Herpes zoster vaccination: an update
One in three people will experience herpes zoster (HZ) in their lifetime, with the risk increasing markedly with age. Vaccination is recommended for older people and those who are immunocompromised. The recombinant zoster vaccine offers excellent protection against HZ and associated postherpetic neuralgia in both these cohorts and is funded on the National Immunisation Program.
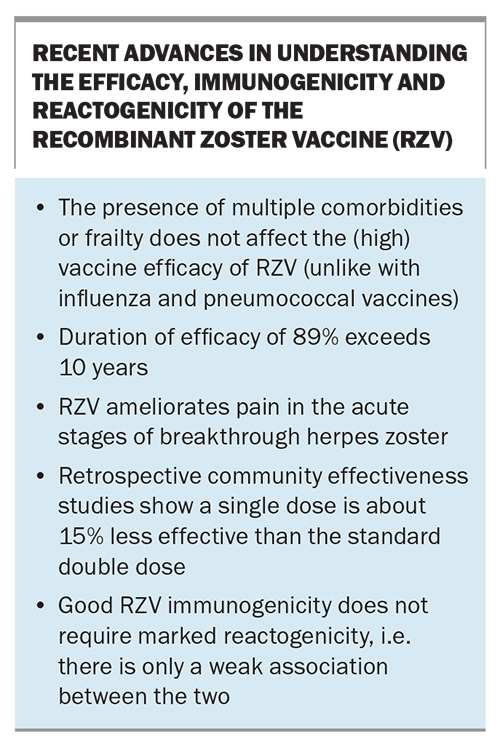
- The recombinant zoster vaccine (RZV) is highly efficacious in immunocompetent patients, irrespective of age and frailty, and this efficacy persists for more than 10 years.
- Two doses of RZV are necessary for optimal effectiveness.
- RZV is safe, including in severely immunocompromised patients.
- RZV has very good efficacy in severely immunocompromised patients with haematological malignancies and after transplantation, as well as very good immunogenicity in those with HIV and other cancers.
- The vaccine is safe and immunogenic after natural herpes zoster infection and attenuated live zoster vaccine.
- RZV is free on the National Immunisation Program for non-Indigenous people aged over 65 years, Indigenous people aged over 50 years and severely immunocompromised patients aged over 18 years.
Herpes zoster: epidemiological and clinical aspects
Herpes zoster (HZ) is caused by reactivation of the varicella zoster virus (VZV) from the dorsal root or trigeminal nerve ganglia. VZV causes inflammation at the dorsal root ganglion and along the nerve as it is transported to the skin dermatome. This results in moderate to severe neuritic pain and, in the innervated dermatome, a characteristic papulovesicular rash.1 This pain may persist beyond 90 days after onset of the rash as postherpetic neuralgia (PHN).2 Reactivation is controlled by specific immunity, especially T-cell mediated immunity, which declines with ageing, commencing beyond 50 years of age, so called ‘immunosenescence’.3 The other major complication is ocular zoster arising from HZ in the first division of the trigeminal nerve, with an incidence of 4 to 25% of all HZ cases and, rarely, causing blindness.4
One in three people will experience HZ in their lifetime, increasing over the age of 50 and rising to an incidence of one in two over the age of 85 years.5 Specifically, the risk of HZ increases markedly with age, from three to five per 1000 personyears for the general adult population to above 10 per 1000 person-years for those aged over 70 years in almost all countries surveyed.6 Recurrent HZ also occurs in all dermatomes, including ocular HZ, commencing about one year after the first episode and reaching an incidence of about 6 to 8% over eight years.7
Herpes zoster in immunocompromised patients
People who are moderately to severely immunocompromised are also at increased risk of HZ, including those with haematological or other malignancies, HIV infection or AIDS, those undergoing solid organ transplantation (SOT) or haemopoietic stem cell transplantation (HSCT) and those receiving immunosuppressive drugs such as 20 mg/day or more of prednisolone or equivalent for over two weeks.8 The incidence and severity of HZ increases proportionately with the degree of immunosuppression. Highly immunocompromised patients receiving allogeneic or autologous HSCT have an HZ incidence of 8 to 28% in the first year after HSCT and are at increased risk of visceral dissemination. HZ incidence is reported as 22 to 32 per 1000 person years in SOT recipients and up to 14 per 1000 person years in patients with a solid tumour receiving chemotherapy. Before the advent of antiretroviral therapy, the risk of HZ in people with HIV infection was 10-fold higher (32 per 1000 person years) than in the age-matched population, and it is still two- to threefold higher than the age-matched population, especially in people with CD4+ T-cell counts below 200 cells/mcL. HZ complications, including recurrent episodes, are still threefold higher than in the age-matched population.8,9
