Seasonal vaccinations: bracing for winter

Seasonal vaccination is an essential part of preventative medicine and should be routinely considered in a GP consultation. In the post-pandemic setting, guidelines have changed. Additionally, several new vaccine developments offer more opportunities to prevent seasonal viral illnesses.
- The epidemiology of influenza has changed since the COVID-19 pandemic, with an earlier commencement of widely circulating virus. Being aware of this helps inform practitioners and patients alike, and optimise immunisation delivery and effectiveness.
- New seasonal respiratory syncytial virus (RSV) vaccines, along with a monoclonal antibody against RSV, are exciting developments, which will hopefully be followed by further vaccines in the seasonal vaccine armamentarium.
- Seasonal vaccines can be coadministered (given at the same time) with many other vaccines, which can increase opportunities to offer vaccination during routine consultations.
- The landscape of both influenza and COVID-19 continues to change, and keeping up to date with the latest epidemiology and vaccine recommendations is a key component to minimise the impact of these diseases.
The COVID-19 pandemic has highlighted the significant burden of disease viral illnesses cause, both in the acute setting and in the significant long-term sequelae that can occur, such as with long COVID. On top of this, the risk of further pandemics and viral threats is highlighted by cases of highly virulent avian influenza being confirmed in various animal species, including sporadic human cases. This requires continuous surveillance, in conjunction with ongoing monitoring of COVID-19 strains and the main circulating influenza viruses A(H1N1)pdm09 and A(H3N2).1
For medical practitioners, the area of seasonal vaccination has expanded beyond influenza to include consideration of ongoing vaccination against COVID-19 and respiratory syncytial virus (RSV). Along with the increased disease burden that has occurred with the advent of COVID-19, during the 2023 influenza season there were 252,296 laboratory confirmed cases and 376 deaths due to influenza. Up until 1st October 2023, influenza vaccine coverage was only 32%, a decrease from the previous year.2
