The battle against RSV in Australia begins – new preventives are here
With winter fast approaching, respiratory syncytial virus (RSV) infections are expected to peak. A range of new preventives against RSV have been approved for use in Australia. These include the monoclonal antibody nirsevimab for infants and two active vaccines for older adults aged 60 years and over, one of which is also approved for pregnant women to offer passive immunisation to the baby.
- Severe respiratory syncytial virus (RSV) infection affects those at the extremes of age.
- New preventive therapies have been approved and are now available to protect against RSV infection.
- The monoclonal antibody nirsevimab offers passive immunisation to infants and is approved for newborns in their first RSV season and at-risk infants up to the age of 24 months.
- Two recombinant RSV vaccines are approved in Australia: both are approved for older adults aged 60 years and over, and one is also approved for pregnant women to provide passive immunisation of infants from birth to 6 months of age.
- Three Australian states have introduced nirsevimab programs, which are free, but they differ in regard to eligibility. A national funding program is not yet established.
As the 2024 Australian autumn stretches into winter, cases of the usual respiratory viruses will once again rapidly rise, affecting all age groups, but the most severely affected populations will be very young children and older adults. This year for the first time ever, new measures are available to battle one of these viruses, respiratory syncytial virus (RSV), so called for its ability to form cellular ‘syncytia’ which enable the virus to fuse infected cells together. It has been a long journey from the discovery of RSV in 1956 to the availability of effective preventives being rolled out in Australia and globally: monoclonal antibody immunoprophylaxis for newborns, vaccines for pregnant women to protect their babies and vaccines for older people will be available for the first time for the Australian 2024 RSV season.
RSV symptoms and severity
RSV is a common cause of upper and lower respiratory tract infection in humans. Because infection with RSV does not confer long-lasting protection, it can cause infections throughout one’s lifetime. The highest burden of RSV disease is among very young children and older adults (those aged 60 years and over). RSV infections can be mild, causing upper respiratory tract symptoms such as cough, sore throat and nasal congestion, or more severe, causing lower respiratory tract symptoms including wheezing and breathing difficulty. Symptoms peak around five days after infection and often improve between seven and 10 days. Increasing age and the presence of certain medical conditions may affect the severity of infection.
In children, the primary RSV infection usually occurs by 2 years of age and is a leading cause of hospitalisation in infants aged less than 6 months due to lower respiratory tract infection and bronchiolitis. Although children with certain medical conditions are at higher risk of severe disease, most RSV-related hospitalisations occur in children who are otherwise healthy.
The major disease burden of RSV in children under 5 years of age occurs in low- and middle-income countries (LMIC), with an estimated 95% of RSV-associated acute lower respiratory infection episodes and more than 97% of the global RSV-attributable deaths across all age bands occurring in LMIC.1 Available preventives are prohibitively expensive and will have to be heavily discounted or subsidised if they are going to be used in LMIC. RSV vaccines for older adults are also likely to be out of reach for most, if not all, LMIC.
There are no effective drugs or therapies for RSV infections and most people with mild symptoms recover without treatment, whereas those who are hospitalised are generally given only supportive care, such as supplementary oxygen, hydration (oral or intravenous) and nutrition, with a small proportion requiring mechanical ventilation or ICU admission.
RSV can be diagnosed by clinical examination in very young children, but older children and adults generally require a diagnostic test such as a respiratory multiplex PCR-based test. Home-use rapid antigen tests for RSV are available, but are not as sensitive as laboratory tests.
