Ulcerative colitis: an update on management
With the continuing evolution of treatments and therapeutic targets for ulcerative colitis (UC), the outlook for patients with this condition has improved greatly in recent years. GPs are a crucial part of shared care for these patients, with an important role in addressing the physical, social and mental health consequences of UC and its treatment.
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) characterised by chronic inflammation of the large intestine. The pathogenesis of IBD is incompletely understood; however, the interaction of genetic, immune, microbiota and environmental factors are thought to lead to immune dysregulation and intestinal damage.1
Australia has one of the highest incidences of IBD in the world, at around 30 per 100,000 population.2,3 The peak age for diagnosis of UC is the third and fourth decades of life, resulting in a rising and compounding prevalence.4,5 Without adequate disease control, UC may result in gastrointestinal symptoms such as bloody diarrhoea, faecal urgency, incontinence, abdominal pain, fatigue, weight loss and nutritional deficiencies, together with extraintestinal manifestations that include arthritis, skin disease and iritis. These manifestations may have a significant impact on quality of life in many patients, reducing productivity and social participation.
The treatment of UC has evolved significantly over the past two decades with the development of biologic-based therapy and small molecule inhibitors. Therapy aims to induce and maintain disease remission to minimise gastrointestinal symptoms and risk of long-term disability, colectomy and colorectal cancer.6 Given the complex management of UC, patients should ideally be managed by a multidisciplinary team comprising gastroenterologists, specialist IBD nurses, dietitians, pharmacists, psychologists, colorectal surgeons and GPs to achieve optimal health outcomes.7
This article provides an update on the management of adult patients with UC, including preventive care, with a focus on the role of the GP. The clinical presentation and diagnosis of UC are beyond the scope of this article and were reviewed in the March 2023 issue of Medicine Today.8
Disease classification
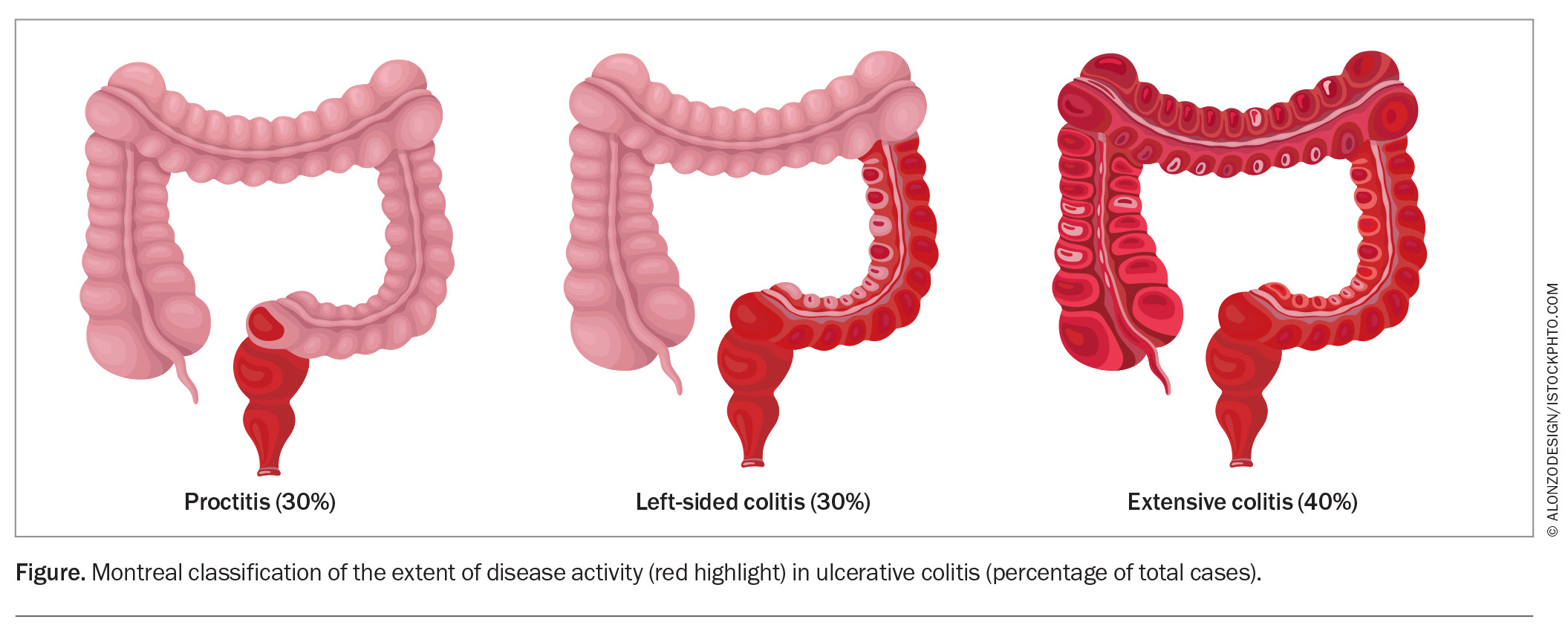
Treatment of UC is guided by the anatomical extent of disease and severity. The Montreal classification categorises UC as:9,10
- proctitis – disease involving only the rectum
- left-sided colitis – disease distal to the splenic flexure
- pancolitis – disease extending proximal to the splenic flexure (Figure).
Over time, the distribution of disease can extend or regress.11
Treatment targets
The treatment targets in most patients with UC comprise:12
- clinical remission – this short-term target to improve quality of life is conventionally defined as stool frequency of three or fewer daily and the absence of rectal bleeding13
- endoscopic healing – this is defined by normalisation of vascular pattern, absence of mucosal friability, erosions and ulceration. It has been increasingly recognised as an independent predictor of reduced risk of long-term complications and is now regarded as an important treatment goal for most patients.12 Recent data suggest histological remission may further improve long-term outcomes; it is an evolving aspirational treatment goal14,15
- normalisation of inflammatory markers
(C-reactive protein [CRP] and faecal calprotectin) – these noninvasive disease activity markers have been shown to correlate with endoscopic activity in UC and have an established role as surrogate indices16 - normal health-related quality of life – improvement in quality of life may require additional measures, such as dietary and psychological therapies, as well as independent management of immune-mediated extraintestinal manifestations.
Assessment of disease activity
Assessment of disease activity requires a detailed history of symptoms, including bowel frequency, consistency, blood and mucus passage, urgency, nocturnal bowel actions and incontinence, as well as assessment of fatigue, weight loss, dietary intake and modifications and extraintestinal manifestations. It is important to complement this history with an assessment of objective markers of disease activity, including serum CRP and albumin levels, iron deficiency with or without anaemia, and faecal calprotectin.