Along with a yearly influenza vaccine, routine booster doses of COVID-19 are recommended at regular intervals. RSV vaccines are now available on private prescriptions for certain at-risk individuals. Additionally, a novel monoclonal antibody, nirsevimab (Beyfortus), has been developed, which has an extended half-life and the potential to give long lasting passive immunity to newborns when they are vulnerable to the disease.3
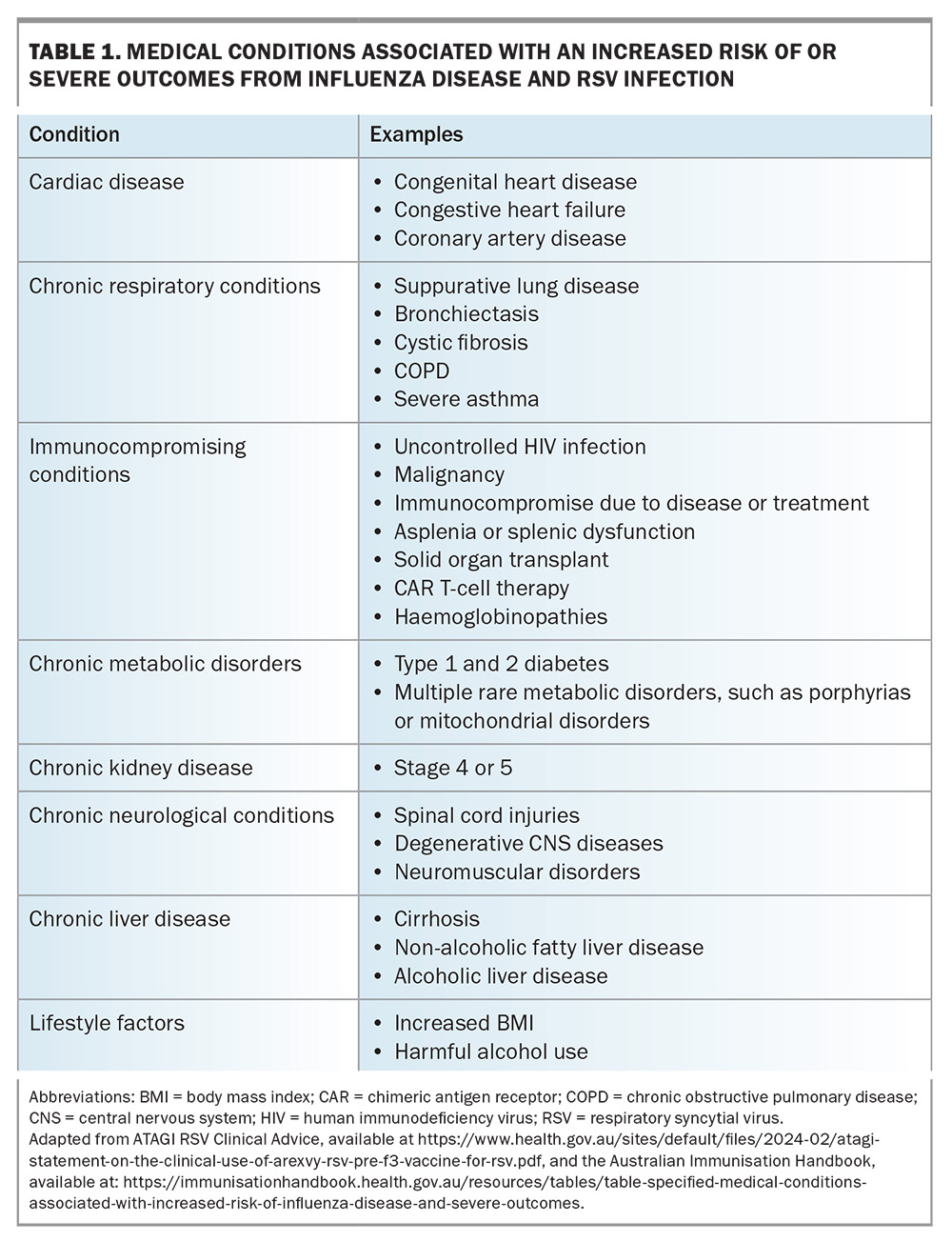
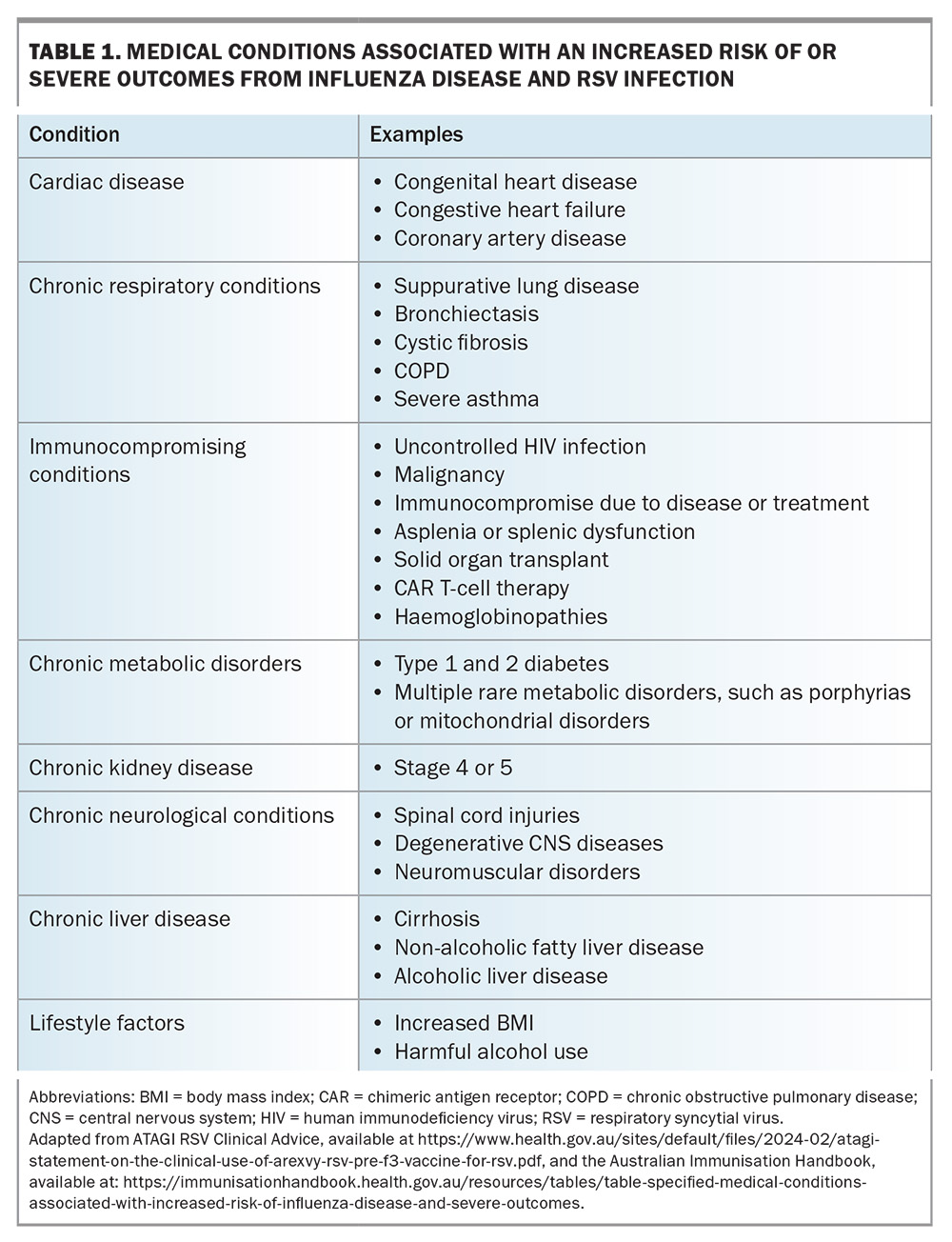
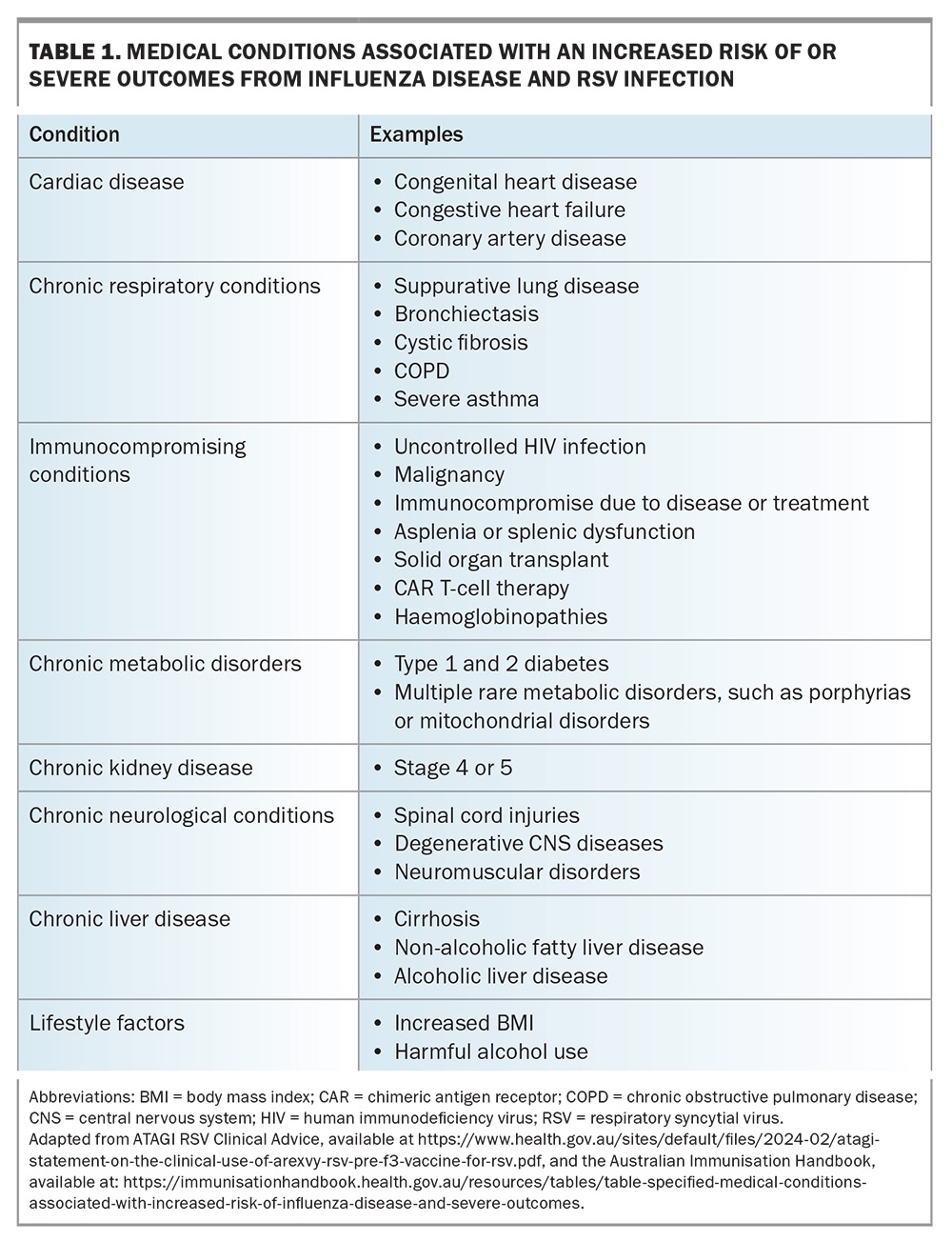
Influenza vaccination is recommended annually for all people aged 6 months and older. In addition, all available seasonal vaccines should be considered on an opportunistic basis during routine consultations, with particular emphasis on high-risk groups. This includes pregnant people, Aboriginal and Torres Strait Islander people, and those with significant comorbidities, such as cardiac disease, chronic respiratory conditions, chronic liver or kidney disease, increased body mass index, harmful substance use and immune compromising conditions (e.g. diabetes). Table 1 provides a comprehensive list of potential diseases that increase the risk of severe disease and poor outcomes with influenza and RSV infections.