RSV epidemiology – the recent situation
In July 2021, RSV was made a notifiable disease in Australia in order to improve surveillance; however, as states were variable in legislating this change, laboratory confirmed RSV as recorded on the National Notifiable Diseases Surveillance System (NNDSS) is only robust from 2023 onwards dashboard (https://nindss.health.gov.au/pbi-dashboard/). Nevertheless, even with this limited data set, the sheer number of cases reported is significant. In 2023, 128,015 cases of RSV were reported, with 50.3% occurring in the 0- to 4-year-old age group, while those aged 60 years and over contributed 27,466 cases (21.5%). Two genetically distinct but clinically similar types of RSV cocirculate (RSV-A and RSV-B); however, the proportion varies across time and locations and is not reported in the NNDSS. In Australia in 2020-2021, RSV-A was the predominant circulating strain, while in 2022-2023, RSV-B was predominant.2
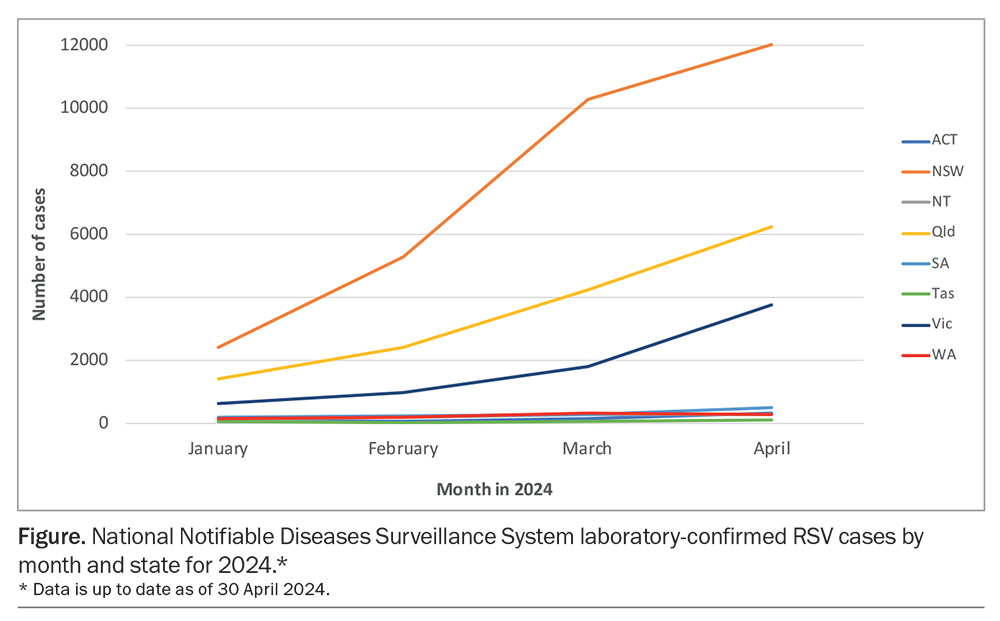
Although RSV cases can be detected all year round, they are most prevalent in the autumn–winter period. By contrast, in the years following the COVID-19 outbreak (2020-2021), major outbreaks of RSV were detected over the summer months in several Australian states.2 Although RSV circulation has returned to its usual seasonal timing, significant variation in peak infections are still seen between states. In 2023, the seasonal peak in New South Wales occurred earlier (April to May) compared with other states, including Queensland (peaking May to June), Victoria (June), South Australia (July) and Western Australia (August). So far in 2024, RSV detection has risen month on month, with rapid increases in cases in NSW, Queensland and Victoria in February and March, along with more modest rises in other states (Figure), with the 0- to 4-year-old age group accounting for most laboratory confirmed cases.
Preventive programs aimed specifically at newborns are unlikely to significantly reduce the overall number of NNDSS laboratory confirmed RSV cases; however, a reduction in paediatric hospitalisations may be seen in those states with full passive immunisation programs. Moving forward, the continued use of the NNDSS database to record cases of RSV, along with data on hospitalisations due to RSV, will be essential to ensure that the new preventives are performing as expected and providing an appropriate public cost benefit. How each state and territory fares for the remainder of the 2024 RSV season will be of intense interest.
What is being introduced; when, where and why?
Passive immunisation of infants with the monoclonal antibody nirsevimab
The major change to the RSV landscape in Australia for 2024 is the adoption in some states of large ‘passive immunisation’ programs in newborns and high-risk infants with the monoclonal antibody nirsevimab. This treatment is based on the delivery of RSV-specific antibodies to newborn babies to provide protection in the first few months of life when the risk of severe disease is greatest. Unlike active immunisation, passive immunisation provides relatively short-lived protection and no immunological memory is developed. Nirsevimab, like most recent human monoclonal antibodies, has been modified to prolong its half-life in vivo to enable protection to last for up to six months.
