Inflammatory bowel disease: causes, symptoms and treatment

Inflammatory bowel disease is characterised by chronic inflammation of the gastrointestinal tract. The recognition of alarm symptoms, raised levels of inflammatory markers or an elevated faecal calprotectin level should prompt referral to a gastroenterologist for appropriate assessment and treatment.
-
Inflammatory bowel disease (IBD) represents a spectrum of chronic inflammatory conditions affecting the gastrointestinal tract and comprises ulcerative colitis and Crohn's disease.
-
The presence of alarm symptoms, elevated inflammatory markers and elevated faecal calprotectin levels should raise suspicion for IBD and warrant referral to a gastroenterologist for further investigation and diagnosis.
-
New treatment targets and improved therapies show efficacy in controlling inflammation, alleviating symptoms and improving patients' quality of life.
-
Patients with IBD are often undertreated or inappropriately given repeated corticosteroid therapy. Early and aggressive treatment for some patients is important to achieve control of inflammation and reduce complications.
-
All patients with IBD who are pregnant should be referred to a gastroenterologist. Most medications can be continued through conception, pregnancy and breastfeeding.
-
Anxiety and depression are common in patients with IBD, particularly in the first year after diagnosis and should be addressed as a part of multidisciplinary and individualised management.
Inflammatory bowel disease (IBD), which comprises ulcerative colitis (UC) and Crohn’s disease, represents a spectrum of chronic inflammatory conditions affecting the gastrointestinal tract. The incidence and prevalence of IBD has been increasing worldwide for the past several decades, and Australia has some of the highest incidence and prevalence rates of IBD in the world.1,2
Over the same time period, several novel therapies have been added to our treatment armamentarium, including biologic agents, small-molecule inhibitors, dietary treatment and microbial therapy, as well as stem cell therapy and laser surgery for perianal disease. As treatments have improved, the treatment targets have evolved, with an emphasis on better disease control and standardisation of objective measures of disease activity. This ‘treat-to-target’ approach aims to achieve biochemical normalisation and endoscopic healing, which in turn reduces corticosteroid use, hospitalisation and surgery in patients with IBD. In the longer term, this approach may alter the natural course of the disease. This article provides an overview of the causal factors and considerations for the diagnosis of IBD, followed by a discussion of the management and treatment of the disease.
Natural history and epidemiology
IBD is increasingly prevalent in developed countries, whereas newly industrialised countries are experiencing an accelerated incidence.1 Australia has one of the highest incidence rates of IBD in the world at 29.5 per 100,000. Between 2010 and 2021, the prevalence almost doubled to 653 per 100,000.2-4 Due to the early onset, chronic nature and low mortality of IBD, its prevalence has been increasing at a faster rate than its incidence, a phenomenon known as compounding prevalence.
IBD is most commonly diagnosed in the third to fifth decades of life.5 Presentations vary widely, with some patients experiencing severe symptoms resulting in hospitalisation, surgery and significant disability, while others experience only occasional mild flares with long periods of remission. In the era of biologic agents, the expectations of disease control in IBD have changed dramatically. More than 50% of patients now enter corticosteroid-free remission or have only mild disease activity within five years of diagnosis.6 Long-term remission, without the use of corticosteroid therapy, is now the aim of treatment and should be the expectation for patients and clinicians.
Biologic use is now increasingly common in IBD and allows superior disease control and quality of life for patients.7 Up to 44% of patients with Crohn’s disease and 16% of patients with UC require biologic therapy to control disease.8 With the increasing use of biologic agents, the rates of hospitalisation and surgery and the incidence of colorectal cancer in patients with IBD have declined compared with those in the pre-biologic era.6
Pathogenesis
A homeostasis exists between the commensal intestinal microbiota, epithelial cells that line the intestine and immune cells within the tissues. Each of these components can be affected by environmental and genetic factors, leading to chronic dysregulated inflammation in a susceptible individual.
Genetic factors
First-degree relatives of patients with IBD are three to 20 times more likely to develop IBD, highlighting the role of genetics in the pathogenesis.9,10 In most patients, IBD is a polygenetic disorder. Genome-wide sequencing has identified over 200 susceptibility loci associated with IBD.11,12 The genes associated with these loci confer an increased risk of IBD by encoding proteins involved in innate immunity, T-cell signalling, epithelial barrier function and autophagy.11
Gut microbiome
The gut microbiome of patients with IBD differs from healthy controls in both diversity and density, a process referred to as dysbiosis.13,14 It is not clear whether dysbiosis is the antigenic driver of IBD, occurs as a result of chronic inflammation or both. However, dysbiosis is likely to contribute to both the initiation and perpetuation of inflammation associated with IBD. Microbial manipulation with faecal microbiota transplantation (FMT) has emerged as a possible treatment for UC in particular.