Endoscopic assessment has the most supporting evidence for predicting future disease course. However, in light of the practical limitations related to cost, access and patient acceptance, it should be used judiciously.17 Intestinal ultrasound examination was recently described as a useful modality for disease assessment and has an increasing role in tertiary IBD centres.18 So far, evidence supporting this modality is limited in patients with isolated proctitis because of the limited views obtained.18
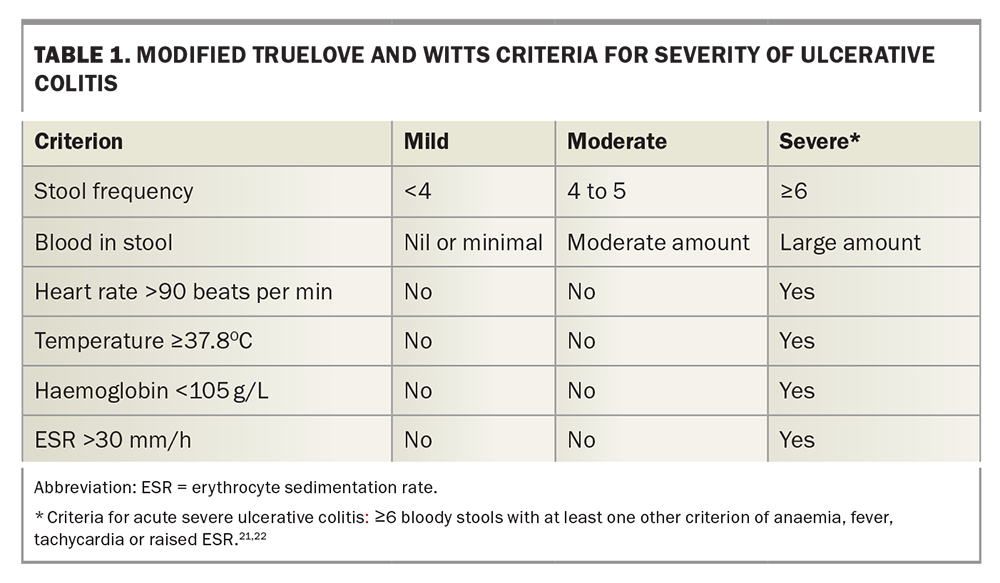
Multiple validated and unvalidated disease indices have been described, including the Mayo Disease Severity Score, Simple Clinical Colitis Activity Index (SCCAI) and modified Truelove and Witts Severity Index, with the general aim of grading current disease activity as mild, moderate or severe (Table 1).19-22 Patients meeting criteria for acute severe UC should be referred urgently for hospital admission and treatment with intravenous corticosteroids.23
Medical therapy
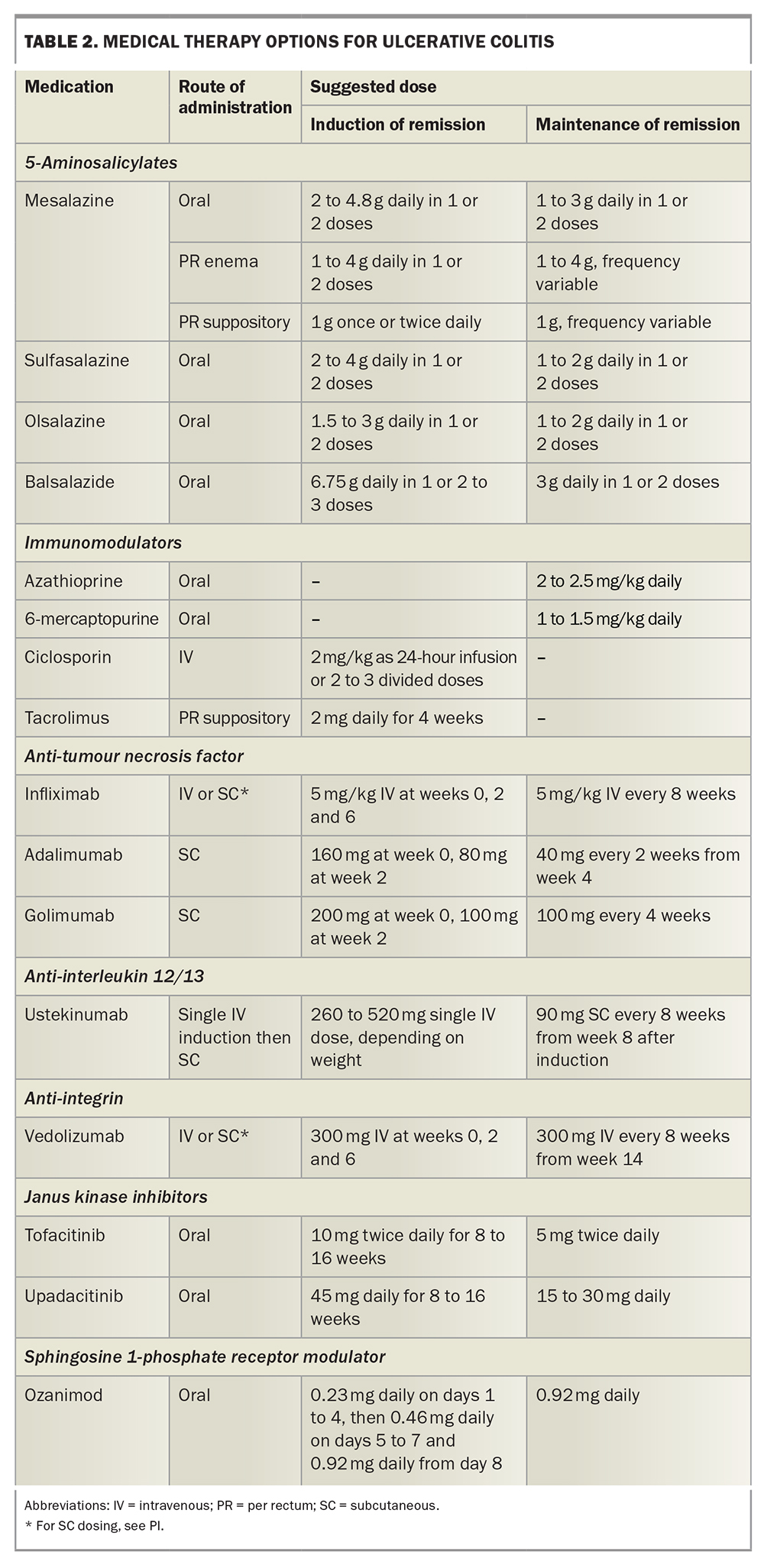
The various options for medical therapy are summarised in Table 2.
