Autoimmune inflammatory rheumatic diseases: vaccines are important
Autoimmune inflammatory rheumatic diseases and immunosuppression increase the risk of infection. Vaccination is fundamental to the prevention of infection by enhancing protective immunity.
Correction
A correction for this article appears in the June 2024 issue of Medicine Today. The online version and the full text PDF of this article (see link above) have been corrected.
- Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk of infection.
- Vaccination enhances protective immunity and is important to prevent infection.
- Some important vaccines to consider in people with AIIRD include the herpes zoster; varicella; measles, mumps and rubella; Japanese encephalitis; yellow fever; COVID-19; influenza; pneumococcal disease; rotavirus; and tuberculosis vaccines.
- The vaccination status of patients with AIIRD should be reviewed regularly to avoid missed vaccination opportunities.
- GPs are ideally placed for this role in partnership with the treating rheumatologist and a vaccine specialist.
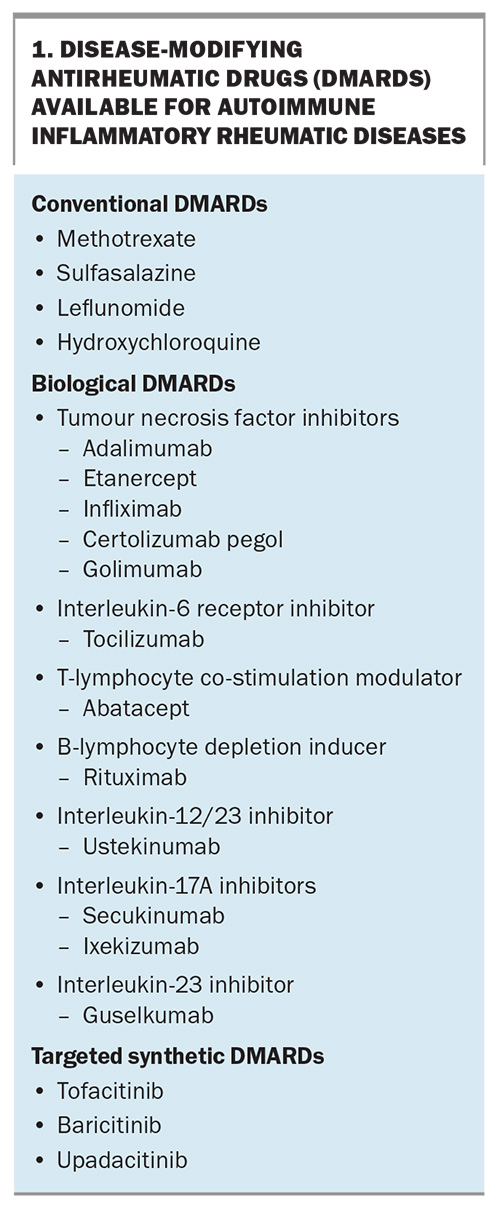
Patients with autoimmune inflammatory rheumatic diseases (AIIRD), such as rheumatoid arthritis, psoriatic arthritis and spondyloarthritis, are often treated with immunosuppressive therapies. These usually comprise corticosteroids and conventional synthetic disease-modifying antirheumatic drugs (DMARDs; csDMARDs), such as methotrexate, sulfasalazine and leflunomide. However, AIIRD treatment has evolved over the past two decades. There are now several parenteral biological DMARDs (bDMARDs) which target specific proinflammatory cytokines or cell types (Box 1). More recently, oral targeted synthetic DMARDs (tsDMARDs), such as tofacitinib, baricitinib and upadacitinib, which target proinflammatory intracellular enzymes, have broadened the therapeutic options available for AIIRD.1
Both AIIRD and immunosuppression are risk factors for infection; thus, vaccination is important to prevent infection in this group of patients.2 This article discusses the use of common vaccines in immunosuppressed patients with AIIRD.