Recent legislative changes in Australia require all immunisation providers to report the administration of influenza and COVID-19 vaccines (along with other non-seasonal vaccines) to the Australian Immunisation Register (AIR).4
Box 1 provides an overview of the three vaccine-preventable seasonal diseases that will be discussed in this article.5,6
Influenza vaccination
The onset of the influenza season has been occurring earlier than usual since the pandemic, requiring a shift in preparation time and administration of the vaccines. Every year, a comprehensive assessment of the epidemiology, antigenic and molecular data of circulating influenza strains is undertaken, along with serological responses from the previous season’s vaccines, to help determine the strains that should be incorporated into the seasonal vaccine for the upcoming season. Of note for the 2024-2025 influenza season, viruses of the B/Yamagata lineage have not been identified globally since March 2020 and, as such, there has been a move away from quadrivalent vaccines to trivalent vaccines, which do not include the B/Yamagata lineage. Despite this, the available vaccines for the 2024 influenza season in Australia will be quadrivalent. Based on the Australian Influenza Vaccine Committee (AIVC) assessment for 2024, the components of influenza vaccinations available in Australia for 2024 are as follows:
- egg-based trivalent influenza vaccines should contain:
– A/Victoria/4897/2022 (H1N1)pdm09-like
virus
– A/Thailand/8/2022 (H3N2)-like virus
– B/Austria/1359417/2021 (B/Victoria linage)
like virus - cell-based trivalent vaccines should contain:
– A/Wisconsin/67/2022 (H1N1)pdm09-like
virus
– A/Massachusetts/18/2022 (H3/N2)-like virus
– B/Austria/1359417/2021 (B/Victoria linage)
like virus.
In addition, quadrivalent vaccines should also contain B/Phuket/3073/2013 (B/Yamagata lineage)-like virus.
Although, rarely, there can be a mismatch in the vaccine strains compared with those that end up circulating in a given season, vaccination is associated with a reduced incidence of influenza from 2.3 to 0.9% (risk ratio, 0.41; 95% confidence interval [CI], 0.36-0.47). This corresponds to a number needed to treat of 71.7 A more recent meta-analysis of influenza vaccine effectiveness looked at 191 studies and included 1,221,809 participants. Although efficacy varied significantly according to age group and virus type, overall pooled efficacy for preventing influenza-related hospital admissions was 43.7%.8 In people with breakthrough influenza, vaccination is associated with reduced mortality and disease severity. Studies have shown a 31% mortality reduction and a 26% reduction in intensive care admission.9 The high dose influenza vaccine has been shown to have increased relative vaccine efficacy compared with standard dose vaccination in adults aged 65 years and over.10
The National Immunisation Program (NIP) funds influenza vaccination in the following groups:
- all children aged 6 months to 5 years
- all adults aged 65 years and above
- specific populations aged 5 to 65 years who have certain medical conditions (Table 1), pregnant women and all Aboriginal and Torres Strait Islander people.
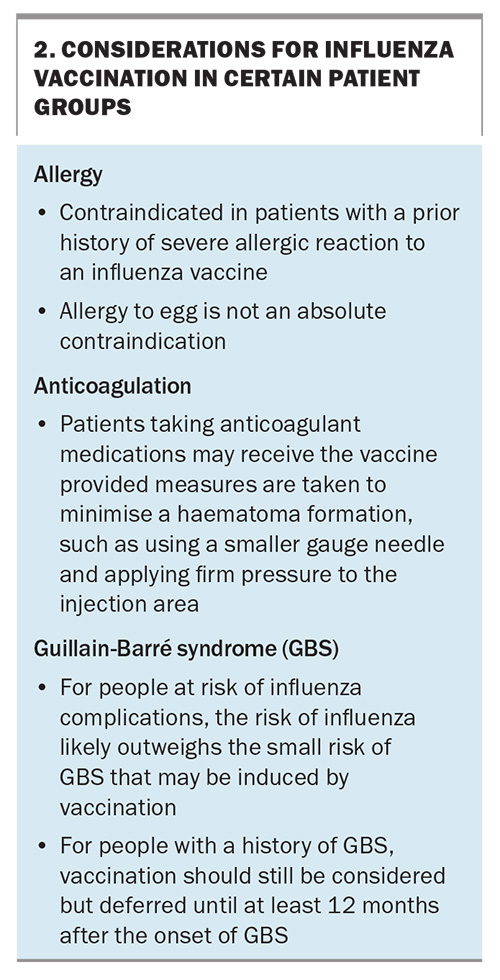
As with all vaccinations, patients should be counselled on the possible adverse reactions to vaccines, including:
- local injection site reactions – the most common adverse reaction; often accompanied by low grade fever, myalgia, headache and fatigue
- shoulder bursitis
- Guillain-Barré syndrome – although it is uncertain delete whether influenza vaccines increase the risk of this condition
- allergic reaction – which is very rare, even among people with an egg allergy.
Influenza vaccines can be coadministered with COVID-19 vaccines, diphtheria, tetanus and pertussis vaccines, pneumococcal and RSV vaccines. In adults aged 65 years and above, in whom the response to vaccines can be reduced because of immunosenescence, both the adjuvanted (Fluad Quad) and high-dose (Fluzone High Dose Quadrivalent) influenza vaccines are preferably recommended over standard dose vaccines.