5-Aminosalicylates
5-Aminosalicylates (5-ASAs) have long been used for the induction and maintenance of remission in people with mild to moderate UC. They include sulfasalazine, mesalazine, balsalazide and olsalazine, all of which are prodrugs of the active aminosalicylate component. All are available as oral preparations. Mesalazine is also available as a topical formulation for rectal administration.24 The median time to clinical response for 5-ASAs is about two to four weeks.25
Induction of remission using 5-ASAs usually requires doses of 4 to 4.8 g of oral mesalazine or equivalent, with daily dosing shown to be as effective as split day dosing, while resulting in improved adherence.26 In people with distal disease (affecting the rectum or rectosigmoid), monotherapy with a topical 5-ASA can be used. If remission is not achieved with topical therapy alone, oral 5-ASAs can be added in combination.27 In people with pancolitis and
left-sided disease, both oral and topical therapy is recommended to induce remission, but maintenance can be sustained with oral
5-ASA monotherapy.28,29 Sulfasalazine may have additional benefit in patients with IBD-related arthritis symptoms.29
Topical mesalazine formulations can be administered as suppositories (concentrating in the rectum), foam enemas (taking effect in the sigmoid colon) or liquid enemas (effect extends to the splenic flexure). To ensure clinical effectiveness and adherence, education about proper administration of these products is crucial. Patients can continue on topical therapy alone; however, patient preference and adherence are often a barrier to ongoing topical treatment.30
Mesalazine is usually well tolerated, with adverse effects that include headache, abdominal pain and, rarely, interstitial nephritis.29 Sulfasalazine may cause deranged liver biochemistry and anaemia, as well as the dose-related effects of headache and nausea, and rare severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis.31 Patients should have routine full blood counts (FBE) and liver function tests (LFTs) performed while using sulfasalazine. Folic acid supplementation may help mitigate these risks.29
Corticosteroids
Topical and oral corticosteroids should be used only for the induction of remission. For patients with moderate to severe UC, systemic corticosteroids are used for induction. In the community, oral prednisolone 40 to 60 mg can be used for induction until symptoms improve, followed by a taper of 5 to 10 mg weekly, typically over six to eight weeks.32 Patients with acute severe UC should be admitted to hospital for intravenous corticosteroids equivalent to a minimum of hydrocortisone 300 mg daily.32
It is important to be vigilant for the well established adverse effects of corticosteroids and to attempt to limit exposure to a maximum of three months.30 Corticosteroid-sparing agents should be considered in patients requiring more than one course of systemic corticosteroids in a 12-month period.33,34
Budesonide multimatrix is a colonic-release corticosteroid that can be used to treat patients with mild to moderate UC refractory to maximal 5-ASA therapy. It has a high first-pass metabolism by the liver, thus reducing systemic effects.35 The typical dose is 9 mg once daily for up to eight weeks.36
Rectal formulations of budesonide and prednisolone can also help in the induction of remission for patients with proctitis or left-sided disease.30
Immunomodulators
Azathioprine and 6-mercaptopurine
Thiopurines (6-mercaptopurine and azathioprine) are immunomodulators that are used for the maintenance of remission in patients who have disease relapse on 5-ASA therapy and those who are corticosteroid-dependent.30,37 They are corticosteroid-sparing agents that are thought to reduce T-cell function. They are metabolised via the thiopurine S-methyltransferase (TPMT) enzyme, and polymorphisms in the gene encoding this enzyme can lead to variable enzyme activity and potential toxicity. Hence, patients should undergo TPMT genotype testing before starting thiopurine therapy.38 Thiopurines can take up to three months to take effect, and a weaning course of corticosteroids can be used concurrently to achieve remission.39
The metabolism of thiopurine medications can be altered by coadministration of xanthine oxidase inhibitors such as allopurinol.40 Coadministration may result in toxicity (myelosuppression) if conventional doses of thiopurines are used. However, in the 25 to 33% of patients who experience ‘shunting’ with thiopurine monotherapy (preferential metabolism to less effective and potentially toxic thiopurine metabolites), low doses of thiopurines in combination with allopurinol may be prescribed to improve therapeutic effect.41,42 Specialist gastroenterologist advice is recommended before pursuing such a strategy.
Adverse effects of thiopurines include pancreatitis, with a prevalence of 3%. If this occurs, the thiopurine should be ceased immediately.43 Other adverse effects include myelosuppression, specifically leukopenia, and hepatotoxicity.39 Consequently, patients should be monitored with FBE, LFTs and measurement of levels of thiopurine metabolites. Testing for variants in the genes that encode TPMT and nudix hydrolase 15 (NUDT15), another enzyme involved in thiopurine metabolism, may be useful to identify patients at risk of myelotoxicity and intolerance.44
A small absolute increased risk of specific malignancies with thiopurines has been described. Patients with IBD treated with thiopurines have a twofold increased risk of nonmelanoma skin cancers and should undergo annual skin checks and be advised on sun protection.45 They also have a slightly increased risk of lymphoma, especially if they are positive for Epstein-Barr virus (EBV); EBV status should be determined before commencing thiopurine treatment.46 There may also be an increased risk of high-grade cervical dysplasia and cervical cancer in patients with IBD treated with thiopurines.47-49
Methotrexate
Methotrexate is commonly used as an immunomodulator in patients with Crohn’s disease, but studies on its use in patients with UC have not shown significant efficacy.50 Methotrexate may be used in selected patients concurrently treated with anti-tumour necrosis factor (TNF) therapies to reduce the risk of anti-drug antibody formation and thus improve durability of effect.51
Advanced therapies
Advanced therapies for patients with UC encompass biologic therapies (monoclonal antibodies) and novel small molecule inhibitors. These medications have revolutionised the management of patients with UC over the past two decades. Clinical response rates in clinical trials have ranged from 30 to 60% over the first eight to 12 weeks, with endoscopic remission usually slower in onset.52-59 Maintenance of response differs across the different agents, but generally 50 to 80% of patients who respond remain in clinical remission at 12 months and beyond. Real-world response rates have generally been reported to be higher than those achieved in clinical trials, which may be related to factors such as dose escalation and less refractory patient cohorts than those participating in clinical trials.60
Despite their effectiveness, these advanced therapies come at significant healthcare expense, ranging from about $10,000 to $25,000 annually.61 Biosimilar medications are now available for some biologics, which has helped reduce cost.62,63
Biologics
Currently available biologic therapies for the treatment of UC include monoclonal antibodies to TNF-alfa, the integrin α4β7 and the interleukin (IL) 12/23. These agents are indicated for patients with moderate to severe UC who have not had an adequate response or are intolerant to immunomodulator therapy; they can be prescribed only by a qualified gastroenterologist. Factors that contribute to the choice of biologic therapy include route of administration, extraintestinal manifestations of disease, side-effect profile and need for rapid induction.
