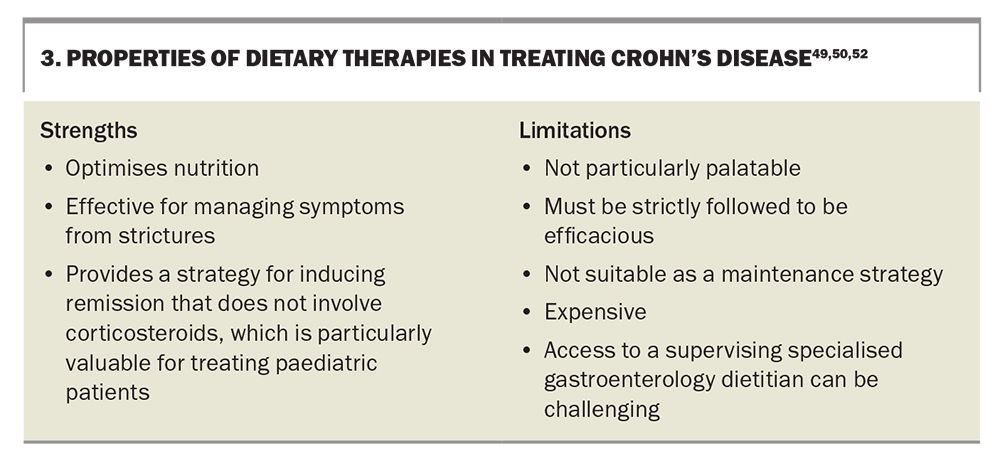
There are multiple diets that are effective in reducing inflammation and inducing remission. The two main diets are exclusive enteral nutrition (EEN) and the Crohn’s disease exclusion diet (CDED).49-51 EEN nutrition is an exclusively liquid diet, consisting entirely of elemental, semielemental or polymeric formulations. It is effective in inducing remission when compared with corticosteroids, particularly in paediatric patients.50 The CDED excludes foods that are thought to drive inflammation.51 The diet is limited to certain unprocessed meats, fruits and vegetables. From a nutrient perspective, the diet is high in protein and resistant starches and low in animal fat, haem, gluten and food additives. The CDED is often implemented in conjunction with partial enteral nutrition. These diets have a duration of between six and 12 weeks and are primarily used to induce remission and improve nutritional status. These diets should be undertaken under the direction of an expert gastroenterology dietitian.52 The strengths and limitations of specific dietary therapies are shown in Box 3.49,50,52
General dietary advice
Emerging evidence suggests that the consumption of ultraprocessed foods is associated with an elevated risk of Crohn’s disease.53 Ultraprocessed foods increase gut inflammation and the ingredients in certain processed foods negatively affect the gut microbiome.54 Robust evidence supporting the avoidance of ultraprocessed foods to reduce disease flares is currently lacking, but it may be sensible to restrict their consumption if patients are willing to pursue dietary therapies.
Furthermore, there are emerging studies indicating the potential benefits of the Mediterranean diet for individuals with Crohn’s disease. One study, for example, showed improved disease activity, enhanced markers of inflammation, and an overall improvement in quality of life for patients on the diet.55 Beyond its utility in managing Crohn’s disease, the Mediterranean diet is widely recognised for its positive impact on various chronic noncommunicable diseases.56
Dietary modifications are only recommended for symptom management in specific situations. Low residue diets are valuable for addressing symptomatic strictures but are no longer routinely advised because of the potential risk of micronutrient deficiencies.57 The low fermentable oligosaccharides, disaccharides, monosaccharides and poly-saccharides (i.e. FODMAP) diet is helpful in managing patients with functional gut symptoms, which is an important consideration given the high prevalence of concomitant irritable bowel syndrome in patients with IBD.58
Nutritional deficiencies
People with Crohn's disease are at risk of nutritional deficiencies for numerous reasons, including malabsorption, hypermetabolism, and restrictive eating patterns.59 Patients with active Crohn’s disease require a higher total caloric and protein intake, given the hypermetabolic state of active disease. Patients with Crohn’s disease are also at risk of micronutrient deficiencies, particularly when there is active disease involving the small intestine, or in cases of intestinal resection. Key micronutrient deficiencies include iron, B12, folate, vitamin D, zinc, selenium and electrolytes in diarrhoeal states.59 These nutritional issues highlight the importance involving a dietitian in the care of patients with Crohn’s disease (particularly in active disease states), as well as the importance of checking for nutritional deficiencies and replacing as appropriate. Simple nutritional optimisation strategies that patients should follow include taking high-quality multivitamins and oral nutritional supplements. As a minimum, checking iron studies, B12, folate and vitamin D levels on an annual basis is sensible.60,61
Conclusion
Crohn’s disease is common in Australia and results from a complex interaction of genetics, environmental factors and the gut microbiome. Its pathophysiology manifests as immune dysregulation and inappropriate gastrointestinal inflammation. There are a growing number of efficacious medical therapies for Crohn’s disease, each with their unique strengths, limitations and safety profiles. The Mediterranean diet has multiple benefits in Crohn’s disease and advising patients to limit the consumption of ultraprocessed foods is prudent. Specific diets can be undertaken to induce remission under the supervision of an expert gastroenterology dietitian. Timely treatment is important to alter the natural course of Crohn’s disease and to improve quality of life in affected patients. MT
COMPETING INTERESTS: Dr Elford: None. Associate Professor Christensen has received speaking fees from Abbvie, Janssen, Pfizer, Takeda and Ferring; research grants from Janssen and Ferring Pharmaceuticals; and served on the advisory board of Gilead and Novartis.