Herpes zoster
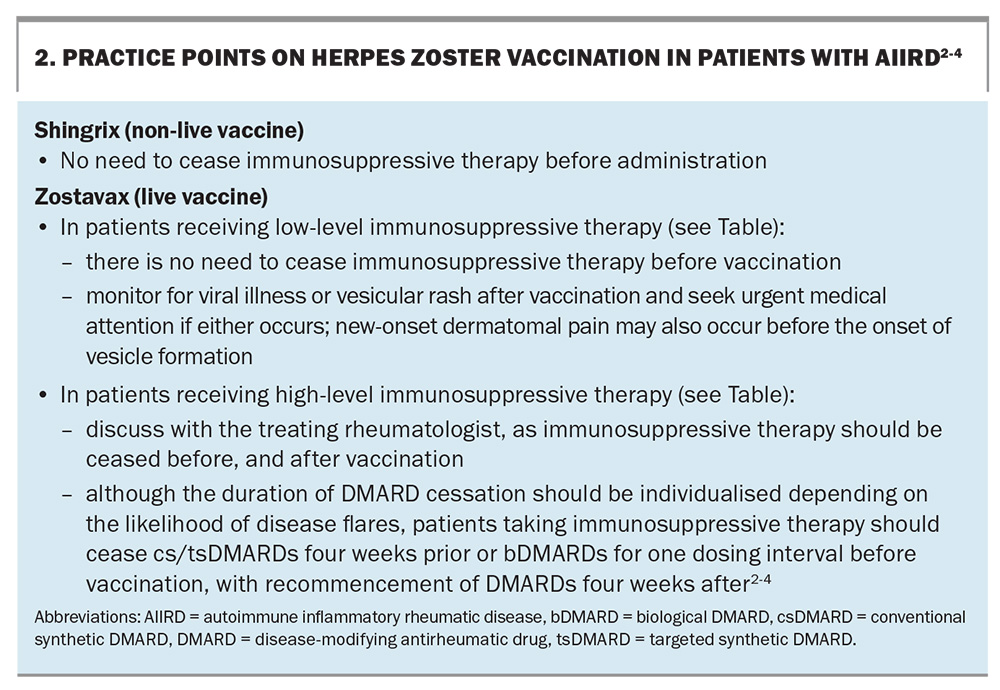
Herpes zoster (HZ) immunisation is a common but complex vaccination scenario in patients with AIIRD. The incidence of HZ (i.e. shingles) in people aged 50 years and older in Australia is about 1 in 100, with an increased risk in those with underlying inflammatory arthritis and an even higher risk in those receiving immunosuppressive therapy for AIIRD.3 Post-herpetic neuralgia and HZ ophthalmicus are potential serious complications of infection. The risk and incidence of viral reactivation increases with age and immunosuppression, especially with tsDMARDs, which are standard therapy in patients with AIIRD.4
Two vaccines are available in Australia to help prevent HZ: the zoster vaccine live (Zostavax), a live attenuated vaccine given as a single dose, and the varicella virus recombinant vaccine (Shingrix), a more effective subunit non-live vaccine given as two doses.3 Under the National Immunisation Program (NIP), Zostavax is subsidised by the government for immunocompetent adults aged 70 years and older, with ‘catch up’ vaccination available for adults aged 71 to 79 years.3
In March 2023, the Pharmaceutical Benefits Advisory Committee recommended that the PBS subsidise Shingrix for non-Indigenous individuals aged 65 years and older, Aboriginal and Torres Strait Islander individuals aged 50 years and older and immunocompromised individuals aged 18 years and older with conditions at ‘high risk’ of HZ infection (e.g. haemopoietic stem cell or solid organ transplants, haematological malignancy and advanced or untreated HIV infection).5 However, a decision for the broader population of immunosuppressed patients was deferred pending advice from the Australian Technical Advisory Group on Immunisation. At its November 2023 meeting, the Committee recommended Shingrix for individuals aged 18 to 64 years at moderate-to-high risk of infection, being treated with csDMARDs (e.g. methotrexate, azathioprine, mycophenolate), bDMARDS (e.g. tumour necrosis factor inhibitors) and tsDMARDs (e.g. Janus kinase inhibitors).
