Bracing for impact: navigating the complexities of the 2024 influenza season in Australia
The 2023 influenza season in Australia presented a unique epidemiological profile, with an earlier onset, extended duration and the second highest number of laboratory-confirmed cases on record. Yet, it demonstrated lower clinical severity and societal disruption compared with many of the influenza seasons observed before the
COVID-19 pandemic. One of the only predictable aspects of seasonal influenza is its fickle nature from year to year.
- Epidemiological vigilance: keeping abreast of local influenza trends is essential for anticipating healthcare needs.
- Rapid diagnosis: utilising the appropriate diagnostic tools promptly can alter the course of the disease and reduce transmission, as well as provide important epidemiological data.
- Strategic treatment: the timely use of antivirals and management of complications are paramount.
- Preventative measures: vaccination remains our best defence against influenza, with nonpharmaceutical measures also being important.
- Addressing gaps: continuous improvement in public health strategies and patient education is crucial for closing existing gaps in influenza management.
With the 2024 influenza season upon us, Australia faces the challenges of anticipating its impact and navigating the complex interplay of viral evolution, public health readiness and varying community and healthcare provider engagement. Each year, as clinicians at the coalface, we are tasked with not only managing the immediate effects of the virus on patients but also mitigating its spread within our community. This article arms GPs and other healthcare providers with a comprehensive guide to managing the upcoming influenza season through an updated understanding of influenza viruses and its local epidemiology. This article provides an overview of the diagnostic process, discusses potential complications and reviews the latest in antiviral treatments and vaccination strategies. Additionally, the significance of nonpharmaceutical interventions and an analysis of the gaps in current clinical practices are covered. This information is crucial for optimising management and ensuring clinicians are prepared for the months ahead.
Virology of influenza: current insights
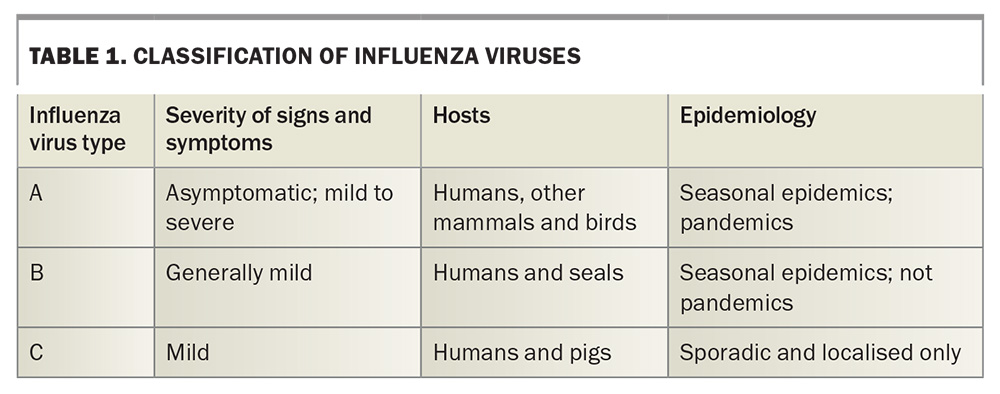
Influenza viruses are a major cause of respiratory illness worldwide,1 belonging to the family Orthomyxoviridae.2 These viruses have an enveloped, segmented, negative-sense RNA genome, characterised by their remarkable adaptability and propensity for mutation, which contributes to their seasonal variability and pandemic potential. Influenza viruses are classified into three main types known to infect humans: A, B and C (Table 1), with influenza A and B responsible for the seasonal epidemics seen in humans.3 Although influenza C may infect humans, the symptoms are usually mild, the incidence is very low and few laboratories test for it.4,5
Influenza A viruses are further subdivided based on the surface proteins haemagglutinin (HA) and neuraminidase (NA). There are at least 16 different HA subtypes and nine different NA subtypes, leading to numerous combinations that can potentially infect humans.6 At present, the subtypes of most concern for public health include A(H1N1) and A(H3N2), known for their role in seasonal influenza epidemics and pandemics.7,8
Avian influenza, arising from influenza A viruses yet distinct from seasonal influenza, is distinguished by varying pathogenicity levels, with low pathogenic avian influenza (LPAI) typically causing no or mild symptoms and high pathogenic avian influenza (HPAI) leading to severe disease and high mortality rates in birds.6 Although primarily affecting birds, certain strains of avian influenza, such as H5N1, can infect humans, with effects ranging from mild symptoms to severe respiratory illness and death. Fortunately, avian influenza viruses generally have limited replicative capacity in human hosts.6 Since 1976, several outbreaks of avian influenza have been reported in domestic poultry across Victoria, New South Wales and Queensland.9 The most recent outbreaks occurred in Victoria in 2020 and 2021, impacting three egg production farms (HPAI H7N7), two turkey farms (LPAI H5N2) and one emu farm (LPAI H7N6).9 These outbreaks were all promptly detected and successfully eradicated, demonstrating the critical role Australia’s domestic and wild bird surveillance program plays in keeping the community safe.