ACKNOWLEDGEMENTS: Dr Elford would like to thank the Australian Commonwealth Government for their support via a Research Training Program Scholarship.
References
1. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet 2017; 389:1741-1755.
2. Bhatia R, Yeoh SW, Vaz K, at al. Inflammatory bowel disease incidence, prevalence and 12-month initial disease course in Tasmania, Australia. Intern Med J 2019; 49: 622-630.
3. Improving inflammatory bowel disease care across Australia. Crohn’s & Colitis Australia (CCA); 2022. Available online at: https://crohnsandcolitis.org.au/advocacy/our-projects/improving-inflammatory-bowel-disease-care-across-australia/ (accessed January 2024).
4. Alfredsson J, Wick MJ. Mechanism of fibrosis and stricture formation in Crohn's disease. Scand J Immunol 2020; 92:e12990.
5. Ashton JJ, Beattie RM Inflammatory bowel disease: recent developments. Arch Dis Child 2023: archdischild-2023-325668. Epub ahead of print.
6. Turner D, Ricciuto A, Lewis A, et al; International Organization for the Study of IBD. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021; 160: 1570-1583.
7. Rieder F, Bettenworth D, Ma C, Parker CE, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn's disease. Aliment Pharmacol Ther 2018; 48: 347-357.
8. Summers RW, Switz DM, Sessions JT Jr, et al. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology 1979; 77: 847-869.
9. Malchow H, Ewe K, Brandes JW, et al. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology 1984; 86:249-266.
10. Singleton JW, Law DH, Kelley ML Jr, Mekhjian HS, Sturdevant RA. National Cooperative Crohn's Disease Study: adverse reactions to study drugs. Gastroenterology 1979; 77: 870-882.
11. Edsbäcker S, Andersson T. Pharmacokinetics of budesonide (Entocort EC) capsules for Crohn's disease. Clin Pharmacokinet 2004; 43: 803-821.
12. Seow CH, Benchimol EI, Griffiths AM, Otley AR, Steinhart AH. Budesonide for induction of remission in Crohn's disease. Cochrane Database Syst Rev 2008; 3: CD000296.
13. Steinhart AH, Ewe K, Griffiths AM, Modigliani R, Thomsen OO. Corticosteroids for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev 2003;4: CD000301.
14. Hanoodi M, Mittal M. Methotrexate. Treasure Island: StatPearls Publishing; 2023. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK556114/ (accessed Jan 2024).
15. Patel V, Wang Y, MacDonald JK, McDonald JW, Chande N. Methotrexate for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev 2014; 8: CD006884.
16. Feagan BG, Rochon J, Fedorak RN, et al; The North American Crohn's Study Group Investigators. Methotrexate for the treatment of Crohn's disease. N Engl J Med 1995; 332: 292-297.
17. Bannwarth B, Labat L, Moride Y, Schaeverbeke T. Methotrexate in rheumatoid arthritis: an update. Drugs 1994; 47: 25-50.
18. Mantzaris GJ. Thiopurines and methotrexate use in IBD patients in a biologic era. Curr Treat Options Gastroenterol 2017; 15: 84-104.