Anti-tumour necrosis factor
Infliximab, adalimumab and golimumab are indicated for patients with moderate to severe UC.64 These agents inhibit the production of TNF-alfa, a proinflammatory cytokine implicated in the pathogenesis of UC.
Infliximab can be administered either subcutaneously or intravenously, with the intravenous formulation shown to rapidly induce remission in patients with moderate to severe UC. Golimumab and adalimumab are administered as subcutaneous injections. Anti-TNF medications are effective for the treatment of many immune-mediated extraintestinal manifestations of UC, such as joint disease and skin and ocular complications.
Anti-TNF drugs are associated with an increased risk of opportunistic and serious infections, especially when they are used in combination with an immunomodulator.65 Anti-TNF drugs may also be associated with a slightly increased risk of lymphoma and melanoma.49 They are contraindicated in patients with multiple sclerosis and in those with New York Heart Association class III to IV heart failure.66
Anti-interleukin 12/23
Ustekinumab is a monoclonal antibody that inhibits the p40 subunit common to the proinflammatory cytokines IL-12 and IL-23. It was listed on the PBS for the treatment of moderate to severe UC in May 2023.67 It is given as a single intravenous induction infusion based on weight, followed by a 90 mg subcutaneous dose every eight weeks. Safety data from trials of up to four years have been reassuring to date, with adverse effects comparable with placebo.55,68,69
Anti-integrin
The anti-integrin antibody, vedolizumab, is indicated for moderate to severe UC when the response to immunomodulator therapy is inadequate.57 Vedolizumab is administered as an infusion every eight weeks. It acts predominantly by inhibiting the interaction between α4β7-integrin on lymphocytes and adhesion molecules on the intestinal vascular endothelium. This prevents the migration of lymphocytes into the intestinal mucosa, reducing intestinal inflammation.70 This mechanism of action gives vedolizumab a favourable safety profile, as it does not have a systemic immunosuppressive effect.
Small molecule agents
Janus kinase inhibitors
Tofacitinib and upadacitinib are oral Janus kinase (JAK) inhibitors that block cytokine signalling and reduce inflammation.71 They have been shown to rapidly induce and maintain remission in patients with UC. They have a dose-dependent association with an increased risk of serious infections, especially herpes zoster (shingles).72,73 JAK inhibitors are associated with increased risk of dyslipidaemia, major adverse cardiovascular events and venous thromboembolism.74 The patient’s lipid profile should be checked at baseline and eight to 12 weeks after commencing JAK inhibitor therapy.75
Sphingosine-1-phosphate receptor inhibitors
Ozanimod is a sphingosine-1-phosphate receptor modulator that regulates lymphocyte migration and blocks infiltration into inflamed mucosa.71 It has been shown to induce and maintain remission in patients with moderate to severe UC. It may be associated with hypertension, bradyarrhythmia and macula oedema in patients with increased risk, such as those with diabetes mellitus.76
Surgery
Despite advances in medical therapy, patients with medically refractory disease – either chronically active disease or an acute severe episode that requires hospitalisation – may need colectomy.77 Further, patients with longstanding active disease are at increased risk for the development of colitis-associated dysplasia and cancer. Surveillance for dysplasia and evolving endoscopic techniques may enable endoscopic resection of foci of dysplasia, but in the case of multifocal dysplasia, especially that deemed to be endoscopically unresectable, colectomy may be the most appropriate option. Such cases are best discussed in a multidisciplinary setting of gastroenterologists, colorectal surgeons and pathologists.77
After colectomy, options for long-term restitution of faecal output include a permanent end ileostomy, ileal pouch anal anastomosis or, in specific cases, an ileorectal anastomosis.78 The choice of option typically involves shared decision making between the patient, surgeon and gastroenterologist, depending on multiple disease- and lifestyle-related factors.
Extraintestinal manifestations
Up to 50% of patients with IBD develop at least one extraintestinal manifestation of disease with a significant impact on quality of life.79,80 Extraintestinal manifestations can precede the diagnosis of IBD or develop later in the disease course. The most common are arthritis, skin disease and eye inflammation; less commonly, the liver, kidneys or pancreas are involved. Some extraintestinal manifestations parallel disease activity and respond to treatment of intestinal inflammation; however, others occur independently of disease state and may require targeted therapy.81
Inflammatory arthropathies are the most common extraintestinal manifestation, with a prevalence of 7 to 25% in patients with IBD. They include ankylosing spondylitis, reactive arthritis and psoriatic arthritis.82 Skin manifestations include erythema nodosum and pyoderma gangrenosum.82 Ocular manifestations occur in about 12% of patients with IBD, with the most common being episcleritis, scleritis and uveitis; they are often independent of bowel disease activity.83
The choice of IBD medication for an individual may be influenced by the presence of specific extraintestinal manifestations, as some biologics and small molecule inhibitors are more effective for controlling these symptoms than others.84 For example, TNF-alfa antagonists and JAK inhibitors may be chosen for patients with axial spondyloarthropathy.84 GPs should screen for extraintestinal manifestations and also consider nonpharmacological management options that can provide symptom relief, such as physical exercise and physiotherapy for arthropathies.82
Managing an acute flare
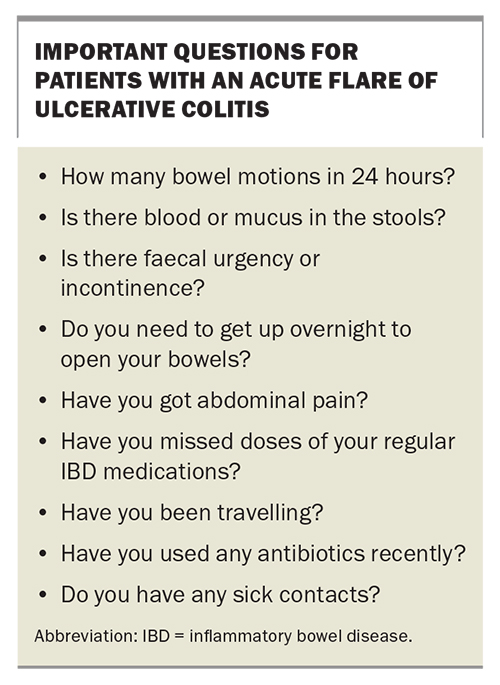
Patients may present to their GP with a change in their bowel symptoms from baseline. This could include increased frequency of bowel motions, bloody diarrhoea, mucus, faecal urgency or abdominal pain. Taking a detailed history is important to help guide investigations and management; suggested questions are shown in the Box. If the patient meets the criteria for acute severe UC, they should be referred urgently to hospital for further management.