Patients who are moderately immunocompromised as a result of treatment for autoimmune diseases with immunosuppressive drugs and biologicals including disease-modifying antirheumatic drugs (DMARDs), (e.g. rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis) are at increased risk of HZ. Treatment with JAK inhibitors shows a fivefold increased risk compared with other therapies.10
The recombinant zoster vaccine
This article focuses on the recombinant zoster vaccine (RZV, or Shingrix) as it is now the only HZ vaccine available to patients free of charge on the Australian National Immunisation Program (NIP), is predominant in most Organisation for Economic Co-operation and Development (OECD) countries, is the only vaccine recommended for severely immunocompromised patients and one for which new data continues to be reported. It has been available for people aged 50 years and older, whether immunocompromised or not, since June 2021 and is now preferred over the live attenuated zoster vaccine (ZVL, or Zostavax).11
RZV consists of only a single highly immunogenic VZV surface protein, glycoprotein E, which is produced efficiently using recombinant technology, and the AS01B adjuvant system. Glycoprotein E is the most abundant protein in VZV-infected cells and is essential for viral replication.12,13 It is the major target for antibody and cellular immune responses to VZV during infection.14,15 AS01B consists of the toll-like receptor 4 agonist, monophosphoryl lipid A (MPL), and the saponin QS-21, all encased within a liposome. After intramuscular injection, RZV drains rapidly to the axillary lymph nodes and initiates a marked immune cascade involving many cell types and molecules to stimulate antiglycoprotein E (gE)-specific antibodies and T cells.16-18 The adjuvant is crucial for the vaccine’s marked immunogenicity and efficacy. Phase 1 and 2 clinical trials showed that RZV could stimulate strong humoral and cellular immune responses to glycoprotein E in adults aged 50 years or older, and especially in those aged over 70 years, and that the adjuvant was essential for these responses, increasing the T-cell response from 10 to 90% in people over the age of 70 years.19,20 As RZV is not a live vaccine, it is safe for immunocompromised patients.18 A single dose of RZV was found to be immunogenic, but two doses administered two months apart were needed for optimal immune responses.19
Efficacy of RZV
Two large phase 3 studies, the Zoster Efficacy Study in Adults 50 Years of Age or Older (ZOE-50) and Zoster Efficacy Study in Adults 70 Years of Age or Older (ZOE-70), were designed and conducted with more than 29,000 participants to assess RZV efficacy in preventing HZ in immunocompetent adults in these two age groups.21,22 These blinded, randomised, placebo-controlled studies were conducted in parallel at the same study sites in 18 countries. Participants were followed up for the development of HZ and PHN for a mean of 3.2 and 3.7 years.21,22 The ZOE-70 study was needed to obtain additional data for the population at highest risk of HZ and with the highest incidence of PHN.21 RZV had an efficacy against HZ of more than 90% in clinical trials, even in people aged over 80 years. Follow-up studies showed this efficacy to be durable at 89% over 10 years, even in the oldest age groups, showing RZV to be the most efficacious and durable vaccine yet for the ageing population.23,24
Two real life studies have confirmed the high effectiveness of RZV, albeit slightly lower (85% and 70%) likely due to difficulties in using databases for diagnosis (e.g. defining HZ according to antiviral drug use). One study showed a decrease in effectiveness of about 15% if only a single dose was administered.25,26
Recombinant vaccine efficacy against PHN