Environmental factors
Certain lifestyle factors and early life exposures to some medications, including antibiotics, may play a role in the development of IBD. Smoking is a well-established risk factor for Crohn’s disease, but not UC.15 Conversely, physical activity reduces the risk of Crohn’s disease, but has no impact on UC.16
Dietary factors play an important role in the development of IBD. Diets high in fruits and vegetables reduce the risk of developing IBD, whereas diets high in fats, particularly polyunsaturated fats, increase the risk.17,18 Antibiotic use at a young age is also associated with the development of IBD.19 Other medications, such as nonsteroidal anti-inflammatories and the oral contraceptive pill, may increase the risk of IBD; however, the risk is likely to be small.20,21
Clinical signs and symptoms
Patients with UC usually present with diarrhoea, which can be accompanied by blood and mucus. Faecal urgency, incontinence and tenesmus are also common, particularly if the rectum is involved. The severity of symptoms can vary from one to two loose non-bloody bowel actions per day to more than 20 bloody bowel actions per day.
The presentation of Crohn’s disease varies depending on the location of the disease and presence or absence of complications, such as perianal disease or intestinal strictures. Crohn’s disease can affect the ileum, the colon, both the ileum and colon (ileocolonic) or the upper gastrointestinal tract. Perianal Crohn’s disease is a relatively common and potentially severe disease phenotype that can occur in isolation or together with luminal disease. Stricturing Crohn’s disease presents with symptoms of obstruction, characterised by crampy abdominal pain and diarrhoea. Diarrhoea, with or without blood, is a common feature of colonic Crohn’s disease. Perianal pain, discharge or recurrent abscess formation is a hallmark of perianal Crohn’s disease.
Both UC and Crohn’s disease are chronic conditions that usually present with gradual and progressive symptoms over many weeks or months. Systemic symptoms such as fatigue and weight loss are common.
Diagnosis
Stool and blood tests
Taking a faecal specimen to rule out infection is important; stool tests include microscopy, culture and sensitivity and the detection of Clostridioides difficile toxin and ova, cysts and parasites (Box). Haematology and biochemistry tests, such as the measurement of blood leukocytes, platelet count and C-reactive protein (CRP) levels, although useful in the assessment of severity in established IBD, are not sufficiently sensitive or specific to be used for diagnosis. More than half of patients with UC and a third of patients with Crohn’s disease will have a normal CRP level at the time of diagnosis. Anaemia and iron deficiency may be seen.
Currently, there is no role for the use of genetic or antibody testing for the diagnosis of IBD. Faecal calprotectin is a nonspecific marker of gastrointestinal inflammation and is useful in differentiating IBD from disorders of gut-brain interaction (functional gut disorders, also often referred to as irritable bowel syndrome [IBS]). A calprotectin level greater than 50 mcg/g is considered abnormal and warrants further investigation with colonoscopy.22 Faecal calprotectin testing is rebateable under the Medicare Benefits Schedule (MBS) for patients younger than 50 years of age who have symptoms consistent with IBD or IBS that persist for longer than six weeks and in whom infectious causes have been excluded. This test can be requested by GPs. Routine blood tests should also be performed (Box).
Imaging
Imaging plays an important role in the initial diagnosis of IBD, as well as in monitoring disease activity and complications, particularly in patients with Crohn’s disease. Small bowel imaging is vital at the time of diagnosis for the complete assessment of disease extent and severity.
Magnetic resonance enterography (MRE) can be used to detect small bowel inflammation and complications, such as strictures and fistulae. MRE avoids the need for radiation and is preferred over CT. One MRE scan per year is covered under the MBS when requested by a gastroenterologist.
Intestinal ultrasound, which is usually performed by a gastroenterologist, is a noninvasive and cost-effective alternative to MRE and is increasingly available in some public hospitals and private clinics. The results are reproducible and the scan can be performed at the point of care to facilitate clinical decision making.23
Colonoscopy
Colonoscopy has three key roles in IBD: initial diagnosis, assessment of disease activity and treatment response and dysplasia assessment. It is the gold standard for diagnosing IBD and also provides information on disease extent, severity and complications, such as strictures.24 Assessing endoscopic healing in response to treatment is vital, as endoscopic healing is associated with improved long-term outcomes.25
Patients with IBD are at an increased risk of developing colorectal cancer. Therefore, colonoscopic surveillance for precancerous lesions (dysplasia) is recommended. Patients with either UC or Crohn’s disease affecting more than one-third of the colon should undergo surveillance colonoscopy eight years after the initial diagnosis.26 Patients with ongoing active inflammation, a family history of colorectal cancer, colonic strictures, previous dysplasia or concomitant primary sclerosing cholangitis require more frequent assessment.