Before starting a course of corticosteroids, patients should have investigations to exclude causes other than IBD, including basic blood tests (FBE; urea, electrolytes and creatinine levels; LFTs; CRP) and faecal tests (faecal microscopy, culture and sensitivity testing, and tests for Clostridioides difficile toxin and faecal calprotectin). After infection has been excluded, therapy for a flare should be commenced, with higher dose 5-ASAs or corticosteroids. Contact with the patient’s gastroenterologist or IBD team would be desirable for further guidance and to ensure early follow up.
Preventive care
The chronic nature of UC coupled with the long-term use of immunomodulators and immunosuppressants places patients at higher risk of multiple adverse health outcomes, including infections, malignancy, nutritional deficiencies, reduced bone health and, understandably, poorer mental health. The role of the GP as part of the multidisciplinary team is crucial in the proactive management of these complications. In general, six-monthly iron studies and measurement of vitamin B12, folate and vitamin D levels are recommended. Replacement should be instituted when a deficiency is identified.
Vaccines
Before commencing immunomodulators and biologic therapy, all patients should:
- be screened for latent infections, including hepatitis B, hepatitis C, HIV infection and tuberculosis
- have their immunity to vaccine-preventable infections confirmed, particularly infections that require live vaccines, as these may be contraindicated in patients receiving immunosuppressant therapy.85
In general, patients with IBD should receive vaccinations in line with the national vaccination schedule, including hepatitis A, hepatitis B, tetanus, human papillomavirus, meningococcus, influenza, pneumococcus, COVID-19, measles, diphtheria and pertussis. Patients with IBD are at increased risk of contracting influenza compared with age-matched controls and should receive annual influenza vaccination.65,86 IBD is also associated with an increased risk of pneumonia.87 Patients with IBD should receive the pneumococcal vaccine in line with national guidelines.86 Immunosuppressed patients with IBD, especially those treated with JAK inhibitors, are also at increased risk of herpes zoster and may benefit from receiving the recombinant shingles vaccine.88
Malignancy screening
Patients with UC have an increased risk of colorectal cancer compared with the normal population.89 Endoscopic surveillance (colonoscopy) for dysplasia should commence at eight years after the diagnosis of UC.49 Patients with concurrent primary sclerosing cholangitis should commence surveillance at diagnosis.49 Surveillance intervals are shortened in patients with moderate to severe active inflammation, a family history of colorectal cancer, colonic strictures and previous dysplasia.49 Women should also be enrolled in age-appropriate national cervical cancer screening programs.49 There is insufficient evidence to support a more intensive screening program. Additionally, patients receiving anti-TNF therapies are at increased risk of lymphoma and skin cancers. GPs plays a crucial role in regular screening for these cancers.49
Nutrition
Patients with UC are at risk of malnutrition, which is associated with poor clinical outcomes.90 Patients should be screened at diagnosis and regularly thereafter for nutritional status, weight changes and serum iron, folate, vitamin B12 and vitamin D levels. Other nutrients, such as vitamin A, vitamin C, vitamin E, potassium, calcium, magnesium, phosphate, zinc and selenium, may be checked in appropriate patients with suspected multiple nutritional deficiencies.33 Inclusion of a dietitian in the patient’s treatment team can help provide tailored dietary advice.
One third of patients with IBD and active disease have iron deficiency, which has a significant impact on quality of life.33 Systemic inflammation may limit the absorption of oral iron; current international guidelines recommend intravenous iron for patients with IBD and active disease, moderate to severe anaemia or intolerance to oral iron.91
Smoking cessation
Although cigarette smoking may reduce the risk of a diagnosis of UC, the effects on disease prognosis and course are less clear. Other adverse effects, including the risk of malignancy, cardiovascular disease and subsequent death, are higher in smokers than nonsmokers.92-94 Patients should be counselled on the benefits of smoking cessation and medications optimised to reduce the risk of worsening of disease activity.33
Cardiovascular health
Although data are inconsistent, patients with IBD have been shown to have a slightly increased risk of cardiovascular disease.84,95 This risk may be greater in patients treated with JAK inhibitors. Monitoring and treatment of elevated serum lipid levels is important in these patients. Ozanimod is associated with hypertension in some patients, and this should be treated appropriately.96
Mental health
Along with recognising the physical symptoms of UC, GPs should regularly screen for psychological symptoms, particularly at the time of UC diagnosis and during flares.97 Rates of anxiety and depression are higher in patients with IBD compared with the general population.98 It has been shown that a higher burden of UC symptoms predicts a greater level of perceived stress in those with UC, but there is no evidence that higher perceived stress predicts worsening symptoms or intestinal inflammation.99
It is crucial to understand the psychological burden of IBD so that collaborative care models can be adequately resourced and utilised by the GP and patient to optimise the patient’s health and quality of life.100 This care model can include the use of psychological therapies, pharmacological interventions, screening measures and physical activity.97 Both active psychotherapeutic treatments such as cognitive behavioural therapy (CBT) and antidepressants have been shown to reduce symptoms of depression and anxiety in patients with IBD.101,102
Bone health
Patients with UC are at increased risk of reduced bone mineral density (BMD) and bony fractures, the pathogenesis of which is multifactorial.86 Although corticosteroid use is a known risk factor for reduced BMD, other factors may contribute.103 Patients should be screened for risk factors for reduced BMD, including low vitamin D and calcium levels, and supplementation implemented as appropriate. Regular weight-bearing exercise and smoking cessation should be encouraged.84 The Gastroenterology Society of Australia recommends a screening dual-energy x-ray absorptiometry (DEXA) scan at diagnosis and then periodically thereafter.104
Pregnancy planning and management
Pregnancy planning is an important consideration for patients with UC. Ideally, patients should be in remission for three to six months before conception, as disease activity during pregnancy is associated with adverse fetal outcomes, including pre-term birth and low birth weight.105 Pre-pregnancy counselling by a specialist IBD service has been shown to improve maternal adherence with medications and maternal and fetal outcomes.106-108
Most medications used to treat UC have excellent safety data in pregnancy and breastfeeding, except for methotrexate, JAK inhibitors and sphingosine 1-phosphate receptor modulators. Cessation of other medications in the context of pregnancy should be avoided. Regular monitoring of disease activity in pregnancy is recommended. The use of noninvasive markers such as faecal calprotectin level and intestinal ultrasound examination is supported by emerging data, with endoscopic assessment also considered safe in selected patients, especially in the second trimester.105,109,110
Complementary and alternative medicines
Alongside conventional therapy, some patients may pursue complementary and alternative medications (CAMs). Many of these therapies lack robust evidence. The European Crohn’s and Colitis Organisation has published consensus statements on CAMs to help clinicians discuss these therapies with patients.111 Curcumin at doses of 1500 to 3000 mg daily has been studied in multiple small randomised controlled trials, with some noting mild effectiveness in preventing relapse.112,113 Curcumin should be prescribed with caution, with rare reports of liver injury associated with medications containing curcumin.114
Conclusion
The number of patients in Australia living with UC continues to increase. Modern therapeutic targets and treatments may result in satisfactory clinical remission and quality of life and reduce the risk of complications in most patients. GPs are a crucial part of shared care for patients with UC, as they are in the position to help address both the direct physical impacts of UC and the indirect physical, social and mental health consequences, to ensure good
long-term outcomes for their patients. MT
COMPETING INTERESTS: Associate Professor Garg has served on the advisory board of Pfizer and has received speaker fees, research or travel grants from Abbvie, Celltrion, Dr Falk, Janssen, Pfizer, Pharmacosmos and Takeda. Dr Pearce, Dr Chauhan, Dr Lucas: None.