19. Demase K, Monitto CK, Little RD, Sparrow MP. The role of low-dose oral methotrexate in increasing anti-TNF drug levels and reducing immunogenicity in IBD. J Clin Med 2023; 12: 4382.
20. Taylor KM, Ward MG, Blaker PA, Sparrow MP. Is there a role for thioguanine therapy in IBD in 2017 and beyond? Expert Rev Gastroenterol Hepatol 2017; 11: 473-486.
21. Van Asseldonk DP, Jharap B, Verheij J, et al. The prevalence of nodular regenerative hyperplasia in inflammatory bowel disease patients treated with thioguanine is not associated with clinically significant liver disease. Inflamm Bowel Dis 2016; 22: 2112-2120.
22. Pagnini C, Cominelli F. Tumor Necrosis Factor's pathway in Crohn's disease: potential for intervention. Int J Mol Sci 2021; 22: 10273.
23. Cushing K, Higgins PDR. Management of Crohn disease: a review. JAMA 2021; 325: 69-80.
24. Schulberg JD, Wright EK, Holt BA, et al. Intensive drug therapy versus standard drug therapy for symptomatic intestinal Crohn's disease strictures (STRIDENT): an open-label, single-centre, randomised controlled trial. Lancet Gastroenterol Hepatol 2022; 7: 318-331.
25. Bouhnik Y, Carbonnel F, Laharie D, et al; GETAID CREOLE Study Group. Efficacy of adalimumab in patients with Crohn's disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut 2018; 67: 53-60.
26. Vasudevan A, Bruining DH, Loftus EV Jr, Faubion W, Ehman EC, Raffals L. Approach to medical therapy in perianal Crohn's disease. World J Gastroenterol 2021; 27: 3693-3704.
27. Gordon H, Burisch J, Ellul P, Karmiris K, et al. ECCO guidelines on extraintestinal manifestations in inflammatory bowel disease. J Crohns Colitis 2023; 17: 827-854.
28. Sattler L, Hanauer SB, Malter L. Immunomodulatory agents for treatment of patients with inflammatory bowel disease (review safety of anti-TNF, anti-integrin, anti IL-12/23, JAK inhibition, sphingosine 1-phosphate receptor modulator, azathioprine / 6-MP and methotrexate). Curr Gastroenterol Rep 2021; 23: 30.
29. Schmitt H, Neurath MF, Atreya R. Role of the IL23/IL17 pathway in Crohn's disease. Front Immunol 2021; 12: 622934.
30. Feagan BG, Sandborn WJ, Gasink C, et al; UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2016; 375: 1946-1960.
31. Verstockt B, Salas A, Sands BE, Abraham C, Leibovitzh H, Neurath MF, Vande Casteele N; Alimentiv Translational Research Consortium (ATRC). IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2023; 20: 433-446.
32. McCall B. Endoscopic remission doubled with risankizumab vs ustekinumab in Crohn’s disease. Newark (NJ): Medscape; 2023. Available online at: https://www.medscape.com/viewarticle/997377?form=fpf (accessed Jan 2024).
33. Chalkias S, Dang X, Bord E, Stein MC, at al. JC virus reactivation during prolonged natalizumab monotherapy for multiple sclerosis. Ann Neurol 2014; 75: 925-934.
34. Sandborn WJ, Panés J, Danese S, et al; BERGAMOT Study Group. Etrolizumab as induction and maintenance therapy in patients with moderately to severely active Crohn's disease (BERGAMOT): a randomised, placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2023; 8: 43-55.
35. Qiu B, Liang JX, Li C. Efficacy and safety of vedolizumab for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2022; 101: e30590.
36. Christensen B, Gibson PR, Micic D, at al. Safety and efficacy of combination treatment with calcineurin inhibitors and vedolizumab in patients with refractory inflammatory bowel disease. Clin Gastroenterol Hepatol 2019; 17: 486-493.
37. Loftus EV Jr, Panés J, Lacerda AP, Peyrin-Biroulet L, et al. Upadacitinib induction and maintenance therapy for Crohn's disease. N Engl J Med 2023; 388: 1966-1980.
38. Herrera-deGuise C, Serra-Ruiz X, Lastiri E, Borruel N. JAK inhibitors: a new dawn for oral therapies in inflammatory bowel diseases. Front Med (Lausanne) 2023; 10: 1089099.
39. Clarke B, Yates M, Adas M, Bechman K, Galloway J. The safety of JAK-1 inhibitors. Rheumatology (Oxford) 2021; 60 Suppl 2: S24-30.