In the absence of a PBS subsidy, Shingrix requires a private prescription at a cost of about $270 per dose.6 Two standard doses are given six months apart in immunocompetent patients and one to three months apart in immunocompromised patients. Cessation of DMARDs is not required as Shingrix is a non-live vaccine.3,6
Shingrix is the preferred vaccine over Zostavax given its better safety and efficacy profile.7 Zostavax should only be used in patients after discussing the risks and benefits compared with Shingrix. Reassuringly, a randomised controlled trial of 617 patients treated with a tumour necrosis factor inhibitor (TNFi; a bDMARD) for autoimmune disease revealed no cases of HZ when Zostavax was given.8
Individuals without prior varicella infection are not at risk of HZ reactivation. In these individuals, Zostavax is contraindicated because of the risk of dissemination from this live vaccine. It is therefore recommended to establish a history of primary varicella infection in those being considered for the vaccine. Serological evaluation can be helpful to establish the serostatus; a positive result confirms previous varicella infection, in which case administration of Shingrix or Zostavax is recommended. This is particularly important for Zostavax, which should be avoided in seronegative immunosuppressed individuals because of the risk of disseminated disease.
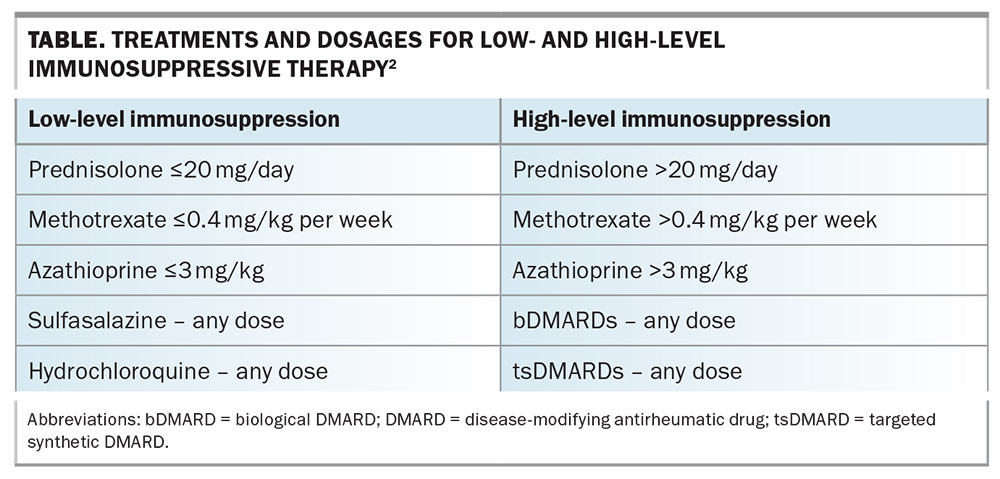
Individuals who test negative on serological evaluation are at risk of primary varicella infection, which can be particularly severe in immunocompromised individuals. For individuals taking low-level immunosuppressive therapy, two doses of a varicella vaccine (monovalent live vaccine Varilrix or Varivax) should be considered to achieve adequate protection against varicella before starting of b/tsDMARD therapy. Combination vaccines (Priorix and ProQuad) should be avoided, as the patient may not require the other vaccines contained in the combination. The two doses should be given at least four weeks apart.3 Box 2 provides practice points related to HZ vaccination in patients with AIIRD.2-4 The Table lists the treatments and dosages for low- and high-level immunosuppressive therapy.2
Measles, mumps and rubella
The measles, mumps and rubella vaccines are live vaccines and, hence, not recommended for immunocompromised patients.9 However, if strongly required (e.g. for occupational reasons), patients taking immunosuppressive therapy should cease cs/tsDMARDs four weeks prior or bDMARDs for one dosing interval before vaccination, with recommencement of DMARDs four weeks after. Postexposure prophylaxis should be given within three days of exposure in nonimmune adults exposed to measles or rubella (but not mumps) with normal human immunoglobulin levels.9
Japanese encephalitis
Japanese encephalitis (JE) is caused by the JE virus (JEV), which is transmitted by infected Culex spp. mosquitoes.10 The infection is endemic to parts of Asia and the Torres Strait region of Australia. However, 37 human cases of JE were reported in early 2022 in four states of Australia.11 This prompted a rollout of JE vaccinaation with a live JE vaccine (Imojev) in regional Australia. Imojev should be avoided in immunosuppressed patients, with the non-live JE vaccine (JEspect) used instead. Numerous wild and domestic animal species can become infected, although most do not develop clinical signs; rarely, animals will develop sufficient viraemia to infect mosquitoes, which can result in further transmission. The virus cannot be transmitted from human to human, or from consuming the meat of an infected animal.10
Yellow fever