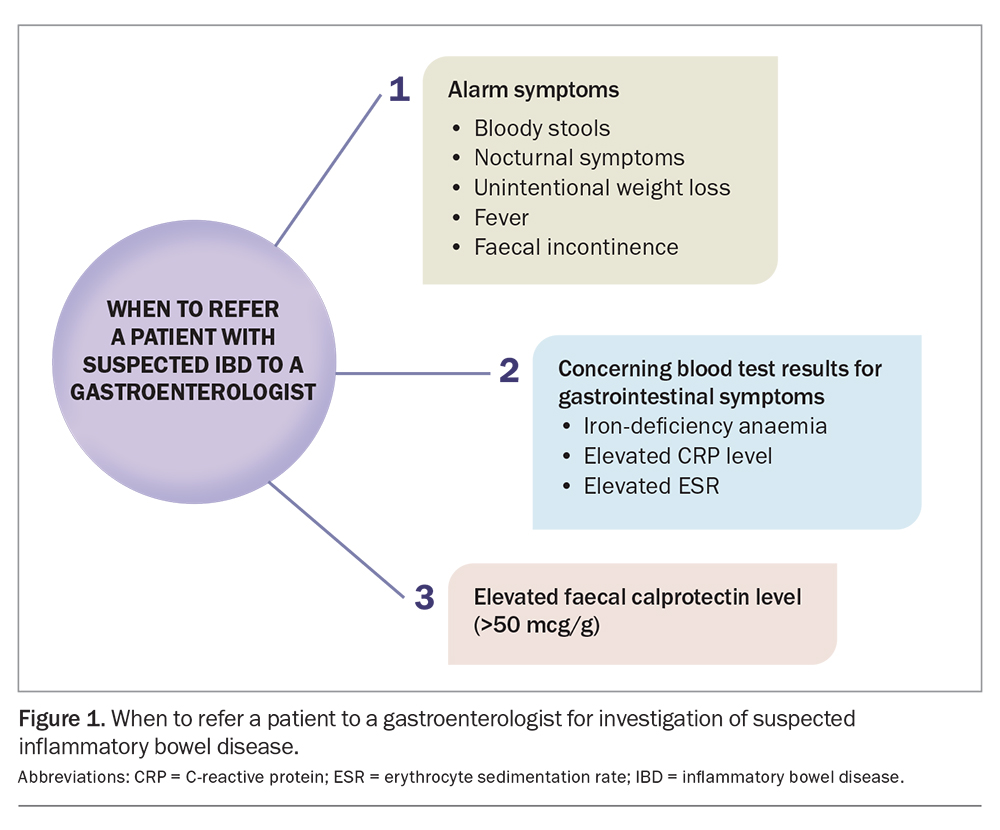
When to refer to a gastroenterologist
Differentiating disorders of gut-brain interaction from IBD can be challenging. Patients with alarm symptoms, an elevated faecal calprotectin level, unexplained iron-deficiency anaemia and elevated levels of inflammatory markers (CRP or erythrocyte sedimentation rate) in the presence of gastrointestinal symptoms should be referred to a gastroenterologist for further investigation. Alarm symptoms include bloody stools, nocturnal symptoms, fever, unintentional weight loss and faecal incontinence (Figure 1).
For all patients with established IBD, consider involving a gastroenterologist to ensure appropriate disease education, treatment and dysplasia surveillance. This is particularly important if the patient has symptoms of active disease, is planning pregnancy or has needed frequent corticosteroid courses for symptom control.
Management
The past few decades have seen several new drug therapies added to the treatment armamentarium for IBD. There have also been major changes to the treatment strategy, which now favours more aggressive treatment earlier in the disease course to induce prompt control of inflammation, symptom resolution and improvement in quality of life. Novel approaches to treatment are emerging, including dietary and microbial therapies.