Nirsevimab is directed to the critical fusion protein (pre-F form) of RSV, a highly conserved viral surface glycoprotein that fuses the viral and host cell membranes during viral entry.3 It is effective against both RSV-A and RSV-B types. The antibody is given as a single intramuscular injection of either 50 mg in 0.5 mL (for infants <5 kg) or 100 mg in 1 mL (for infants ≥5 kg). A single dose of nirsevimab, given via injection, is expected to protect infants for at least five months, covering them for the duration of an average RSV season, which is supported by clinical trial results.3
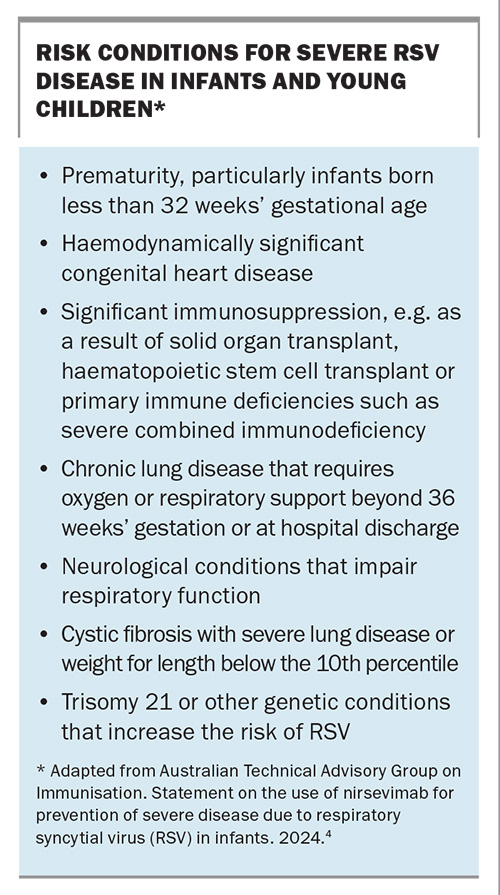
The cost of these treatments in the US is about US$495 for either dosage form, but is lower in Europe with government sponsored programs. In Australia, three states to date – WA, Queensland and NSW – have announced subsidised nirsevimab programs for the 2024 RSV season. Unlike in the US, nirsevimab is not currently available outside of these programs or on the private market in Australia. An application for nirsevimab to be listed on the PBS is pending the July 2024 Pharmaceutical Benefits Advisory Committee meeting (https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/agenda/pdf/2024/PBAC-meeting-agenda-July-2024-v2.pdf) and the outcome will determine whether a national program will be introduced for the 2025 RSV season. Risk conditions for severe RSV disease in infants and young children are listed in the Box.4
In an Australian first, the WA Department of Health announced on 5 March 2024 that it was providing free nirsevimab immunisation for eligible children (those born on or after 1 October 2022) from 1 April 2024 to 30 September 2024.5 They also noted that: ‘While the program is available until September, providers are urged to prioritise immunisation in April and May to ensure children whom are eligible receive the nirsevimab prior to or at the start of the RSV season.’ The program will be delivered through participating birth hospitals, general practices, Aboriginal Medical Services and community health immunisation clinics, but will not be offered through community pharmacies.6 The cost of this program was estimated to be about AUD$11 million and is expected to prevent around 700 RSV-related infant hospitalisations in 2024 in WA.
The Queensland government followed shortly after WA, announcing on 25 March 2024 that they would be introducing a free, state-wide nirsevimab immunisation program for 2024 beginning in April (until 31 October 2024) for all eligible infants and young children at a cost of $31 million. Those who are eligible include all newborn infants, who will be offered a dose at birth or prior to discharge from hospital, infants under 8 months of age born on or after the program commencement date who were not immunised in hospital and infants aged 8 to 19 months inclusive with certain complex medical conditions.7
On the same day as the Queensland government’s announcement, the NSW government announced a more limited free nirsevimab rollout for 2024 costing $3.1 million. Eligible children are those most vulnerable to RSV and include premature infants (less than 37 weeks’ gestation at birth) born after 31 October 2023 and other vulnerable infants, including those with neonatal lung disease, congenital heart disease and other immunodeficiency and genetic disorders.8 In addition, infants and children in certain circumstances can replace palivizumab (a first-generation monoclonal antibody directed to the F protein of RSV) with nirsevimab. Infants who have had a prior laboratory-confirmed RSV infection in 2024 are excluded from the program.