References
1. Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res 2019; 2019: 7247238.
2. Bhatia R, Yeoh SW, Vaz K, et al. Inflammatory bowel disease incidence, prevalence and 12‐month initial disease course in Tasmania, Australia. Intern Med J 2019; 49: 622-630.
3. Wilson J, Hair C, Knight R, et al. High incidence of inflammatory bowel disease in Australia: a prospective population-based Australian incidence study. Inflamm Bowel Dis 2010; 16: 1550-1556.
4. Busingye D, Pollack A, Chidwick K. Prevalence of inflammatory bowel disease in the Australian general practice population: a cross-sectional study. PLoS One 2021; 16: e0252458.
5. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011; 140: 1785-1794.
6. Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol 2018; 16: 343-356.e3.
7. Ricci C, Lanzarotto F, Lanzini A. The multidisciplinary team for management of inflammatory bowel diseases. Dig Liver Dis 2008; 40 Suppl 2: S285-S288.
8. Tambakis G, Wright E. Inflammatory bowel disease: causes, symptoms and treatment. Med Today 2023; 24(3): 14-20.
9. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19(Suppl A): 5A-36A.
10. Baghaei A, Emami MH, Adibi P, et al. Inflammatory bowel disease registry and monitoring: Feasibility study and application (Isfahan Inflammatory Bowel Disease Surveillance Project. Int J Prev Med 2019; 10: 190.
11. Burisch J, Katsanos KH, Christodoulou DK, et al; Epi-IBD Group. Natural disease course of ulcerative colitis during the first five years of follow-up in a European population-based inception cohort-an Epi-IBD study. J Crohns Colitis 2019; 13: 198-208.
12. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021; 160: 1570-1583.
13. Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 2013; 7: 827-851.
14. Canavan C, Card T, West J. The incidence of other gastroenterological disease following diagnosis of irritable bowel syndrome in the UK: a cohort study. PLoS One 2014; 9: e106478.
15. Battat R, Duijvestein M, Guizzetti L, et al. Histologic healing rates of medical therapies for ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials. Am J Gastroenterol 2019; 114: 733-745.
16. Yoon JY, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Correlations of C-reactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig Dis Sci 2014; 59: 829-837.
17. Singh S, Ananthakrishnan AN, Nguyen NH, et al. AGA clinical practice guideline on the role of biomarkers for the management of ulcerative colitis. Gastroenterology 2023; 164: 344-372.
18. Smith RL, Taylor KM, Friedman AB, Gibson RN, Gibson PR. Systematic review: clinical utility of gastrointestinal ultrasound in the diagnosis, assessment and management of patients with ulcerative colitis. J Crohns Colitis 2020; 14: 465-479.
19. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987; 317: 1625-1629.
20. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut 1998; 43: 29-32.
21. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; preliminary report on a therapeutic trial. BMJ 1954; 2: 375-378.
22. Conrad K, Roggenbuck D, Laass MW. Diagnosis and classification of ulcerative colitis. Autoimmun Rev 2014; 13: 463-466.
23. Walsh AJ, Bryant RV, Travis SP. Current best practice for disease activity assessment in IBD. Nature Rev Gastroenterol Hepatol 2016; 13: 567-579.
24. Drug utilisation sub-committee (DUSC). 5-Aminosalicylic acids. Pharmaceutical Benefits Scheme; 2017. Available online at: https://www.pbs.gov.au/pbs/industry/listing/participants/public-release-docs/2017-09/5-aminosalicylic-acids-for-ulcerative-colitis (accessed September 2023).
25. Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2006; (2): CD000543.
26. Prantera C, Rizzi M. 5-ASA in ulcerative colitis: improving treatment compliance. World J Gastroenterol 2009; 15: 4353-4355.
27. Feagan BG, MacDonald JK. Oral 5‐aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2012; 10: CD000544. Update in: Cochrane Database Syst Rev 2016; (5): CD000544.
28. Ford AC, Khan KJ, Achkar J-P, Moayyedi P. Efficacy of oral vs. topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol 2012; 107: 167-176.
29. Ko CW, Singh S, Feuerstein JD, et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology 2019; 156: 748-764.
30. Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis 2022; 16: 2-17.
31. Loftus EV Jr, Kane SV, Bjorkman D. Systematic review: short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther 2004; 19: 179-189.
32. Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2010; 105: 501-523.
33. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019; 68(Suppl 3): S1-S106.
34. Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc 2014; 89: 1553-1563.
35. Danese S, Hart A, Dignass A, et al. A multicentre prospective cohort study assessing the effectiveness of budesonide MMX (Cortiment MMX) for active, mild-to-moderate ulcerative colitis. United European Gastroenterol J 2019; 7: 1171-1182.