Aminosalicylates
Aminosalicylates are indicated for the induction and maintenance of clinical remission in patients with mild to moderate UC. Patients with proctitis and proctosigmoiditis should be commenced on topical therapy. Aminosalicylates are not recommended for patients with Crohn’s disease.27
Patients with left-sided or extensive UC should use a combination of oral and topical therapy, which is more effective than either alone.28 Unfortunately, these medications are often prescribed at inadequate doses, which affects efficacy. Higher doses of oral mesalazine (3 g or more daily) are recommended for patients with active disease or patients who do not respond to standard dosing. In these patients, the addition of daily rectal mesalazine therapy is also recommended, which can be delivered via enemas or suppositories, depending on the disease distribution and patient preference and tolerability. Lower doses of oral mesalazine of around 2 g daily are appropriate for the maintenance of remission.
Mesalazine is the most widely used aminosalicylate. It is generally well tolerated and has few side effects; however, up to 5% of patients experience a hypersensitivity reaction with worsening of diarrhoea.29 Sulfasalazine, on the other hand, has high rates of adverse effects and is rarely used. Balsalazide and olsalazine are also rarely used.
Corticosteroids
Corticosteroids act rapidly to reduce acute inflammation and are indicated to induce remission in patients with moderate to severe IBD. Corticosteroids should only be used for short periods and act as a bridge to more appropriate corticosteroid-sparing therapy. Initial doses range from 40 to 50 mg daily in adults, and doses are weaned over six to eight weeks.
Corticosteroids are associated with several side effects. Short-term side effects include insomnia, mood disturbance, weight gain and glucose intolerance. Long-term corticosteroid therapy should not be used in patients with IBD, as it is associated with osteoporosis, osteonecrosis, cataracts and myopathy.
Immunomodulators
Azathioprine and mercaptopurine are the main immunomodulators used for patients with IBD and are indicated for maintenance therapy. They have modest efficacy as monotherapy and are often used in combination with anti-tumour necrosis factor (TNF) biologic therapy to improve efficacy and to reduce the risk of the formation of antibodies to anti-TNF drugs, which, once developed, can lead to a loss of treatment response.
Thiopurine metabolite levels can be checked to ensure that therapeutic drug levels are achieved. Thiopurines have a slow onset of action and take eight to 12 weeks to be effective. Xanthine oxidase inhibitors (allopurinol) affect the metabolism of these drugs and should not be used in people taking thiopurines without the advice of a gastroenterologist. Side effects to thiopurine use are common and include bone marrow suppression, hepatotoxicity, nausea, anorexia, rash, pancreatitis and febrile illness. Blood test monitoring is required while taking these medications. Thiopurines also increase the risk of lymphoma and nonmelanoma skin cancers, and patients on these drugs require annual skin checks.30
Biologic agents
Biologic agents encompass anti-TNF, anti-integrin and anti-interleukin (IL)12/23 agents. They are indicated for the induction and remission of moderate to severe IBD when patients do not respond to immunomodulators.
Anti-TNF agents
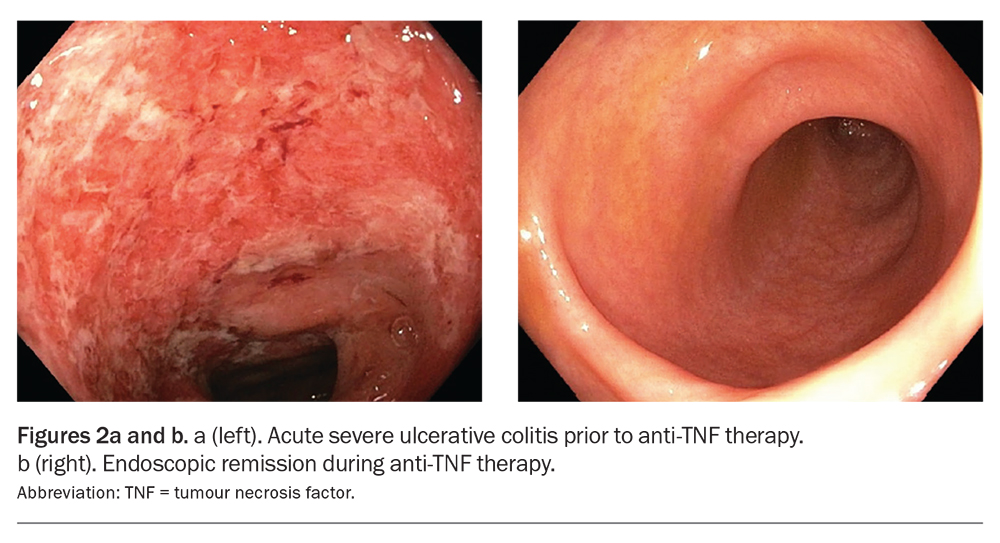
The most common anti-TNF agents are infliximab, adalimumab and golimumab. They are indicated for the induction and maintenance therapy of moderate to severe UC and Crohn’s disease (Figures 2a and b).31,32 Infliximab and golimumab are administered via infusion, while adalimumab is administered via subcutaneous injection. Anti-TNF medications are associated with an increased risk of severe infections and some malignancies, although the risk is low.33,34
Anti-integrin agents
Vedolizumab is a monoclonal antibody directed against the alpha-4/beta-7 integrin and prevents lymphocytes from binding to the endothelium, specifically in the gastrointestinal tract, which reduces gastrointestinal inflammation. This gut-specific mechanism of action means that systemic immunosuppression does not occur in patients, giving this drug an excellent safety profile. It is indicated in patients with moderate to severe UC or Crohn’s disease where the disease is active despite treatment with corticosteroids and an immunomodulator.35,36 It is administered via infusion every eight weeks.