Yellow fever virus is endemic to tropical and subtropical regions of Africa and South America.12 Given the resurgence of post-coronavirus disease (COVID-19) travel, patients with AIIRD may require yellow fever vaccination with a live attenuated vaccine. Although live vaccines are generally contraindicated in immunocompromised patients, the need for documented evidence of yellow fever vaccination to allow entry to some countries means that consideration may be required on a case-by-case basis. However, there is provision for a certificate of medical contraindication to yellow fever vaccination by vaccination centres.12 If required, patients taking immunosuppressive therapy should cease cs/tsDMARDs four weeks prior or bDMARDs for one dosing interval before live vaccination, with recommencement of DMARDs four weeks after.13
COVID-19
Patients with AIIRD may have a higher risk of severe COVID-19 infection (odds ratio, 1.6; 95% CI, 1.13-2.26) compared with patients with other conditions.14 Some factors, including high disease activity, chronic lung disease or immunosuppressive treatments (especially rituximab, sulfasalazine and >10 mg/day prednisolone-equivalent dosages of glucocorticoids), are associated with poorer outcomes.14 Immune responses following COVID-19 vaccination are impaired in immunosuppressed patients with AIIRD.15,16
Although advice regarding COVID-19 vaccination continues to evolve, certain principles remain. Most patients with AIIRD in Australia should have received their primary course of a COVID-19 vaccine (either two or three doses) and a subsequent booster dose. Many people with AIIRD will also have had a 2022 winter booster dose (the second booster dose). A third, 2023 winter booster dose is recommended to reduce severe disease from the emerging surge of Omicron BA.4 and BA.5 subvariant infections (this means that some people with AIIRD will have had their sixth COVID-19 vaccine dose).17 These recommendations are targeted at those aged 65 years and older, or aged between 18 and 64 years with comorbidities that increase the risk of severe COVID-19.17
The Australian Rheumatology Association advises that cs/b/tsDMARDs should not routinely be interrupted at the time of COVID-19 vaccination.18 The one exception is abatacept, a T-lymphocyte costimulatory inhibitor, which should be withheld one week before, and for one week after, each COVID-19 vaccine dose.18 Administration of the anti-CD20 antibody rituximab requires vaccines to be given at least two weeks before administration because of the effect of B-lymphocyte depletion.18
A suboptimal humoral response to COVID-19 vaccination in patients with AIIRD may prompt pre-exposure prophylaxis with tixagevimab–cilgavimab, a neutralising monoclonal antibody against certain strains of COVID-19.19 However, changes in dominant COVID-19 strains have impacted the effectiveness of this strategy, and monoclonal antibody therapies for the prevention or treatment of COVID-19 are no longer recommended.20 Prompt antiviral use (e.g. with nirmatrelvir–ritonavir) provides an alternative strategy against severe infection in immunosuppressed patients with AIIRD.21 Caution may be required as nirmatrelvir–ritonavir is a strong inhibitor of CYP3A and may increase the plasma concentrations of many medications that are metabolised by CYP3A, including corticosteroids or tsDMARDS, such as tofacitinib or upadacitinib.22
Given the evolving COVID-19 vaccination advice from the Australian Government Department of Health and Aged Care, discussion with the patient's treating rheumatologist and a vaccination specialist is recommended. As immunosuppressed patients with AIIRD have a higher risk of severe COVID-19 infection, patient education should particularly target this group to ensure they are up to date with the latest vaccination recommendations. Prompt antiviral use may prevent severe COVID-19 infection in these patients.