Pooled data from the ZOE-50 and ZOE-70 studies showed an RZV efficacy against PHN of 91% in adults aged 50 years or older.21,22 Efficacy was similar (89%) for those aged 70 years or older. Its efficacy against PHN was due to prevention of HZ. Follow-up studies also showed attenuation of acute pain and reduced pain medication during the uncommon breakthrough case of HZ in RZV immunised patients.27 The number needed to vaccinate with RZV to prevent one HZ case ranged from eight to 11, and to prevent one case of PHN ranged from 39 to 53.28
RZV also significantly protected against other complications of HZ, including disseminated disease, ocular HZ and neurological disease, including vasculitis and stroke, and visceral diseases. Unlike some other vaccines for the ageing population, RZV also showed no diminution in efficacy against HZ in those patients with multiple comorbidities or frailty, common in those aged over 70 years.29,30
Safety and reactogenicity of recombinant vaccine
In the ZOE-50 and ZOE-70 studies, more than 14,000 adults aged 50 years or older received RZV and a similar number received the saline placebo. There were no differences in serious adverse events (10.1% vs 10.4%) or fatalities (4.3% vs 4.6%) between participants receiving vaccine and placebo.21,22,31 There was also no significant difference in the incidence of potential immune-mediated diseases between those receiving vaccine and placebo (1.1% vs 1.3%), a potential concern with a new immunomodulating adjuvant such as AS01B.31 Post-licensure studies monitoring safety for potential immune-mediated diseases showed that there is an extremely low incidence (3 per one million) of Guillain-Barré syndrome in the first 42 days after each RZV dose, and is similar to that of adenovirus-based and influenza vaccines.32
A recent retrospective study also suggested a minor increase in recurrent ocular HZ if patients were immunised with RZV within 56 days after their previous episode of ocular HZ.33 However, in Australia RZV is recommended to be administered one year after a prior episode of HZ (see below).
Reactogenicity may be local at the injection site or systemic. In the first seven days after RZV administration, injection site reactions were much more frequent in RZV recipients than in those who received placebo (82% vs 12%; Box).22 The most common local reaction was pain. Systemic reactions, most often myalgia and fatigue, were also more frequent after RZV than placebo (66% vs 30%). There was less reactogenicity with advancing age. The more important reactogenicities are the severe or ‘grade 3’ local and systemic reactions, defined as those preventing normal daily activity. These occurred much more frequently with RZV than placebo (local reactions, 9.5% vs 0.4%). However, these reactions to vaccine were transient, lasting one to three days overall, and one to two days for grade 3 reactions. Thus, the vaccine was generally well tolerated as 95% of recipients returned for a second dose, including 91% of recipients with grade 3 reactions. The incidence of grade 3 reactogenicity was similar after first and second doses, and only 34% of those with such reactogenicity after the first dose had grade 3 reactions after the second dose.34 Furthermore, good RZV immunogenicity does not require marked reactogenicity, i.e. there is only a weak association between the two.35
Safety of RZV when administered after prior herpes zoster or after live attenuated zoster vaccine
When RZV was administered five years after natural herpes zoster (physician documented), the vaccine was found to be safe, with similar immunogenicity to that seen in the ZOE50/70 trials (in recipients without prior HZ), inducing antibody to the vaccine in more than 90% of patients aged 50 years or over.36 RZV administered to patients five years after ZVL was found to be equally immunogenic and safe compared with those without prior immunisation.37 These results are important in the Australian context, where there is high ZVL uptake in the community. In Australia (and some other countries), RZV is recommended to be given at one year or more after natural HZ or ZVL; however, RZV is not funded on the NIP until five years after ZVL.11 In some other countries, including the USA, RZV is recommended soon after full recovery from HZ; however, in the special case of ocular HZ this is unwise until further studies clarifying the risk of inducing recurrent ocular HZ are available.33