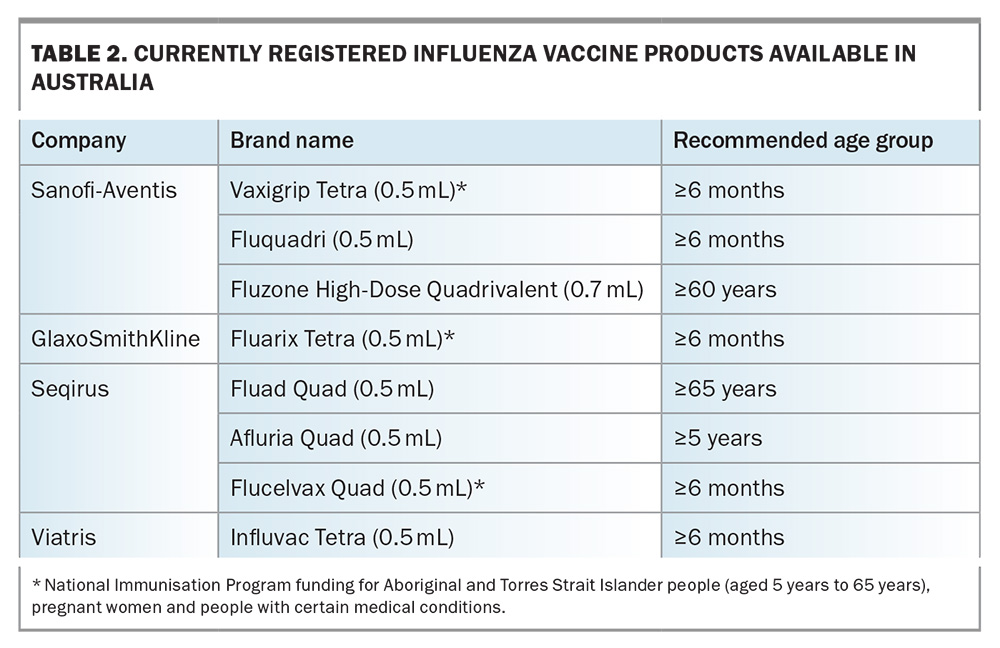
A relatively new development for influenza vaccination is the introduction of cell-based influenza vaccines, with Flucelvax being the first one to be available on the NIP for vulnerable patients.11 These vaccines can more closely match recommended vaccine strains than egg-based vaccines, as the possibility of adaptation occurring in the eggs in which the virus is traditionally grown is eliminated. A recent meta-analysis of 18 studies over three influenza seasons (between 2017 and 2020) showed a relative increased pooled efficacy of 8.4% (95% CI, 6.5-10.2%) compared with egg-based vaccines.12Table 2 lists the currently available influenza vaccines in Australia. Some considerations required for certain patient groups are outlined in Box 2. More detail on influenza vaccines can be found in the article ‘Bracing for impact: navigating the complexities of the 2024 influenza season in Australia’ in this same issue of Medicine Today pages 39-47.
COVID-19 vaccination
COVID-19 vaccination remains highly efficacious despite changing circulating viral strains. A systematic review and meta-analysis of 28 randomised controlled trials published between 1 January 2020 and 12 September 2022 included 286,915 people in the vaccination group and 233,236 people in the placebo group. The efficacy of preventing hospitalisation was 95.3% (95% CI, 88.0-98.7), preventing severe infection was 90.8% (CI, 85.5-95.1)and preventing death was 85.8% (CI, 68.7-94.6).13
It is not currently clear how the burden of COVID-19 will change and whether a clear seasonal pattern of disease will emerge, with many new variants evolving and changing the epidemiology of the virus. Currently, COVID-19 vaccination can be considered for people aged 18 years and older, and for children aged six months to 18 years with severe immunocompromise or increased risk of severe disease. Ongoing booster doses are recommended with a frequency that depends on a person’s age and level of immunocompromise. Currently, this is as follows:
- children younger than 5 years: not recommended
- children and adolescents aged between 5 and 17 years: every 12 months if severely immunocompromised
- people aged 18 to 64 years: every 12 months (or six-monthly if severely immunocompromised)
- people aged 65 to 74 years: every 12 months (or six-monthly if there is increased concern)
- people aged 75 years and older: every six months.14
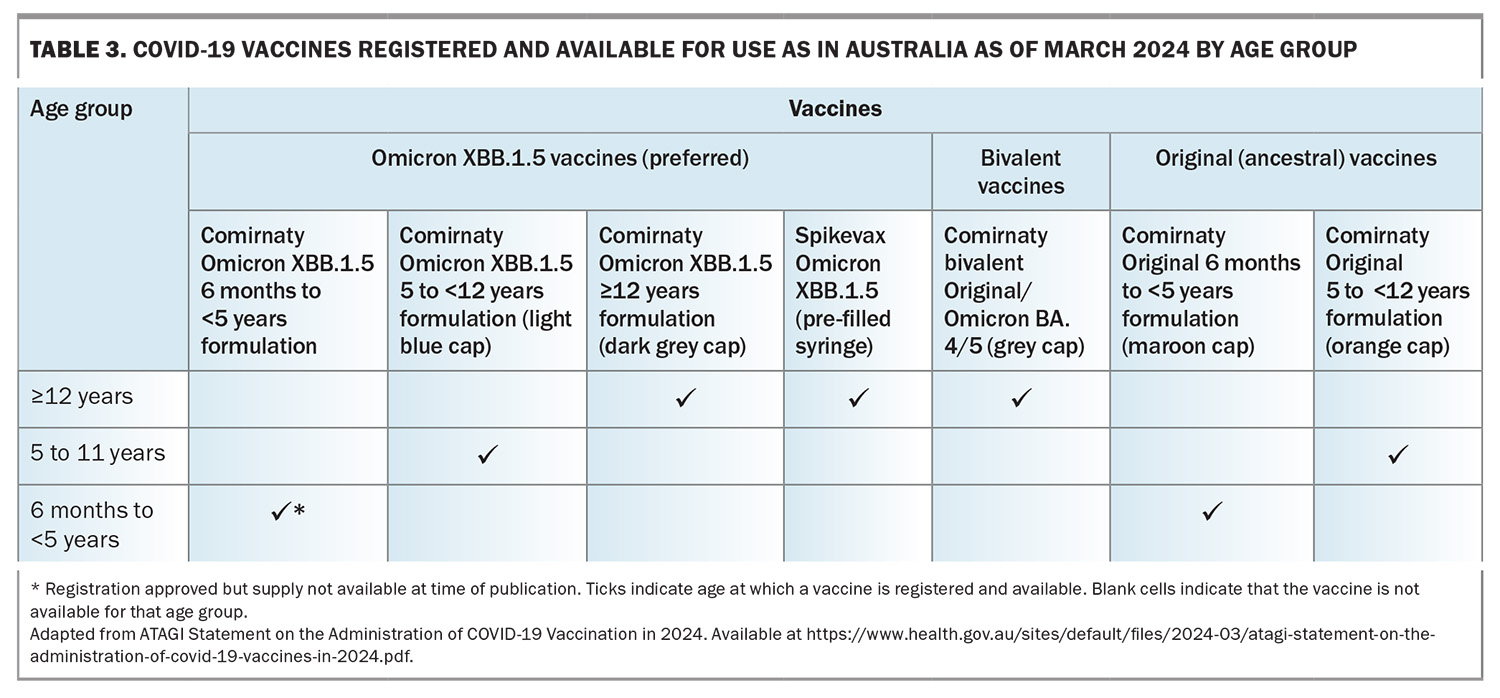
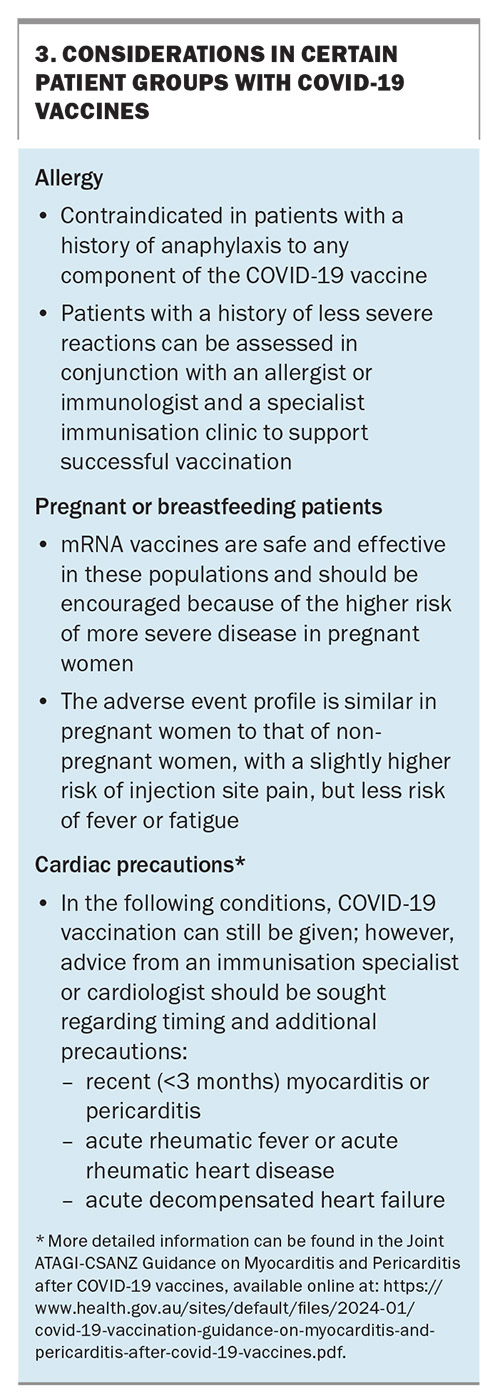
Currently, COVID-19 vaccines are funded for all eligible individuals and a Medicare card is not required. Given the change in circulating strains that are present in Australia going into winter 2024, Omicron XBB.1.5-based vaccines are the preferred vaccines to be administered as a booster dose. These vaccines continue to have good efficacy against new variants, such as JN.1.15 Despite this, currently an XBB.1.5-containing vaccine is not available for children aged 6 months to 5 years; however, a formulation has been approved for use in this age group and is expected to become available during 2024. The currently available COVID-19 vaccines are summarised in Table 3. Considerations of certain patient groups and COVID-19 vaccines are listed in Box 3.16