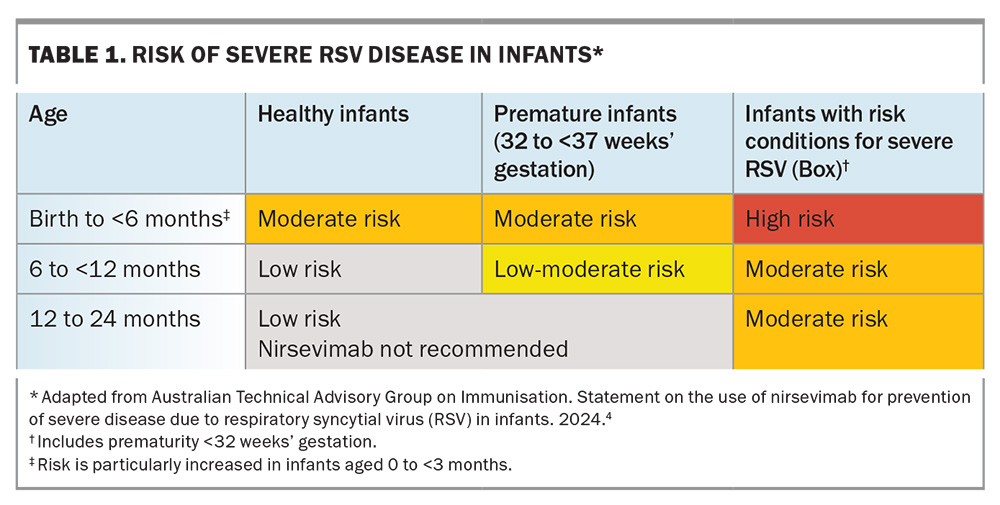
On 26 March 2024, the Australian Technical Advisory Group on Immunisation (ATAGI) issued a statement on the clinical use of nirsevimab for the prevention of severe diseases due to RSV in infants.4 This document also included an assessment of the relative risks of RSV infection by age, health status and prematurity (Table 1).4
So why this apparent rush to rollout nirsevimab in some states of Australia? The answer is simple: it works incredibly well in preventing RSV infections and subsequent hospitalisations in infants. The HARMONIE phase 3b clinical trials, showed that nirsevimab protected infants against hospitalisation for RSV-associated lower respiratory tract infection (efficacy, 83.2%; 95% confidence interval [CI], 67.8-92.0; p<0.001) and against very severe RSV-associated lower respiratory tract infection (efficacy, 75.7%; 95% CI, 32.8-92.9; p=0.004).9
Similar real-world results from the use of nirsevimab in the Northern Hemisphere 2023-2024 RSV season supported the clinical trials results. In the US, among infants entering their first RSV season (between October 2023 and February 2024) nirsevimab was reported as 90% effective (95% CI, 75-96) against RSV-associated hospitalisation, with a median time from receipt to symptom onset of 45 days (interquartile range, 19-76 days).10 Similar studies in Spain over the same time period showed a pooled nirsevimab effectiveness against RSV-associated lower respiratory tract infection hospital admissions of 84.4% (95% CI, 76.8-90.0).11
It is also worth noting that the proof of principle for monoclonal antibody-derived passive immunity has been long established with the use of palivizumab, a first-generation monoclonal antibody RSV immunoprophylaxis. Although palivizumab is effective, its monthly dosage requirements and high cost make its inclusion in RSV prevention programs difficult and its use is limited to only the highest at-risk infants.12