Anti-IL12/23 agents
Ustekinumab inhibits the proinflammatory cytokines IL12/23. It is indicated for patients with moderate to severe Crohn’s disease and is administered subcutaneously every four to eight weeks.37 Ustekinumab has an excellent safety profile and does not increase the risk of malignancy or serious infection.38
Small-molecule inhibitors
Janus kinase inhibitors
Tofacitinib is a nonselective Janus kinase (JAK) inhibitor and its use is indicated for patients with moderate to severe UC who have lost response to anti-TNF therapy.39 It is administered orally twice daily and has a rapid onset of action (as quickly as three days). In patients with rheumatoid arthritis, tofacitinib is associated with an increased risk of major adverse cardiac events.40 It is not clear whether this risk is applicable to patients with IBD.
Upadacitinib, a selective JAK 1 inhibitor, is a once-daily oral treatment with efficacy in both moderate to severe UC and Crohn’s disease. It is TGA approved for use in patients with moderate to severe Crohn’s disease and UC, although it is not available on the PBS for these indications.
JAK inhibitors are mostly well tolerated, although increased rates of herpes zoster have been described, as well as a small increased risk of deep vein thrombosis.
Sphingosine 1-phosphate receptor modulators
Ozanimod is a sphingosine 1-phosphate receptor modulator that is TGA approved for the treatment of UC, although it is not available on the PBS for this indication. It prevents lymphocytes from migrating away from lymph nodes and entering the lymphatic circulation, thus reducing inflammation. This class of medications is associated with bradycardia, and all patients require an ECG before starting treatment; however, they are otherwise well tolerated.
Emerging therapies
Some patients with IBD may fail to respond to or lose response to conventional treatments. FMT and dietary therapy are alternative treatment options.
Faecal microbiota transplantation
FMT refers to the transplantation of stool from a healthy donor to the patient. FMT products are currently approved by the TGA to repopulate the recipient’s bowel with a healthy microbial population and there is good evidence of their efficacy for the treatment of recurrent C. difficile infection.
FMT aims to restore the balance of gut microbiota to reduce inflammation. Randomised controlled trials have demonstrated the efficacy of FMT in inducing clinical remission of UC.42,43 An Australian trial has, for the first time, shown the benefit of capsule (lyophilised) FMT for the induction and maintenance of remission of UC, although the trial included only a small number of patients.43 There are no published placebo-controlled randomised trials examining the efficacy of FMT in Crohn’s disease. At the time of writing, a randomised trial is under way at St Vincent’s Hospital Melbourne. There are Australian recommendations for donor screening, the route of administration, indications and production of FMT products.45
Despite enthusiasm for FMT from some patients and practitioners, there are several limitations to the widespread use of this therapy. Broadly, these include the complexities of FMT preparation and delivery, likely need for long-term use to maintain efficacy in patients with IBD and difficulties and uncertainties related to optimal donor and patient selection.
Dietary therapy
Dietary therapy has a role in the general health and wellbeing of patients with IBD and in the treatment of active disease, maintenance of remission and management of malnutrition. Dietary advice for patients in remission should focus on eating a well-balanced diet comprising fresh foods and avoiding processed foods and artificial sweeteners, which may have deleterious effects on the gut microbiota, thereby promoting inflammation.46
Dietary therapy is an effective treatment in patients with active Crohn’s disease. Exclusive enteral nutrition, a formula-based diet, reduces inflammation and can be used in place of corticosteroid therapy for remission induction in patients with Crohn’s disease.47 Exclusive enteral nutrition should only be used as a bridge to an alternative maintenance agent (such as a thiopurine or biologic agent), and an experienced dietitian must appropriately supervise its use.