Coadministration with other vaccines
Recent studies have now clarified that RZV is safe and retains similar immunogenicity after coadministration with unadjuvanted inactivated seasonal influenza, pneumococcal (PPV23), COVID-19 RNA and diphtheria-tetanus-pertussis (DTaP) vaccines.38-40
RZV efficacy and immunogenicity in immunocompromised patients
Five key phase 2 and 3 clinical trials have provided evidence for the immunogenicity, reactogenicity, safety and/or efficacy of RZV in moderately to severely immunocompromised patients.41-45 In patient groups receiving autologous HSCT, SOT and haematological malignancy or solid tumours with chemotherapy, two vaccine doses were administered at zero and one to two months, with follow up at 12 months. In patients aged 18 years and older (of whom 73% were aged over 50 years) with haematological malignancies (chronic lymphatic leukaemia, Hodgkin’s disease, non-Hodgkin’s lymphoma, myeloma), RZV was administered during or after chemotherapy. Significant immune responses were observed after RZV, with T-cell and gE antibody responses seen in 84% and 60% of patients, respectively. Vaccine efficacy against HZ was excellent at 87% in a post hoc analysis.41
In the largest trial, more than 1800 patients were administered RZV 50 to 70 days after receiving autologous HSCT for acute myeloid leukaemia, Hodgkin’s disease, non-Hodgkin’s lymphoma or multiple myeloma. Excellent immune responses to RZV were observed, with T-cell responses seen in 89% and gE antibody in 71% of patients. The efficacy was very good against HZ (68%) and excellent against PHN (89%), other complications (78%) and hospitalisation (85%). Vaccine efficacy was unchanged in patients receiving rituximab for B-cell lymphomas and chronic lymphocytic leukaemia.42 In the SOT group, a T-cell response occurred in 64% and gE antibody in 74% of recipients.43 Immunogenicity was lowest in patients with solid tumours receiving chemotherapy, with a T-cell response seen in 46%, although a gE antibody response was seen in 92%.44
In the HIV trial, patients with HIV infection were stratified into three subgroups according to CD4+ T-cell count and received three vaccine doses at zero, two and six months, with follow up at 18 months. RZV induced strong gE antibody and T-cell responses that persisted until the end of the 18-month study, irrespective of CD4+ T-cell level or whether patients were on antiretroviral therapy. The third dose did not add to immunogenicity.45
Where patients cannot immediately receive RZV after induction of immunosuppression, antiviral prophylaxis for HZ with valaciclovir or famciclovir should be continued until peak immunogenicity is reached. For example, in the HSCT trials RZV was administered 50 to 70 days after receiving HSCT and then the second dose one to two months later; peak immunogenicity was reached one month after the second dose.42 In practice, the US Centers for Disease Control and Prevention recommends that patients receive RZV at least three to 12 months after HSCT or before transplantation or chemotherapy, if possible. If this is not possible then RZV should be administered when immunosuppression is reduced.46
The next target group for RZV are moderately immunocompromised patients on immunosuppressive medication for autoimmune diseases, including chemical and biological agents, some of which are antirheumatic drugs or DMARDS. As JAK inhibitors are one of the most potent agents in predisposing to HZ, several trials are underway to examine efficacy in such people.10 Some studies of immunogenicity are reassuring in indicating that RZV induces increased VZV/gE antibody in 80 to 100% of people on JAK inhibitors. T-cell responses were not studied.47,48 The results of current efficacy trials are eagerly awaited.