RSV vaccination and monoclonal antibody
Nirsevimab is a novel long-acting monoclonal antibody, which has recently received TGA approval for use in infants from birth, and for children in their second year of life who have conditions that increase their risk of severe RSV disease. Currently, there are state-based RSV programs and for 2024, the funded groups are:
- Queensland and Western Australia: all newborn infants and medically at-risk children
- New South Wales: medically at-risk infants and children.17
Nirsevimab is a long-acting monoclonal antibody that binds to the prefusion conformation of the F protein of RSV and has been modified with an amino acid substitution in the Fc region to extend its half-life.3 Clinical trial data for nirsevimab among healthy infants born preterm (between 29 and 35 weeks’ gestation) or late preterm to term (35 weeks’ and above gestation), entering their first RSV season, has shown a reduced risk of hospitalisation for RSV-associated lower respiratory tract disease (LRTD) by 78.4% and 76.8%, respectively, over 150 days post administration.18
For older adults (aged 60 years and over), Arexvy is now available via a private prescription. This is an adjuvanted recombinant RSV vaccine targeting the prefusion F protein and is administered as a single dose. Its efficacy over two seasons following one dose of the vaccine has been found to be 67.2% (97.5% CI, 48.2-80.0) against RSV-LRTD and 78.8% (95% CI, 52.6-92.0%) against severe RSV-LRTD.19 Pain at the injection site and fatigue have been the most reported adverse effects. No difference in rates of serious adverse events has been found in trial participants aged 60 years and over compared with placebo.20 Studies are currently ongoing to determine its efficacy over multiple seasons, the need for and timing of revaccination, and the potential benefit in younger age groups.
Currently, ATAGI recommends RSV vaccination in the following groups:
- all adults aged 75 years and over
- Aboriginal and/or Torres Strait Islander people aged between 60 and 74 years
- adults aged 60 to 74 years with medical conditions that increase the risk of severe disease due to RSV (Table 1).
Although the development of an RSV vaccine is an exciting advancement, there will likely be some challenges to overcome because it is a completely new vaccine for a disease that previously had no approved vaccines. First, there may be an underappreciation of the significance of RSV, which can be coupled with concerns about the possible adverse effects of the vaccine. Second, optimal timing of vaccine administration, along with the durability of protection, needs to be defined to help determine ongoing RSV vaccination strategies. Lastly, the cost of the vaccine, which has a list price of US$280 per dose, will be a significant hurdle to patients initially, prior to any potential NIP funding.21