Other RSV preventives on offer in 2024 in Australia
Active immunisation for older adults and pregnant women
RSV vaccines for selected groups are or will soon be available. These vaccines differ from the passive immunity provided by nirsevimab and are designed to generate a more traditional ‘active’ immunity, along with immunological memory after vaccination. To date, the TGA has approved two RSV vaccines:
- a vaccine for older adults aged 60 years and over, approved 8 January 2024 (RSVPreF3 OA; Arexvy)13
- a vaccine for older adults (aged 60 years and over) and pregnant women (24 to 36 weeks’ gestation) for the prevention of RSV lower respiratory tract disease in infants from birth through 6 months of age by passive immunisation through the placenta from the mother, approved March 2024 (RSVpreF vaccine; Abrysvo).14
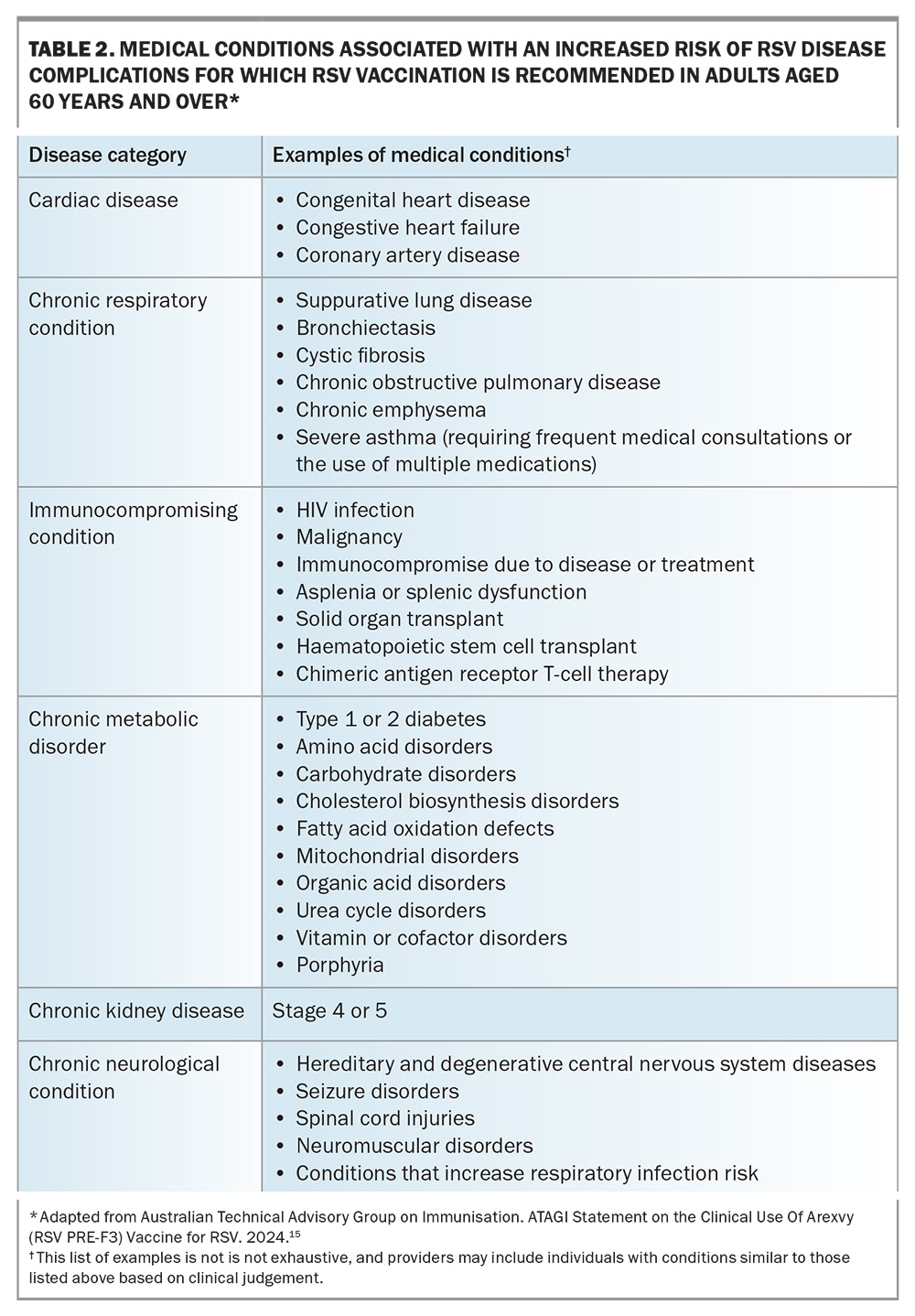
Both vaccines contain a recombinant, stabilised RSV pre-F protein (120 mcg per dose) given as a single intramuscular dose, and are effective against RSV-A and RSV-B. Arexvy also contains the adjuvant ASO1E (25 mcg of detoxified monophosphoryl lipid A [MPL] and 25 mcg of detoxified saponin derivative [QS-21]), which is mixed with the pre-F freeze dried material before administration. Medical conditions that ATAGI recommend RSV vaccination for are listed in Table 2.15
Arexvy is now available on the private market in time for the 2024 Australian RSV season and costs between $310 and $350 a dose. It is registered for adults aged 60 years and over and is not approved for use in people aged less than 60 years or in pregnancy. ATAGI have published a statement on the clinical use of Arexvy for RSV.15 It remains to be seen if Abrysvo will make it to the market in time for this RSV season due to its later approval.