Some exclusion diets have been studied as maintenance therapy and show promise, particularly for Crohn’s disease. An example is the Crohn’s disease exclusion diet, which has been shown to induce and maintain remission in patients with mild to moderate Crohn’s disease.48 Other dietary therapies, including the specific carbohydrate diet and IBD anti-inflammatory diet, may also be beneficial; however, the data are limited.
There have been no randomised trials studying dietary therapy in patients with UC, although at the time of writing, studies are in progress in Australia to examine the role of sulfide reduction in patients with UC.49
Patients with IBD are at risk of malnutrition, which can lead to weight loss, bone disease and nutritional deficiencies. Referral to a dietitian with experience in IBD is crucially important. Any exclusion diet in patients with IBD should only be attempted by motivated patients and with the support and supervision of a dietitian and gastroenterologist.
Special considerations
Diagnostic and treatment decisions should be individualised to each patient. GPs must be aware of several unique cases when investigating patients with IBD.
Perianal Crohn’s disease
Perianal Crohn’s disease can manifest as perianal fistulae, perianal abscesses or anal strictures. Patients can present with perianal pain, discharge or difficulty defaecating. The goals of treatment are to drain areas of infection, treat luminal Crohn’s colitis and achieve fistula closure. Patients often require multimodal therapy administered by a multidisciplinary team and may include examination under anaesthesia, surgical drainage, seton placement, antibiotic therapy and biologic agents. Anti-TNF therapy is the most effective medical therapy for patients with perianal Crohn’s disease, and the addition of antibiotics may provide even greater benefit.50,51
Stricturing disease
Strictures are the most common complication of Crohn’s disease. Patients with strictures present with abdominal pain, nausea, vomiting and an inability to pass flatus or faeces. Most strictures have an inflammatory component, and therefore respond, at least partially, to medical therapy.52 Fibrotic strictures are not responsive to medical therapy and can be managed either by endoscopic dilation (usually when strictures are 5 cm or less' in length) or surgically (when greater than 5 cm in length or if endoscopic dilatation is not possible or unsuccessful) The decision requires multidisciplinary discussion and is made on a case-by-case basis.
Pregnancy
Most of the medications used to treat IBD are safe to use during pregnancy and breastfeeding. Methotrexate, however, is strictly contraindicated in pregnancy and must be ceased before conception.53 At the time of writing, there is insufficient evidence to support the use of JAK inhibitors or sphingosine 1-phosphate receptor modulators in pregnancy or breastfeeding, and these medications should be ceased before conception. Active IBD is associated with adverse pregnancy and neonatal outcomes, including spontaneous abortion, pre-term birth and low birthweight. Therefore, it is important that disease remission is achieved prior to conception and that IBD drug treatment is continued throughout pregnancy to ensure good disease control and to optimise maternal and fetal health.54
Mental health
The impact of IBD on a patient’s quality of life is well documented. The condition negatively impacts personal relationships, the ability to perform day-to-day activities and social interaction. In this context, patients with IBD have higher rates of anxiety and depression compared with the general population.55 The rates of anxiety and depression are particularly high in the first year after diagnosis with IBD and in patients with active disease.55,56 Early referral for psychological care is paramount and reinforces the importance of a multidisciplinary approach when caring for patients with IBD.
Preventative care
Patients with IBD should have iron, vitamin B12, folate and vitamin D levels measured every six months. Supplementation is recommended if there is a deficiency. Monitoring of bone mineral density by a dual-energy X-ray absorptiometry is recommended every two years in those with a history of prolonged corticosteroid exposure.
The recommended vaccines for patients with IBD include those for tetanus, diphtheria, pertussis, human papilloma virus, influenza, pneumococcal disease, hepatitis A, hepatitis B, COVID-19 and meningococcal disease. The use of corticosteroids, immunomodulators and biologic agents, particularly the newer JAK inhibitors and sphingosine 1-phosphate receptor modulators, increases the risk of shingles infection. Therefore, vaccination against herpes zoster is also recommended for all patients with IBD aged 50 years and older, and should be considered for those younger than 50 years of age who are receiving particular biologic agents, such as JAK inhibitors.56 The recombinant zoster vaccine is preferred over the live zoster vaccine, as it has greater efficacy and is safe to administer to patients on immunosuppressive therapy, although it is not currently rebated via the PBS.56 The live zoster vaccine should not be given to any patient taking an immunomodulator or biologic agent or any person in whom these therapies are to be commenced within three months of the date of vaccination because of the risk of virus reactivation or development of disseminated varicella zoster virus infection.57
Patients receiving a thiopurine should have an annual skin check because of the increased risk of nonmelanoma skin cancer conferred by these drugs. Adequate sun protection is strongly encouraged.29
Smoking is strongly associated with negative disease outcomes in Crohn’s disease.58 Smoking cessation is actively encouraged in those with Crohn’s disease or UC, and the use of pharmaceutical and psychological approaches should be considered.