36. Lichtenstein GR. Budesonide multi-matrix for the treatment of patients with ulcerative colitis. Dig Dis Sci 2016; 61: 358-370.
37. Solitano V, D’Amico F, Fiorino G, Paridaens K, Peyrin-Biroulet L, Danese S. Key strategies to optimize outcomes in mild-to-moderate ulcerative colitis. J Clin Med 2020; 9: 2905.
38. Liu YP, Wu HY, Yang X, et al. Association between thiopurine S-methyltransferase polymorphisms and thiopurine-induced adverse drug reactions in patients with inflammatory bowel disease: a meta-analysis. PLoS One 2015; 10: e0121745.
39. Warner B, Johnston E, Arenas-Hernandez M, Marinaki A, Irving P, Sanderson J. A practical guide to thiopurine prescribing and monitoring in IBD. Frontline Gastroenterol 2018; 9: 10-15.
40. Krynetski EY, Krynetskaia NF, Yanishevski Y, Evans WE. Methylation of mercaptopurine, thioguanine, and their nucleotide metabolites by heterologously expressed human thiopurine S-methyltransferase. Mol Pharmacol 1995; 47: 1141-1147.
41. Ansari A, Patel N, Sanderson J, O’Donohue J, Duley J, Florin T. Low‐dose azathioprine or mercaptopurine in combination with allopurinol can bypass many adverse drug reactions in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2010; 31: 640-647.
42. Sparrow MP, Hande SA, Friedman S, et al. Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther 2005; 22: 441-446.
43. Moon W, Loftus Jr E. Recent advances in pharmacogenetics and pharmacokinetics for safe and effective thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther 2016; 43: 863-883.
44. Goh LL, Lim CW, Leong KP, Ong KH. TPMT and NUDT15 testing for thiopurine therapy: a major tertiary hospital experience and lessons learned. Front Pharmacol 2022; 13: 837164.
45. Ariyaratnam J, Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: a meta-analysis. Am J Gastroenterol 2014; 109: 163-169.
46. Smith MA, Irving P, Marinaki AM, Sanderson J. Malignancy on thiopurine treatment with special reference to inflammatory bowel disease. Aliment Pharmacol Ther 2010; 32: 119-130.
47. Allegretti JR, Barnes EL, Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflamm Bowel Dis 2015; 21: 1089-1097.
48. Kreijne J, Goetgebuer R, Erler N, et al; Dutch Initiative on Crohn and Colitis (ICC). Cumulative exposure to immunomodulators increases risk of cervical neoplasia in women with inflammatory bowel disease. Aliment Pharmacol Ther 2023; 58: 207-217.
49. Gordon H, Biancone L, Fiorino G, et al. ECCO guidelines on inflammatory bowel disease and malignancies. J Crohns Colitis 2023; 17: 827-854.
50. Singh S, Allegretti JR, Siddique SM, Terdiman JP. AGA technical review on the management of moderate to severe ulcerative colitis. Gastroenterology 2020; 158: 1465-1496.e17.
51. Strik AS, van den Brink GR, Ponsioen C, Mathot R, Lowenberg M, D'Haens GR. Suppression of anti-drug antibodies to infliximab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2017; 45: 1128-1134.
52. Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol 2012; 10: 1002-1007.
53. Sandborn WJ, Feagan BG, D’Haens G, et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2021; 385: 1280-1291.
54. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723-1736.
55. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019; 381: 1201-1214.
56. Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022; 399: 2113-2128.
57. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699-710.
58. Sandborn WJ, Van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012; 142: 257-265.e3.
59. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462-2476.
60. Barberio B, Zingone F, Frazzoni L, et al. Real-life comparison of different anti-TNF biologic therapies for ulcerative colitis treatment: a retrospective cohort study. Digest Dis 2021; 39: 16-24.
61. Department of Health and Aged Care (DHAC). Making more life-changing medicines available to all Australians [media release]. DHAC; 2017. Available online at: https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/making-more-life-changing-medicines-available-to-all-australians (accessed September 2023).
62. Department of Health and Aged Care (DHAC). Biosimilar medicines subsidised on the Pharmaceutical Benefits Scheme. DHAC; 2022 (updated March 2023). Available online at: https://www.health.gov.au/resources/publications/biosimilar-medicines-subsidised-on-the-pharmaceutical-benefits-scheme?language=en (accessed September 2023).
63. Haifer C, Srinivasan A, An YK, et al. Switching Australian patients with moderate to severe inflammatory bowel disease from originator to biosimilar infliximab: a multicentre, parallel cohort study. Med J Aust 2021; 214: 128-133.
64. Cholapranee A, Hazlewood GS, Kaplan GG, Peyrin‐Biroulet L, Ananthakrishnan AN. Systematic review with meta‐analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn's disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther 2017; 45: 1291-1302.
65. Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis 2021; 15: 879-913.
66. TNF-alpha antagonists. In: Australian medicines handbook; 2023. Available online at: https://shop.amh.net.au/ (accessed September 2023).
67. Pharmaceutical Benefits Scheme. Ustekinumab. Department of Health and Aged Care; 2023. Available online at: https://www.pbs.gov.au/medicine/item/13261Y-13273N (accessed September 2023).
68. Panaccione R, Danese S, Sandborn WJ, et al. Ustekinumab is effective and safe for ulcerative colitis through 2 years of maintenance therapy. Aliment Pharmacol Ther 2020; 52: 1658-1675.
69. Ghosh S, Feagan B, Ott E, et al. OP39 safety of ustekinumab in IBD: final pooled long-term safety analysis through 5 years in CD and 4 years in UC. J Crohns Colitis 2023; 17(Suppl 1): i55-i56.
70. Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis 2016; 10: 1437-1444.
71. Lucaciu LA, Seicean R, Seicean A. Small molecule drugs in the treatment of inflammatory bowel diseases: which one, when and why? A systematic review. Eur J Gastroenterol Hepatol 2020; 32: 669-677.
72. Sandborn WJ, Panés J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2019; 17: 1541-1550.
73. Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L. Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology 2020; 158: 1554-1573.e12.
74. Ytterberg SR, Bhatt DL, Connell CA. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis [reply]. N Engl J Med 2022; 386: 1768.
75. Agrawal M, Kim ES, Colombel J-F. JAK inhibitors safety in ulcerative colitis: practical implications. J Crohns Colitis 2020; 14(Suppl 2): S755-S760.