RZV reactogenicity and safety in immunocompromised people
In the above trials, no vaccination-related severe adverse effects were reported.41-45 Local reactogenicity at the injection site in patients in all five of these trials was only moderately increased (75 to 85%) compared with immunocompetent people in the ZOE50 and 70 trials (84% in those aged over 50 years and 79% in those over 70 years.)21,22 However, systemic reactogenicity was markedly increased in all five immunocompromised groups (67 to 83%), and was mostly because of the underlying disease, with a marked increase in systemic reactogenicity also seen in placebo recipients (46 to 79%). Compared with immunocompetent people, severe or ‘grade 3’ local or systemic reactions in immunocompromised groups were relatively mildly elevated (10 to 20%).41,49
Future research
Follow-up studies on the durability of the efficacy of RZV in immunocompetent people out to 15 years are continuing. Studies on the safety of RZV coadministration with other adjuvanted vaccines are also urgently needed. Future research on the mechanism of action of RZV may lead to the development of RZV-like vaccines that have reduced reactogenicity but retain immunogenicity and efficacy, as there is only a weak correlation between the two effects.35
Further research on the efficacy, immunogenicity and reactogenicity of RZV in immunocompromised patients is still needed and underway. In particular, the completion of phase 3 trials in patients receiving SOTs and those with solid tumours receiving chemotherapy are of major importance. For all five groups of severe immunosuppression, the durability of immunogenicity and vaccine efficacy beyond 12 to 18 months needs to be defined. Currently, there are few data on the safety and efficacy of RZV in patients with autoimmune diseases who are being treated with biological and synthetic DMARDs, especially agents with an immunosuppressive effect on T cell responses; however, phase 3 trials of RZV in patients on JAK inhibitors are in progress. Studies on the use of RZV to prevent varicella/chickenpox in severely immunocompromised children are worth pursuing in view of the contraindication to live attenuated vaccines and recent data showing RZV can induce naïve T cell responses to VZV gE.50,51
Indications for the recombinant zoster vaccine in Australia
In Australia, RZV is recommended for immunocompetent people over the age of 50 years and in patients aged 18 years and older who are moderately to severely immunocompromised or soon expected to be so. Household contacts are also recommended to be immunised. RZV is funded through the NIP for non-Indigenous immunocompetent people aged 65 years or over, Indigenous people aged 50 years or over and patients aged 18 years or over who are severely immunocompromised.10
Conclusion
RZV is highly efficacious against herpes zoster and complications including PHN, regardless of age (including in people more than 80 years of age), comorbidities and frailty. Protection remains stable up to 10 years after vaccination, with longer-term efficacy trials in progress. Two doses given two to six months apart, are required for optimal efficacy. RZV is associated with a high level of local (injection site) and systemic reactogenicity; however, the incidence of reactogenicity that impairs everyday activity is low and lasts one to three days, with only one-third experiencing similar severity with the second dose. The vaccine is very safe, with an extremely low incidence of Guillain-Barré syndrome. It is the only safe vaccine for HZ in moderate-severely immunocompromised patients with very good, albeit lower, efficacy in patients with transplants and haematological malignancy. Local reactogenicity is similar to immunocompetent people and systemic reactogenicity is increased due to underlying disease. MT
COMPETING INTERESTS: Professor Cunningham has received honoraria for consultancies from GSK, Moderna, Seqirus and Merck, paid to his institution.
References
1. Mitchell BM, Bloom DC, Cohrs RJ, Gilden DH, Kennedy PG. Herpes simplex virus-1 and varicella-zoster virus latency in ganglia. J Neurovirol 2003; 9: 194-204.
2. Mallick-Searle T, Snodgrass B, Brant JM. Postherpetic neuralgia: epidemiology, pathophysiology, and pain management pharmacology. J Multidiscip Health 2016; 9: 447-454.
3. Gershon AA, Gershon MD. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin Microbiol Rev 2013; 26: 728-743.
4. Yawn BP, Saddier P, Wollan PC, et al. A populations-based study of the incidence of and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007: 82: 1341-1349.
5. Centers for Disease Control and Prevention. Prevention of herpes zoster. MMWR 2008 June; 57(RR-5): 1-30.
6. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4: e004833.
7. Yawn BP, Wollan PC, Kurland MJ,et al. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86; 88-93.
8. Liu B, Heywood AE, Reekie J, et al. Risk factors for herpes zoster in a large cohort of unvaccinated older adults: a prospective cohort study. Epidemiol Infect 2015; 143: 2871-2881.
9. Dagnew AF, Vink P, Drame M, Willer DO, Salaun B, Schuind AE. Immune responses to the adjuvanted recombinant zoster vaccine in immunocompromised adults: a comprehensive overview. Hum Vaccin Immunother 2021; 17: 4132-4143.
10. Winthrop K, Nash P, Yamaoka K, et al. Incidence and risk factors for herpes zoster in patients with rheumatoid arthritis receiving upadacitinib: a pooled anlysis of six phase III clinical trials. Ann Rheum Dis 2022; 81: 206-213.