Influenza
Influenza vaccination is the most important intervention for the prevention of infection-associated hospitalisation and mortality.23 Patients on immunosuppressive therapy have an increased risk of influenza-related complications and should receive the influenza vaccine annually before the start of the influenza season, as protection is the greatest in the first four months postvaccination.24 Although a lower seroprotective response may be seen in the presence of DMARDs, most patients have an adequate immune response postinfluenza vaccination. However, as rituximab can cause prolonged B-cell depletion with marked impairment of humoral immunity, vaccination should occur before rituximab administration.25 If necessary, COVID-19 vaccines can be coadministered with influenza vaccines.17 Some evidence suggests that patients with AIIRD who receive the influenza vaccine for the first time should receive two doses at least four weeks apart, and one dose annually thereafter.26 If possible, discontinuation of methotrexate for two weeks after seasonal influenza vaccination may improve the humoral vaccine response.27,28
If possible, immunosuppressed patients with AIIRD should receive an adjuvanted or high-dose formulation, as these induce a greater postvaccination immunological response.4 However, standard-dose influenza vaccines should be administered if a high-dose or adjuvanted formulation is not available. Oseltamivir or zanamivir can be used for treatment and postexposure prophylaxis; these treatments are generally underutilised.29
Pneumococcal disease
Immunosuppressive therapy can increase the risk of invasive pneumococcal disease (meningitis, pneumonia or bacteraemia). The two types of pneumococcal vaccines available in Australia are the pneumococcal conjugate vaccines (PCVs; 13-valent [v] [13vPCV], 15vPCV and 20vPCV) and pneumococcal polysaccharide vaccine (23vPPV).30 Immunocompromised patients with AIIRD should receive a dose of one of the PCVs, followed by the 23vPPV at least two months later. An additional 23vPPV dose should be given a minimum of five years later. Pneumococcal vaccination should ideally occur before DMARD initiation to maximise the immune response.30 The Australian pneumococcal vaccination program is currently under review.
Pregnancy and vaccination
Although shared decision-making between patients with AIIRD and the treating rheumatologist can result in DMARDs being stopped prepregnancy, bDMARDs, particularly TNFi drugs, may be continued in some cases to avoid disease flares. Almost all bDMARDs cross the placenta, leading to concern about the newborn being immunosuppressed. This has implications for in vivo replication of a live vaccine given to infants in the first 12 months of life.31 In Australia, the only live vaccine listed on the NIP given in the first 12 months of life is the rotavirus vaccine, which is given at 2 and 4 months of age (live attenuated human rotavirus vaccine, Rotarix), or 2, 4 and 6 months of age (live attenuated pentavalent human–bovine rotavirus vaccine, RotaTeq), depending on the preparation.32
Live vaccines for infants should be avoided if the mother received a TNFi (or other bDMARD) after 16 weeks of gestation. However, not all international guidelines are this conservative.4 Rotavirus can cause severe diarrhoea in babies, leading to dehydration and hospitalisation. Some guidelines suggest that the rotavirus vaccine can be given in the first six months of life to babies of mothers who have continued taking a TNFi during pregnancy.34 However, this should be discussed with the treating rheumatologist or infectious diseases physician before administration. The likelihood of rotavirus infection is low in infants younger than 6 months of age; therefore, a ‘catch-up’ rotavirus vaccine dose is probably not needed. The one exception is if the mother is taking certolizumab pegol, a TNFi that lacks a fragment crystal portion and hence, does not traverse the placenta.31 Live vaccines can thus be given to infants born to mothers treated with certolizumab pegol during pregnancy.31