Resources for GPs
Crohn’s and Colitis Australia have an online program to help support GPs in managing patients with IBD more effectively. The GP Aware program provides online webinars, resources and self-directed modules and workshops that have CPD points allocated (https://crohns andcolitis.org.au/advocacy/our-projects/gp-aware/).
Conclusion
IBD is an increasingly common disease with a high prevalence in Australia. Early referral to a gastroenterologist for assessment and management is important as many effective treatment options are available. Many patients will benefit from biologic agents, and the early use of these disease-modifying drugs to better control inflammation can reduce complications from active disease and improve a patient’s quality of life. All patients require monitoring of disease activity, which includes disease assessment and cancer surveillance. Most medications for IBD are safe for use in pregnant patients. Achieving disease remission before conception and maintaining it during pregnancy is important to optimise outcomes for the mother and baby.
GPs have an important role in assisting with prompt diagnosis and appropriate referral, preventative health care and mental health care for patients with IBD. The management of IBD is increasingly complex and requires a multidisciplinary approach. MT
COMPETING INTERESTS: Dr Tambakis: None. Assistant Professor Wright has acted as an advisor to, been a speaker for, and received research support from AbbVie, Falk, Ferring, Janssen, Pfizer and Takeda.
References
1. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017; 390: 2769-2778.
2. Studd C, Cameron G, Beswick L, et al. Never underestimate inflammatory bowel disease: high prevalence rates and confirmation of high incidence rates in Australia. J Gastroenterol Hepatol 2016; 31: 81-86.
3. Bhatia R, Yeoh SW, Vaz K, et al. Inflammatory bowel disease incidence, prevalence and 12-month initial disease course in Tasmania, Australia. Intern Med J 2019; 49: 622-630.
4. Busingye D, Pollack A, Chidwick K. Prevalence of inflammatory bowel disease in the Australian general practice population: a cross-sectional study. PLoS One 2021; 16: e0252458.
5. Shivashankar R, Tremaine WJ, Harmsen WS, Loftus, EV Jr. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol 2017; 15: 857-863.
6. Aniwan S, Loftus EV. The natural history of inflammatory bowel disease. Curr Treat Options Gastro 2021; 19: 597-607.
7. Ford A, Sandborn W, Khan K, Hanauer S, Talley N, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 644-659.
8. Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther 2018; 47: 364-370.
9. Monsén U, Broström O, Nordenvall B, Sörstad J, Hellers G. Prevalence of inflammatory bowel disease among relatives of patients with ulcerative colitis. Scand J Gastroenterol 1987; 22: 214-218.
10. Fielding JF. The relative risk of inflammatory bowel disease among parents and siblings of crohn’s disease patients. J Clin Gastroenterol 1986; 8: 655-657.
11. Liu J, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015; 47: 979-986.
12. McGovern DP, Kugathasan S, Cho JH. Genetics of inflammatory bowel diseases. Gastroenterology 2015; 149: 1163-1176.
13. Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006; 55: 205-211.
14. Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014; 15: 382-392.
15. Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc 2006; 81: 1462-1471.
16. Khalili H, Ananthakrishnan AN, Konijeti GG, Liao X, Higuchi LM, Fuchs CS. Physical activity and risk of inflammatory bowel disease: prospective study from the Nurses’ Health Study cohorts. BMJ 2013; 347: f6633.
17. Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013; 145: 970-977.
18. Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 2011; 106: 563-573.
19. Ungaro R, Bernstein CN, Gearry R, et al. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol 2014; 109: 1728-1738.
20. Ananthakrishnan AN, Higuchi LM, Huang ES, et al. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann Intern Med 2012; 156: 350-359.
21. Cornish JA, Tan E, Simillis C, Clark SK, Teare J, Tekkis PP. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol 2008; 103: 2394-2400.
22. An Y, Prince D, Gardiner F, et al. Faecal calprotectin testing for identifying patients with organic gastrointestinal disease: systematic review and meta-analysis. Med J Aust 2019; 211: 461-467.
23. Bryant RV, Friedman AB, Wright EK, et al. Gastrointestinal ultrasound in inflammatory bowel disease: an underused resource with potential paradigm-changing application. Gut 2018; 67: 973-985.