A second RSV vaccine, Abrysvo, has been approved for older adults (aged 60 years and over) and pregnant women (24 to 36 weeks’ gestation) for the prevention of RSV LRTD in infants from birth through 6 months of age by passive immunisation through the placenta from the mother; however, at the time of writing, it is not available on the market.22 More detail on RSV vaccines and monoclonal antibody can be found in the article ‘The battle against RSV in Australia begins: new preventives are here’ in this same issue of Medicine Today pages 49-54.
Strategies to increase vaccination
Before we enter the winter season, we have an opportunity to offer patients a chance to reduce their risk of severe disease. Unfortunately, vaccine hesitancy and misinformation has increased since the COVID-19 pandemic, and being well-informed is a key component of countering this. Some suggested strategies to help increase vaccination rates in general practice include: recommending vaccination to all eligible patients opportunistically, coadminister vaccines when safe, implement a practice-based notification and recall program, and record all vaccines on the AIR (which is now a legislative requirement for many vaccines).23
Conclusion
Vaccination remains one of our most effective medical interventions to prevent infectious diseases. In particular, influenza, COVID-19 and RSV are important vaccine-preventable diseases against which efficacious vaccines and monoclonal antibodies are available. Armed with the most updated knowledge to counter growing misinformation, clinicians are uniquely placed to educate, encourage and recommend vaccination to their patients. MT
COMPETING INTERESTS: Professor Griffin has received speaker honoraria from Seqirus. Dr Armstrong and Dr Spence: None.
ACKNOWLEDGEMENT: The authors would like to thank Lydia McLennan for reviewing the manuscript.
References
1. World Health Organisation (WHO). Influenza at the human-animal interface. Geneva: WHO; 2024. Available online at: https://cdn.who.int/media/docs/default-source/influenza/human-animal-interface-risk assessments/influenza_summary_ira_ha_interface_march_2024.pdf sfvrsn=a60a050c_3&download=true (accessed April 2024).
2. Australian Government Department of Health and Aged Care (DoHAC). Australian Influenza Surveillance Report – 2023 End of Season Summary. Canberra: Australian Government DoHAC; 2024. Available online at: https://www.health.gov.au/sites/default/files/2023-12/aisr-2023-national-influenza-season-summary.pdf (accessed April 2024).
3. Australian Technical Advisory Group on Immunisation (ATAGI). Statement on the use of Nirsevimab for prevention of severe disease due to respiratory syncytial virus (RSV) in infants. Canberra: ATAGI; 2024. Available online at: https://www.health.gov.au/sites/default/files/2024-03/atagi-statement-on-nirsevimab-2024.pdf (accessed April 2024).