76. Sandborn WJ, Feagan BG, Hanauer S, et al. Long-term efficacy and safety of ozanimod in moderately to severely active ulcerative colitis: results from the open-label extension of the randomized, phase 2 TOUCHSTONE study. J Crohns Colitis 2021; 15: 1120-1129.
77. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019; 114: 384-413.
78. Oresland T, Bemelman WA, Sampietro GM, et al. European evidence based consensus on surgery for ulcerative colitis. J Crohns Colitis 2015; 9: 4-25.
79. Greuter T, Vavricka SR. Extraintestinal manifestations in inflammatory bowel disease -epidemiology, genetics, and pathogenesis. Expert Rev Gastroenterol Hepatol 2019; 13: 307-317.
80. Sange AH, Srinivas N, Sarnaik MK, et al. Extra-Intestinal Manifestations of Inflammatory Bowel Disease. Cureus 2021; 13: e17187.
81. Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal manifestations of inflammatory bowel disease: current concepts, treatment, and implications for disease management. Gastroenterology 2021; 161: 1118-1132.
82. Danese S, Semeraro S, Papa A, et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol 2005; 11: 7227-7236.
83. Veloso FT. Extraintestinal manifestations of inflammatory bowel disease: do they influence treatment and outcome? World J Gastroenterol 2011; 17: 2702-2707.
84. Gordon H, Burisch J, Ellul P, et al. ECCO guidelines on extraintestinal manifestations in inflammatory bowel disease. J Crohns Colitis 2023; jjad108.
85. Rahier J-F, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014; 8: 443-468.
86. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol 2017; 112: 241-258.
87. Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol 2013; 108: 240.
88. Clarke B, Yates M, Adas M, Bechman K, Galloway J. The safety of JAK-1 inhibitors. Rheumatology (Oxford) 2021; 60(Suppl 2): ii24-ii30.
89. Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis 2013; 19: 789-799.
90. Nguyen GC, Munsell M, Harris ML. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm Bowel Dis 2008; 14: 1105-1111.
91. Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis 2015; 9: 211-222.
92. Bastida G, Beltran B. Ulcerative colitis in smokers, non-smokers and ex-smokers. World J Gastroenterol 2011; 17: 2740-2747.
93. Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 2009; 124: 2406-2415.
94. Masala G, Bagnoli S, Ceroti M, et al. Divergent patterns of total and cancer mortality in ulcerative colitis and Crohn's disease patients: the Florence IBD study 1978-2001. Gut 2004; 53: 1309-1313.
95. Fumery M, Xiaocang C, Dauchet L, Gower-Rousseau C, Peyrin-Biroulet L, Colombel J-F. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: a meta-analysis of observational studies. J Crohns Colitis 2014; 8: 469-479.
96. Sands BE, Schreiber S, Blumenstein I, Chiorean MV, Ungaro RC, Rubin DT. Clinician’s guide to using ozanimod for the treatment of ulcerative colitis. J Crohns Colitis 2023 Jul 12:jjad112. Epub ahead of print.
97. Ananthakrishnan AN, Kaplan GG, Bernstein CN, et al. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus. Lancet Gastroenterol Hepatol. 2022; 7: 666-678.
98. Mikocka-Walus A, Knowles SR, Keefer L, Graff L. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis 2016; 22: 752-762.
99. Sexton KA, Walker JR, Graff LA, et al. Evidence of bidirectional associations between perceived stress and symptom activity: a prospective longitudinal investigation in inflammatory bowel disease. Inflamm Bowel Dis 2017; 23: 473-483.
100. Bernstein CN, Hitchon CA, Walld R, et al. Increased burden of psychiatric disorders in inflammatory bowel disease. Inflamm Bowel Dis 2019; 25: 360-368.
101. Szigethy E, Craig AE, Iobst EA, et al. Profile of depression in adolescents with inflammatory bowel disease: implications for treatment. Inflamm Bowel Dis 2009; 15: 69-74.
102. Kristensen MS, Kjaerulff TM, Ersboll AK, Green A, Hallas J, Thygesen LC. The influence of antidepressants on the disease course among patients with Crohn's disease and ulcerative colitis - a Danish nationwide register-based cohort study. Inflamm Bowel Dis 2019; 25: 886-893.
103. Zhou T, Pan J, Lai B, Cen L, Jiang W, Yu C, Shen Z. Bone mineral density is negatively correlated with ulcerative colitis: a systematic review and meta-analysis. Clin Transl Med 2020; 9: 18.
104. Gastroenterological Society of Australia (GESA). Management overview of IBD. Melbourne: GESA; 2021. Available online at: https://www.gesa.org.au/public/13/files/Education%20%26%20Resources/Clinical%20Practice%20Resources/IBD/Management%20overview%20of%20IBD%20update.pdf (accessed September 2023).
105. Van der Woude C, Ardizzone S, Bengtson M, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis 2015; 9: 107-124.
106. de Lima A, Zelinkova Z, Mulders AG, van der Woude CJ. Preconception care reduces relapse of inflammatory bowel disease during pregnancy. Clin Gastroenterol Hepatol 2016; 14: 1285-1292.e1.
107. Watanabe C, Nagahori M, Fujii T, et al. Non-adherence to medications in pregnant ulcerative colitis patients contributes to disease flares and adverse pregnancy outcomes. Dig Dis Sci 2021; 66: 577-586.
108. Broms G, Granath F, Linder M, Stephansson O, Elmberg M, Kieler H. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm Bowel Dis 2014; 20: 1091-1098.
109. Laube R, Paramsothy S, Leong RW. Review of pregnancy in Crohn's disease and ulcerative colitis. Therap Adv Gastroenterol 2021; 14: 17562848211016242.
110. Flanagan E, Wright EK, Begun J, et al. Monitoring inflammatory bowel disease in pregnancy using gastrointestinal ultrasonography. J Crohns Colitis 2020; 14: 1405-1412.
111. Torres J, Ellul P, Langhorst J, Mikocka-Walus A, et al. European Crohn’s and Colitis Organisation topical review on complementary medicine and psychotherapy in inflammatory bowel disease. J Crohns Colitis 2019; 13: 673-685e.
112. Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol 2015; 13: 1444-1449.e1.
113. Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol 2006; 4: 1502-1506.
114. Australian Government Department of Health and Aged Care. Therapeutic Goods Administration (TGA). Medicines containing turmeric or curcumin - risk of liver injury. Safety advisory. TGA; 2023. Available online at: https://www.tga.gov.au/news/safety-alerts/medicines-containing-turmeric-or-curcumin-risk-liver-injury (accessed Sep 2023).