11. Zoster. In: Australian immunisation Handbook. Canberra: Australian Government Department of Health; 2023. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/zoster-herpes-zoster (accessed June 2024).
12. Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Ann Rev Microbiol 1990; 44: 59-80.
13. Mallory S, Sommer M, Arvin AM. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J Virol 1997; 71: 8279-8288.
14. Bergen RE, Sharp M, Sanchez A, Judd AK, Arvin AM. Human T cells recognize multiple epitopes of an immediate early/tegument protein (IE62) and glycoprotein I of varicella zoster virus. Viral Immunol 1991; 4: 151-166.
15. Malavige GN, Jones L, Black AP, Ogg GS. Varicella zoster virus glycoprotein E-specific CD4+ T cells show evidence of recent activation and effector differentiation, consistent with frequent exposure to replicative cycle antigens in healthy immune donors. Clin Exp Immunol 2008; 152: 522-531.
16. Garcon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines 2011; 10: 471-486.
17. Didierlaurent A, Laupeze, Di Pasquale A, et al. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines 2017; 16: 55-63
18. Cunningham AL, McIntyre P, Subbarao K, Booy R, Levin MJ. Vaccines for older adults. BMJ 2021; 372: n188.
19. Chlibek R, Bayas JM, Collins H, et al. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults >=50 years of age. J Infect Dis 2013; 208: 1953-1961.
20. Chlibek R, Smetana J, Pauksens K, et al. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine 2014; 32: 1745-1753.
21. Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375: 1019-1032.
22. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372: 2087-2096.
23. Boutry C, Hastie A, Diez-Domingo J, et al; Zoster-049 Study Group. The adjuvanted recombinant zoster vaccine confers long-term protection against herpes zoster: interim results of an extension study of the pivotal phase iii clinical trials (ZOE-50 and ZOE-70). Clin Infect Dis 2022; 74: 1459-1467.
24. Strezova A, Diez-Domingo J, Al Shawafi K, et al. Long-term protection against herpes zoster by the adjuvanted recombinant zoster vaccine: interim efficacy, immunogenicity and safety up to 10 years after vaccination. Open Forum Infect Dis 2022; 9: ofac485.
25. Sun Y, Kim E, Kong CL, et al. Effectiveness of the recombinant zoster vaccine in adults aged 50 and over in the United States: a claims-based cohort study. Clin Infect Dis 2021;73: 949-956.
26. Izurieta HS, Wu HX Forshee R, et al. Recombinant zoster vaccine (Shingrix): real-world effectiveness in the first two years post-licensure. Clin Infect Dis 2021; 73: 941-948.
27. Kim JH, Johnson R, Kovac M, Cunningham AL, Amakrane M, Sullivan KM, Dagnew AF, Curran D, Schuind A. Adjuvanted recombinant zoster vaccine decreases herpes zoster-associated pain and the use of pain medication across 3 randomized, placebo-controlled trials. Pain 2023; 164: 741-748.
28. Curran D, Van Oorschot D, Varghese L, et al. Assessment of the potential public health impact of herpes zoster vaccination in Germany. Hum Vaccin Immunother 2017; 13: 2213-2221.
29. Kovac M, Lal H, Cunningham AL, et al; ZOE-50/70 Study Group. Complications of herpes zoster in immunocompetent older adults: incidence in vaccine and placebo groups in two large phase 3 trials. Vaccine 2018; 36: 1537-1541.
30. Curran D, Kim JH, Matthews S, et al; Zoster-064 Study Group. Recombinant zoster vaccine is efficacious and safe in frail individuals. J Am Geriatr Soc 2021; 69: 744-752.
31. López-Fauqued M, Campora L, Delannois F, et al. Safety profile of the adjuvanted recombinant zoster vaccine: pooled analysis of two large randomised phase 3 trials. Vaccine 2019; 37: 2482-2493.