4. Australian Government DoHAC. Mandatory reporting changes to the Australian Immunisation Register (AIR) from 1 March 2024. Canberra, DoHAC; 2024. Available online at: https://www.health.gov.au/news/mandatory-reporting-changes-to-the-australian-immunisation-register-from-1-March-2024 (accessed April 2024).
5. WHO. Coronavirus Disease (COVID-19). Geneva: WHO; 2024. Available online at: https://www.who.int/news-room/fact-sheets/detail/coronavirus-disease-(covid-19) (accessed April 2024).
6. WHO. Coronavirus disease (COVID-19) pandemic overview. Geneva: WHO; 2024. Available online at: https://www.who.int/europe/emergencies/situations/covid-19 (accessed April 2024).
7. Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2: CD001269.
8. Guo J, Chen X, Guo Y, et al. Real-world effectiveness of seasonal influenza vaccination and age as effect modifier: A systematic review, meta-analysis and meta-regression of test-negative design studies. Vaccine 2024; 42: 1883-1891.
9. Ferdinands JM, Thompson MG, Blanton L, Spencer S, Grant L, Fry AM. Does influenza vaccination attenuate the severity of breakthrough infections? A narrative review and recommendations for further research. Vaccine 2021; 39: 3678-3695.
10. Lee JKH, Lam GKL, Shin T, Samson SI, Greenberg DP, Chit A. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: An updated systematic review and meta-analysis. Vaccine 2021; 39 Suppl 1: A24-a35.
11. News GP. First cell-based flu vaccine added to National Immunisation Program. Sydney: News GP; 2024. Available online at: https://www1.racgp.org.au/newsgp/clinical/first-cell-based-flu-vaccine-added-to-national-imm#:~:text=First%20cell%2Dbased%20flu%20vaccine%20added%20to%20National
%20Immunisation%20Program,-Jolyon%20Attwooll&text=The%20locally%20made%20Flucelvax%20
QUAD,vulnerable%20cohorts%20aged%205%E2%80%9364 (accessed April 2024).
12. Coleman BL, Gutmanis I, McGovern I, Haag M. Effectiveness of cell-based quadrivalent seasonal influenza vaccine: a systematic review and meta-analysis. Vaccines 2023; 11: 1607.
13. Yang ZR, Jiang YW, Li FX, et al. Efficacy of SARS-CoV-2 vaccines and the dose-response relationship with three major antibodies: a systematic review and meta-analysis of randomised controlled trials. The Lancet Microbe 2023; 4: e236-e246.
14. Australian Government DoHAC. COVID-19 vaccine advice and recommendations for 2024. Canberra: DoHAC; 2024. Available online at: https://www.health.gov.au/our-work/covid-19-vaccines/getting-your-vaccination/booster-doses (accessed April 2024).
15. Infectious Diseases Society of America. Will COVID Vaccines Continue to Work Against JN.1 and Other New Variants? Available online at: https://www.idsociety.org/covid-19-real-time-learning-network/vaccines/
will-covid-vaccines-continue-to-work-against-jn.1-and-other-new-variants#/+/0/publishedDate_na_dt/desc/ (accessed April 2024)
16. Australian Immunisation Handbook. COVID-19. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/covid-19#recommendations (accessed April 2024).
17. National Centre for Immunisation Research and Surveillance Australia. Respiratory syncytial virus (RSV): Frequently asked questions (FAQs). Available online at: https://ncirs.org.au/ncirs-fact-sheets-faqs-and-other-resources/respiratory-syncytial-virus-rsv-frequently-asked (accessed April 2024).
18. Muller WJ, Madhi SA, Seoane Nuñez B, et al. Nirsevimab for prevention of RSV in term and late-preterm infants. N Engl J Med 2023; 388: 1533-1534.
19. Ison MG, Papi A, Athan E, et al. Efficacy and safety of Respiratory Syncytial Virus (RSV) prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons. Clin Infect Dis 2024; ciae010. Online ahead of print.
20. ATAGI. Statement on the Clinical use of Arexvy (RSV Pre-F3) Vaccine for the Prevention of Respiratory Syncytial Virus (RSV) Disease in Older Adults in Australia. Canberra; ATAGI: 2024. Available online at: https://www.health.gov.au/sites/default/files/2024-02/atagi-statement-on-the-clinical-use-of-arexvy-rsv-pre-f3-vaccine-for-rsv.pdf (accessed April 2024).
21. GSK for you. AREXVY. Available online at: https://www.gskforyou.com/gsk-pricing-information/ (accessed April 2024).
22. Therapeutic Goods Administration (TGA). ABRYSVO recombinant respiratory syncytial virus pre-fusion F protein 120 microgram/0.5 mL bivalent vaccine powder for injection vial + diluent syringe (406624). TGA; Canberra, 2024. Available online at: https://www.tga.gov.au/resources/artg/406624 (accessed April 2024).
23. Van Buynder P. Enhancing influenza vaccination in older people. Medicine Today 2019; 20: 42-47.