The live Bacille Calmette–Guérin vaccine to prevent tuberculosis (TB) is not routinely used in Australia given the low TB rates. However, the vaccine is considered for Aboriginal and Torres Strait Islander infants younger than 5 years of age, in children born to mothers with TB or in people travelling to TB endemic areas.33
Conclusion
Vaccination prevents severe disease and complications associated with a wide variety of infections. As this is particularly important in immunosuppressed patients with AIIRD, the vaccination status of these patients should be reviewed regularly to avoid missed vaccination opportunities. GPs are ideally placed for this role, in partnership with the treating rheumatologist and a vaccine specialist. Live vaccines are generally contraindicated in severely immunocompromised patients because of the risk of vaccine-induced disease. Inactive vaccines are safe, but vaccine effectiveness may be reduced in the setting of immunosuppression. Vaccination should ideally occur before starting immunosuppressive therapy. However, this is often not practical in clinical practice. Ceasing DMARDs before vaccination can be a complex decision and discussion with the treating rheumatologist may be required. MT
COMPETING INTERESTS: Dr Yoon and Dr O’Sullivan: None. Associate Professor Wong has received unrestricted research grants in the last four years from Abbvie, UCB and Janssen to investigate vaccine effectiveness in rheumatology patients.
References
1. Wong PKK, Bagga H, Barrett C, et al. A practical approach to vaccination of patients with autoimmune inflammatory rheumatic diseases in Australia. Intern Med J 2017; 47: 491-500.
2. Australian Immunisation Handbook. Vaccination for people who are immunocompromised. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccination-for-special-risk-groups/vaccination-for-people-who-are-immunocompromised (accessed February 2024).
3. Australian Immunisation Handbook. Zoster (herpes zoster). Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/zoster-herpes-zoster (accessed February 2024).
4. Bass AR, Chakravarty E, Akl EA, et al. 2022 American College of Rheumatology guideline for vaccinations in patients with rheumatic and musculoskeletal diseases. Arthritis Care Res (Hoboken) 2023; 75: 449-464.
5. Pharmaceutical Benefits Scheme. Pharmaceutical Benefits Advisory Committee (PBAC) meeting outcomes March 2023 PBAC meeting. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/pbac-outcomes (accessed February 2024).
6. Australian Immunisation Handbook. Changes to the recommended use of herpes zoster vaccine to prevent herpes infection. Canberra: Australian Government Department of Health and Aged Care; 2022. Available online at: https://immunisationhandbook.health.gov.au/resources/publications/changes-to-the-recommended-use-of-herpes-zoster-vaccine-to-prevent-herpes-infection (accessed February 2024).
7. Harbecke R, Cohen JI, Oxman MN. Herpes zoster vaccines. J Infect Dis 2021; 224: S429-S442.
8. Curtis JR, Cofield SS, Bridges SL Jr., et al. The safety and immunologic effectiveness of the live varicella-zoster vaccine in patients receiving tumor necrosis factor inhibitor therapy: a randomized controlled trial. Ann Intern Med 2021; 174: 1510-1518.
9. Australian Immunisation Handbook. Measles. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/measles (accessed February 2024).
10. Mulvey P, Duong V, Boyer S, et al. The ecology and evolution of Japanese encephalitis virus. Pathogens 2021; 10: 1534.
11. Australian Government Department of Health and Aged Care. Japanese encephalitis. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://www.health.gov.au/diseases/japanese-encephalitis#:~:text=On%204%20March%202022%2C%20Australia’s,multiple%20states%20on%20mainland%20Australia (accessed February 2024).
12. National guidelines for yellow fever vaccination centres and providers. Canberra: Australian Government Department of Health and Aged Care; 2022. Available online at: https://www.health.gov.au/sites/default/files/2022-12/national-guidelines-for-yellow-fever-vaccination-centres-and-providers.pdf (accessed February 2024).
13. Australian Immunisation Handbook. Yellow fever. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/yellow-fever (accessed February 2024).