32. Goud R, Lufkin B, Duffy, J et al. Risk of Guillain-Barre syndrome following recombinant zoster vaccine in Medicare beneficiaries. JAMA Intern Med 2021; 181: 1623-1630.
33. Walia A, Sun Y, Acharya NR. Risk of herpes zoster ophthalmicus recurrence after recombinant zoster vaccination JAMA Ophthalmol 2024; 142: 249-256.
34. Colindres R, Wascotte V, Brecx, et al. A post hoc analysis of reactogenicity trends between dose 1 and dose 2 of the adjuvanted recombinant zoster vaccine in two randomised trials Hum Vacc Immunother 2020; 16: 2628-2633.
35. Callegaro A, Burny W, Heve C, et al. Association between immunogenicity and reactogenicity; a post hoc analysis of two phase 3 studies with the recombinant zoster vaccine J. Infect Dis 2022; 226: 1943-1948.
36. Godeaux O, Kovac M, Shu D, et al. Immunogenicity and safety of an adjuvanted herpes zoster subunit candidate vaccine in adults >/= 50 years of age with a prior history of herpes zoster: a phase III, non-randomized, open-label clinical trial. Human Vaccin Immunother 2017; 13: 1051-1058.
37. Grupping K, Campora L, Douha M, et al. Immunogenicity and safety of the HZ/su adjuvanted herpes zoster subunit vaccine in adults previously vaccinated with a live attenuated herpes zoster vaccine. J Infect Dis 2017; 216: 1343-1351.
38. Schwarz TF, Aggarwal N, Moeckesch B, et al. Immunogenicity and safety of an adjuvanted herpes zoster subunit vaccine coadministered with seasonal influenza vaccine in adults aged 50 years or older. J Infect Dis 2017; 216: 1352-1361.
39. Marechal C, Lal H, Poder A, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine co-administered with the 23-valent pneumococcal vaccine in adults greater than 50 years of age: a randomized trial. Vaccine 2018; 38: 4278-4286.
40. Strezova A, Lal H, Enweonye I, et al. The adjuvanted recombinant zoster vaccine co-administered with a tetanus diphtheria and pertussis vaccine in adults aged ≥50 years: a controlled trial. Vaccine 2019; 37: 5877-5885.
41. Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis 2019; 19: 988-1000.
42. Bastidas A, de la Serna J, El Idrissi M, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA 2019; 322: 123-133.
43. Vink P, Ramon Torrell JM, Sanchez Fructuoso A, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase 3, randomized clinical trial. Clin Infect Dis 2020; 70: 181-190.
44. Vink P, Delgado Mingorance I, Maximiano Alonso C, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer 2019; 125: 1301-1312.
45. Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211: 1279-1287.
46. Centers for Disease Control and Prevention (CDC). Clinical considerations for use of recombinant zoster vaccine (RZV, Shingrix) in immunocompromised adults aged ≥19 years. USA: CDC; 2022. Available online at: https://www.cdc.gov/shingles/vaccination/immunocompromised-adults.html (accessed June 2024).
47. Venerito V, Stefanizzi P, Cantarini L, et al. Immunogenicity and safety of adjuvanted recombinant zoster vaccine in rheumatoid arthritis patients on anti-cellular biologic agents or JAK inhibitors: a prospective observational study. Int J Med Sci 2023; 24: 6967-6977.
48. Kallmark H, Bergstrom T, Nagel, et al. Serologic immunogenicity and safety of herpes zoster subunit vaccine in patients with rheumatoid arthritis receiving Janus kinase inhibitors. Rheumatology 2023; kead552.
49. López-Fauqued M, Co-van der Mee M, Bastidas A, et al. Safety profile of the adjuvanted recombinant zoster vaccine in immunocompromised populations: an overview of six trials. Drug Safety 2021; 44: 811-823.
50. Laing K, Ford ES, Johnson M, et al. Recruitment of naïve CD4 T cells by the recombinant zoster vaccine correlates with persistent immunity J.Clin Invest 2023; 133: e172634.
51. Cunningham AL, Sandgren K, Truong N. Advances in understanding the mechanism of action of adult vaccines J Clin Invest 2023; 133: e175378.