40. Denesh D, Carbonell J, Kane JS, Gracie D, Selinger CP. Patients with inflammatory bowel disease (IBD) prefer oral tablets over other modes of medicine administration. Expert Rev Gastroenterol Hepatol. 2021 Sep;15(9):1091-1096. doi: 10.1080/17474124.2021.1898944. Epub 2021 Mar 10. PMID: 33653185.
41. Honap S, Chee D, Chapman TP, Patel M, et al; LEO IBD Research Consortium. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis 2020; 14: 1385-1393.
42. Chaparro M, Garre A, Mesonero F, et al. Tofacitinib in ulcerative colitis: real-world evidence from the ENEIDA registry. J Crohns Colitis 2021; 15: 35-42.
43. Raine T, Danese S. Breaking through the therapeutic ceiling: what will it take? Gastroenterology 2022; 162: 1507-1511.
44. Santiago P, Braga-Neto MB, Loftus EV. Novel therapies for patients with inflammatory bowel disease. Gastroenterol Hepatol (NY) 2022; 18: 453-465.
45. Feagan BG, Sands BE, Sandborn WJ, et al; VEGA Study Group. Guselkumab plus golimumab combination therapy versus guselkumab or golimumab monotherapy in patients with ulcerative colitis (VEGA): a randomised, double-blind, controlled, phase 2, proof-of-concept trial. Lancet Gastroenterol Hepatol 2023; 8: 307-320.
46. Ahmed W, Galati J, Kumar A, et al. Dual biologic or small molecule therapy for treatment of inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2022; 20: e361-e379.
47. Lee H, Jeon JH, Kim ES. Mitochondrial dysfunctions in T cells: focus on inflammatory bowel disease. Front Immunol 2023; 14:1219422.
48. Microbial Restoration in Inflammatory Bowel Diseases (MIRO II). Melbourne: National Library of Medicine; 2021. Available from: https://clinicaltrials.gov/study/NCT04970446 (accessed Jan 2024).
49. Caio G, Lungaro L, Caputo F, et al. Nutritional treatment in Crohn's disease. Nutrients 2021; 13: 1628.
50. Szczubełek M, Pomorska K, Korólczyk-Kowalczyk M, Lewandowski K, Kaniewska M, Rydzewska G. Effectiveness of Crohn's disease exclusion diet for induction of remission in Crohn's disease adult patients. Nutrients 2021; 13: 4112.
51. Yanai H, Levine A, Hirsch A, et al. The Crohn's disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn's disease (CDED-AD): an open-label, pilot, randomised trial. Lancet Gastroenterol Hepatol 2022; 7: 49-59.
52. Elford AT, Leong RW, Halmos EP, et al. IBD barriers across the continents: a continent-specific analysis - Australasia. Therap Adv Gastroenterol 2023;16: 17562848231197509.
53. Chen J, Wellens J, Kalla R, et al. Intake of ultra-processed foods is associated with an increased risk of Crohn's disease: a cross-sectional and prospective analysis of 187 154 participants in the UK biobank. J Crohns Colitis 2023;17: 535-552.
54. Bolte LA, Vich Vila A, Imhann F, et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021; 70: 1287-1298.
55. Chicco F, Magrì S, Cingolani A, et al. Multidimensional impact of Mediterranean diet on IBD patients. Inflamm Bowel Dis 2021; 27: 1-9.
56. Dominguez LJ, Di Bella G, Veronese N, Barbagallo M. Impact of Mediterranean diet on chronic non-communicable diseases and longevity. Nutrients 2021; 13: 2028.
57. Charlebois A, Rosenfeld G, Bressler B. The impact of dietary interventions on the symptoms of inflammatory bowel disease: a systematic review. Crit Rev Food Sci Nutr.2016; 56: 1370-1378.
58. Popa SL, Pop C, Dumitrascu DL. Diet advice for Crohn's disease: FODMAP and beyond. Nutrients 2020; 12: 3751.
59. Jabłońska B, Mrowiec S. Nutritional status and its detection in patients with inflammatory bowel diseases. Nutrients 2023; 15: 1991.
60. Lomer MCE, Wilson B, Wall CL. British Dietetic Association consensus guidelines on the nutritional assessment and dietary management of patients with inflammatory bowel disease. J Hum Nutr Diet 2023; 36: 336-377.
61. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol 2017; 112: 241-258.