In clinical trials of adults aged 60 years and over who received a single dose of Arexvy, vaccine efficacy after the first RSV season was 82.6% (95% CI, 57.9-94.1) against laboratory confirmed RSV-related lower respiratory tract disease (LRTD), 94.1% (95% CI, 62.4-99.9) against severe RSV-related LRTD and 71.7% (95% CI, 56.2-82.3) against RSV-related acute respiratory infection.16 Follow-up trials showed that one Arexvy dose was still efficacious, albeit reduced, over a second RSV season, with vaccine efficacies of 67.2% (97.5% CI, 48.2-80.0) against RSV-LRTD, 78.8% (95% CI, 52.6-92.0) against severe RSV-LRTD and 52.7 (95% CI, 40.0-63.0) against RSV-related acute respiratory infection over a median follow-up of 6.3 months during season two. Revaccination before the second RSV season did not improve the efficacy in any of these groups.17
Similarly, the efficacy of one dose of Abrysvo against severe RSV-associated LRTD, defined as having three or more symptoms, in adults aged 60 years and over extended over two RSV reasons, with an efficacy of 88.9% (95% CI, 53.6-98.7) in the first and 77.8% (95% CI, 51.4-91.1) in the second season.18 Vaccine efficacy against less severe RASV-LRTD, defined as having two or more symptoms, was 65.1% (95% CI, 35.9-82.0) after season one and 55.7% (95% CI, 34.7-70.4) after the end of the second season.18
Questions remain as to whether older adults will accept and take up these new RSV vaccines, given both vaccines had reduced efficacy in the second year after the initial vaccination, and no increased protection was seen after revaccination with Arexvy. The US Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) benefit–risk analysis compared the risk of a range outcomes as a result of RSV infections, from seeking medical care to hospitalisation and death, with the estimated small increase in risk of Guillain–Barré syndrome (GBS) associated with both vaccines.19 The benefits of vaccination increased with age, with those aged 80 years and over having the greatest benefit. Their summary based on available data for both vaccines includes the following:19
- from a population perspective, the estimated benefits of RSV vaccination outweigh the potential risk of GBS in adults aged 60 years and over
- 10 to 25 cases of GBS per million doses administered are estimated (95% CI, 7-43) compared with a background rate of five cases per million doses administered
- estimated benefits of RSV vaccination vary by age group and RSV incidence
- individual-level risk for severe RSV disease and timing of vaccination relative to the RSV season likely affect estimated benefits
- there is substantial uncertainty in estimates of both benefit and risk.
A report presented to the CDC ACIP on 29 February 2024 on the post-licensure safety monitoring of RSV vaccines in adults aged 60 years and over concluded: ‘Early data from VSD [Vaccine Safety Datalink] suggest the potential for an increased rate for GBS after GSK (Arexvy) RSV vaccine, but additional analyses are needed to further assess this potential risk; insufficient doses of Pfizer (Abrysvo) RSV vaccine used in VSD to inform risk.’20 Therefore, although the overall risk profile for older adults appears favourable, with both products having mostly minor local and systemic adverse side effects,21,22 longer-term neurological issues such as GBS will need to be monitored to ensure these vaccines provide sufficient health benefits to justify their use in all older age groups.