24. Kim YG, Jang BI. The role of colonoscopy in inflammatory bowel disease. Clin Endosc 2013; 46: 317-320.
25. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021; 160: 1570-1583.
26. Shergill AK, Lightdale JR, Bruining DH, et al. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc 2015; 81: 1101-1121.
27. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in crohn’s disease: medical treatment. J Crohns Colitis 2020; 14: 4-22.
28. Ko CW, Singh S, Feuerstein JD, et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology 2019; 156: 748-764.
29. Chakraborty TK, Bhatia D, Heading RC, Ford MJ. Salicylate induced exacerbation of ulcerative colitis. Gut 1987; 28: 613-615.
30. Ariyaratnam J, Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: a metaanalysis. Am J Gastroenterol 2014; 109: 163-169.
31. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462-2476.
32. Cholapranee A, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther 2017; 45: 1291-1302.
33. D’Haens G, Reinisch W, Colombel JF, et al. Five-year Safety Data From ENCORE, a European observational safety registry for adults with Crohn’s disease treated with infliximab [remicade(R)] or conventional therapy. J Crohns Colitis 2017; 11: 680-689.
34. Mariette X, Matucci-Cerinic M, Pavelka K, Taylor P, van Vollenhoven R, Heatley R. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis 2011; 70: 1895-1904.
35. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699-710.
36. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711-721.
37. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016; 375: 1946-1960.
38. Hanauer SB, Sandborn WJ, Feagan BG, et al. IM-UNITI: three -year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn’s disease. J Crohns Colitis 2020; 14: 23-32.
39. Sandborn WJ, Su C, Panes J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 377: 496-497.
40. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022; 386: 316-326.
41. Danese S, Vermeire S, Zhou W, et al. OP24 efficacy and safety of upadacitinib induction therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 U-ACHIEVE study. J Crohns Colitis 2021; 15: S022-S024.
42. Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017; 389: 1218-1228.
43. Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA 2019; 321: 156-164.
44. Haifer C, Paramsothy S, Kaakoush NO, et al. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol 2022; 7: 141-151.
45. Haifer C, Kelly CR, Paramsothy S, et al. Australian consensus statements for the regulation, production and use of faecal microbiota transplantation in clinical practice. Gut 2020; 69: 801-810.
46. Levine A, Rhodes JM, Lindsay JO, et al. Dietary guidance from the International Organization for the Study of Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2020; 18: 1381-1392.
47. Narula N, Dhillon A, Zhang D, et al. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2018; 4(4) CD000542.
48. Yanai H, Levine A, Hirsch A, et al. The Crohn’s disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn’s disease (CDED-AD): an open-label, pilot, randomised trial. Lancet Gastroenterol Hepatol 2022; 7: 49-59.
49. Day AS, Yao CK, Costello SP, et al. Therapeutic potential of the 4 strategies to SUlfide-REduction (4-SURE) diet in adults with mild to moderately active ulcerative colitis: an open-label feasibility study. J Nutr 2022; 152: 1690-1701.
50. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999; 340: 1398-1405.
51. Dewint P, Hansen BE, Verhey E, et al. Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn’s disease: a randomised, double-blind, placebo controlled trial (ADAFI). Gut 2014; 63: 292-299.
52. Schulberg JD, Wright EK, Holt BA, et al. Intensive drug therapy versus standard drug therapy for symptomatic intestinal Crohn’s disease strictures (STRIDENT): an open-label, single-centre, randomised controlled trial. Lancet Gastroenterol Hepatol 2022; 7: 318-331.
53. van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis 2015; 9: 107-124.
54. Laube R, Paramsothy S, Leong RW. Use of medications during pregnancy and breastfeeding for Crohn’s disease and ulcerative colitis. Expert Opin Drug Saf 2021; 20: 275-292.
55. Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021; 6: 359-370.
56. Umar N, King D, Chandan JS, et al. The association between inflammatory bowel disease and mental ill health: a retrospective cohort study using data from UK primary care. Aliment Pharmacol Ther 2022; 56: 814-822.
57. Schreiner P, Mueller NJ, Fehr J, Maillard MH, Brand S, Michetti P. Varicella zoster virus in inflammatory bowel disease patients: what every gastroenterologist should know. J Crohns Colitis 2021; 15: 316-325.
58. Lunney PC, Kariyawasam VC, Wang RR, et al. Smoking prevalence and its influence on disease course and surgery in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther 2015; 42: 61-70.