14. Strangfeld A, Schafer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2021; 80: 930-942.
15. Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021; 80: 1098-1099.
16. Medeiros-Ribeiro AC, Bonfiglioli KR, Domiciano DS, et al. Distinct impact of DMARD combination and monotherapy in immunogenicity of an inactivated SARS-CoV-2 vaccine in rheumatoid arthritis. Ann Rheum Dis 2022; 81: 710-719.
17. Australian Immunisation Handbook. COVID-19. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/covid-19 (accessed February 2024).
18. Australian Rheumatology Association. Australian clinician guide for the use of immunomodulatory drugs in autoimmune rheumatic diseases at the time of COVID-19 vaccination. Leeton: Australian Rheumatology Association; 2021. Available online at: https://rheumatology.org.au/For-Healthcare-Professionals/Clinical-Resources/COVID-Information (accessed February 2024).
19. Goulenok T, Delaval L, Delory N, et al. Pre-exposure anti-SARS-CoV-2 monoclonal antibodies in severely immunocompromised patients with immune-mediated inflammatory diseases. Lancet Rheumatol 2022; 4: e458-e461.
20. Guidance for the use of anti-SARS-CoV-2 monoclonal antibodies and antiviral agents as prophylaxis or to prevent severe infection from COVID-19 in NSW. Sydney: NSW Agency for Clinical Innovation; 2023. Available online at: https://aci.health.nsw.gov.au/__data/assets/pdf_file/0010/698005/ACI-Guidance-for-use-of-anti-SARS-CoV-2-monoclonal-antibodies-and-antiviral-agents.pdf (accessed February 2024).
21. Sun F, Lin Y, Wang X, et al. Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection. Lancet Infect Dis 2022; 22: 1279.
22. National Institutes of Health (NIH). Drug-drug interactions between ritonavir-boosted nirmatrelvir (Paxlovid) and concomitant medications. Maryland: NIH; 2023. Available online at: https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/ritonavir-boosted-nirmatrelvir--paxlovid-/paxlovid-drug-drug-interactions/ (accessed February 2024).
23. Australian Immunisation Handbook. Influenza. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/influenza-flu (accessed February 2024).
24. Kaine JL, Kivitz AJ, Birbara C, Luo AY. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol 2007; 34: 272-279.
25. Kapetanovic MC, Saxne T, Nilsson JA, Geborek P. Influenza vaccination as model for testing immune modulation induced by anti-TNF and methotrexate therapy in rheumatoid arthritis patients. Rheumatology (Oxford) 2007; 46: 608-611.
26. Gabay C, Bel M, Combescure C, et al; H1N1 Study Group. Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: a prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum 2011; 63: 1486-1496.
27. Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2017; 76: 1559-1565.
28. Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77: 898-904.
29. Influenza and seasonal prophylaxis with oseltamivir. Sydney: NSW Agency for Clinical Innovation; 2022. Available online at: https://aci.health.nsw.gov.au/__data/assets/pdf_file/0012/731010/Evidence-check-Influenza-and-seasonal-prophylaxis-with-oseltamivir.pdf (accessed February 2024).
30. Australian Immunisation Handbook. Pneumococcal disease. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/pneumococcal-disease (accessed February 2024).
31. Clowse ME, Forger F, Hwang C, et al. Minimal to no transfer of certolizumab pegol into breast milk: results from CRADLE, a prospective, postmarketing, multicentre, pharmacokinetic study. Ann Rheum Dis 2017; 76: 1890-1896.
32. Australian Immunisation Handbook. Rotavirus. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/rotavirus (accessed February 2024).
33. Australian Immunisation Handbook. Tuberculosis. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/tuberculosis (accessed February 2024).
34. Australian Rheumatology Association. Vaccinations in rheumatology. Leeton: Australian Rheumatology Association; 2023. Available online at: https://rheumatology.org.au/For-Patients/Vaccinations-in-Rheumatology (accessed February 2024).