The introduction of the maternal RSV vaccine, including whether it will be used alongside or instead of nirsevimab, and the individual and state/federal government provider preferences for use over or with nirsevimab remains to be seen and may differ between states depending on availability, cost, logistics of rollout, continued effectiveness and accumulated safety data.
Other RSV preventives
A third mRNA-based vaccine for older adults that encodes the pre-F protein is currently under consideration by the TGA.23 New monoclonal antibodies are also in development, including clesrovimab, which is completing phase 3 trials in 2024, and a candidate human IgG1 monoclonal antibody, RSM01 that targets site Ø of the pre-F of RSV and has the added half-life extended mutations.24,25 Other RSV vaccines and monoclonal antibodies are currently undergoing clinical trials to cover older adults and pregnant women, as well as other groups susceptible to RSV in the community who are not covered by the current range of preventives. The PATH RSV website provides a summary of the global developments in preventive and therapeutic approaches to RSV, including new vaccine and monoclonal antibody candidates (https://www.path.org/our-impact/resources/rsv-vaccine-and-mab-snapshot/).
Conclusion
2024 is now a watershed year for the prevention of RSV in Australia, with state-funded passive immunisation programs available for newborn infants in three states, as well as the availability of active immunisation for older adults and potentially pregnant women nationally. An interesting experiment is evolving this RSV season in infants, with five states and territories opting not to offer nirsevimab to any infants, while two states (WA and Queensland) will have universal programs and NSW will offer a very limited program focussing on the highest risk infants. Data from these programs will be instrumental in determining the future use of RSV immunoprophylaxis for infants and the cost-benefit of these programs. The current lack of government subsidies and poor public knowledge of RSV and its clinical consequences are likely to hamper the uptake of maternal and older adult-targeted RSV vaccines, and further education will be needed in the future to ensure that the public better understands the relative benefits and risks involved. Government funding will also be essential to avoid inequities in the availability of these products. MT
COMPETING INTERESTS: None.
References
1. Li Y, Wang X, Blau DM, et al; Respiratory Virus Global Epidemiology Network; Nair H; RESCEU investigators. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022; 399: 2047-2064.
2. Eden JS, Sikazwe C, Xie R, et al; Australian RSV study group. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat Commun 2022; 13: 2884.
3. McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol. 2013; 372: 83-104.
4. Australian Technical Advisory Group on Immunisation (ATAGI). Statement on the use of nirsevimab for prevention of severe disease due to respiratory syncytial virus (RSV) in infants. Department of health and aged care; Canberra, 2024. Available online at: https://www.health.gov.au/sites/default/files/2024-03/atagi-statement-on-nirsevimab-2024.pdf (accessed April 2024).
5. Western Australian children first to access protection from RSV. Government of Western Australia; Perth, 2024. Available online at: https://www.wa.gov.au/government/media-statements/Cook-Labor-Government/Western-Australian-children-first-to-access-protection-from-RSV-20240305 (accessed April 2024).
6. Respiratory Syncytial Virus (RSV) immunisation. Government of Western Australia; Perth, 2024. Available online at: https://www.health.wa.gov.au/Articles/N_R/Respiratory-syncytial-virus-RSV-immunisation (accessed April 2024).
7. Free RSV immunisation program for Queensland infants and young children. Queensland Government; 2024. Available online at: https://statements.qld.gov.au/statements/99963 (accessed April 2024).
8. NSW Health. Frequently asked questions about Beyfortus™ (nirsevimab) for health professionals. NSW Government; Sydney,2024. Available online at: https://www.health.nsw.gov.au/immunisation/pages/nirsevimab-professionals.aspx (accessed April 2024).
9. Drysdale SB, Cathie K, Flamein F, et al; HARMONIE Study Group. Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N Engl J Med 2023; 389: 2425-2435.
10. Moline HL, Tannis A, Toepfer AP, et al. New Vaccine Surveillance Network Product Effectiveness Collaborators. Early estimate of nirsevimab effectiveness for prevention of respiratory syncytial virus-associated hospitalization among infants entering their first respiratory syncytial virus season - New Vaccine Surveillance Network, October 2023-February 2024. MMWR Morb Mortal Wkly Rep 2024; 73: 209-214.
11. López-Lacort M, Muñoz-Quiles C, Mira-Iglesias A, et al. Early estimates of nirsevimab immunoprophylaxis effectiveness against hospital admission for respiratory syncytial virus lower respiratory tract infections in infants, Spain, October 2023 to January 2024. Euro Surveill 2024; 29: 2400046.
12. Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev. 2013; (4): CD006602.
13. Therapeutic Goods Administration (TGA). Arexvy. Australian Prescription Medicine Decision Summary. TGA; Canberra, 2024. Available online at: https://www.tga.gov.au/resources/auspmd/arexvy (accessed April 2024).
14. Therapeutic Goods Administration (TGA). ABRYSVO recombinant respiratory syncytial virus pre-fusion F protein 120 microgram/0.5 mL bivalent vaccine powder for injection vial + diluent syringe (406624). TGA; Canberra, 2024. Available online at: https://www.tga.gov.au/resources/artg/406624 (accessed April 2024).
15. Australian Technical Advisory Group on Immunisation (ATAGI). ATAGI Statement on the Clinical Use Of Arexvy (RSV PRE-F3) Vaccine for RSV. ATAGI; Canberra, 2024. Available online at: https://www.health.gov.au/resources/publications/atagi-statement-on-the-clinical-use-of-arexvy-rsv-pre-f3-vaccine-for-rsv?language=en (accessed April 2024).
16. Papi A, Ison MG, Langley JM, et al; AReSVi-006 Study Group. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N Engl J Med 2023; 388: 595-608.
17. Ison MG, Papi A, Athan E, et al; AReSVi-006 study group. Efficacy and safety of respiratory syncytial virus prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons. Clin Infect Dis 2024: ciae010.
18. Pfizer Announces Positive Top-Line Data for Full Season Two Efficacy of ABRYSVO® for RSV in Older Adults. Press release; February 29, 2024. Available online at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two#:~:text=Pfizer%20
currently%20is%20the%20only,regardless%20of%20the%20virus%20subgroup (accessed April 2024).
19. Centers for Disease Control and Prevention (CDC). RSV vaccination in older adults: benefit-risk discussion. CDC; 2024. Available online at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2024-02-28-29/07-RSV-Adults-Melgar-508.pdf (accessed April 2024).
20. Centers for Disease Control and Prevention (CDC). Post-licensure safety monitoring of respiratory syncytial virus (RSV) vaccines in adults aged ≥60 years. CDC; 2024. Available online at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2024-02-28-29/05-RSV-Adults-Shimabukuro-508.pdf (accessed April 2024).
21. Australian product information. AREXVY (Recombinant Respiratory Syncytial Virus pre-fusion F protein) powder and suspension for suspension for injection. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2024-PI-01071-1 (accessed April 2024).
22. Australian product information – Abrysvo® (recombinant respiratory syncytial virus pre-fusion f protein) vaccine. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2024-PI-01489-1 (accessed April 2024).
23. Therapeutic Goods Administration (TGA). Notice for mRNA-1345 (for Respiratory Syncytial Virus (RSV) pre-fusion F protein (Moderna Australia Pty Ltd). TGA; Canberra, 2023. Available online at: https://www.tga.gov.au/resources/designations-determinations/notice-mrna-1345-respiratory-syncytial-virus-rsv-pre-fusion-f-protein-moderna-australia-pty-ltd (accessed April 2024).
24. Merk Clincial Trials. Clesrovimab in Infants and children at increased risk for severe respiratory syncytial virus disease https://www.merckclinicaltrials.com/trial/nct04938830/ (accessed May 2024).
25. Levi M, Watson S, Anderson AB, et al. Pharmacokinetics and safety in healthy adults of RSM01, a novel RSV monoclonal antibody, and population pk modeling to support pediatric development. Open Forum Infect Dis 2023 Nov; 10(Suppl 2): ofad500.453.