Influenza B viruses are not divided into subtypes but are instead separated into two lineages: B/Yamagata and B/Victoria.6 These viruses generally cause less severe epidemics than those caused by influenza A but can still have a significant impact, particularly in children and adolescents.10 Since the onset of the COVID-19 pandemic, an intriguing surveillance phenomenon has been the apparent disappearance of the B/Yamagata lineage.11,12 Global surveillance data have shown no documented cases of the B/Yamagata lineage since early 2020, suggesting a potential extinction of this lineage from human circulation. Further research is required to understand the full implications of this absence, including potential shifts in vaccine formulation strategies and influenza virus ecology.
Causes of the changing face of influenza
Influenza virus replication begins when the HA protein binds to sialic acid receptors on the surface of a host cell, typically in the upper respiratory tract.13 The virus then enters the cell via receptor-mediated endocytosis, and the viral RNA segments are released into the cytoplasm. These segments move into the nucleus, where viral RNA transcription and replication take place. The RNA-dependent RNA polymerase in the virus lacks proofreading ability, which leads to frequent errors during RNA synthesis. This high mutation rate, combined with the segmented nature of the genome, facilitates rapid evolution of the virus through two main mechanisms: antigenic drift and antigenic shift.14
Antigenic drift involves minor changes in the HA and NA proteins due to point mutations in the viral RNA. This results in seasonal influenza epidemics, as the immune system may not fully recognise the new variants, reducing the effectiveness of prior immunity or vaccination. Antigenic shift is a more dramatic change that occurs only in influenza A viruses and involves the reassortment of RNA segments between different viruses. If a single cell is infected with two different influenza A viruses, new hybrid viruses can emerge with a mixture of RNA segments. This process can lead to the emergence of a significantly different virus that can cause pandemics, as the human population often has little to no pre-existing immunity to these new viral combinations.
Understanding these mechanisms is crucial for vaccine formulation and predicting the effectiveness of the seasonal influenza vaccine.
Unravelling the spread: how influenza moves among us
The primary mode of transmission for seasonal influenza is through large respiratory particles (≥5 mcm), and smaller particles (<5 mcm) that are able to travel further and remain in the air longer than can be generated when an infected person coughs, sneezes or talks, requiring close contact generally within 1 to 2 m.15 Transmission can also occur by touching surfaces contaminated with the virus and then touching the mouth, nose or eyes.16,17 For this reason, frequent handwashing and sanitising surfaces are important in controlling the spread of the virus. Furthermore, influenza can spread rapidly in crowded areas such as schools, residential aged care facilities and workplaces, especially when poorly ventilated.
The pathogenesis of influenza involves the initial infection of epithelial cells in the upper respiratory tract. The virus binds to sialic acid receptors on the surface of host cells, facilitating entry and subsequent replication.6 Between 18 and 72 hours following inoculation,6,18 the immune system responds to this invasion, often resulting in the classic symptoms of influenza such as high fever, chills, cough, sore throat, malaise, headache and myalgia.19 However, not all patients will display symptoms,20 with a recent study finding 44% of all influenza infections were asymptomatic.21 This is an important point often lost on many who view influenza as nothing more than the common cold, dismissing the impact their transmission may have on others in the community. The degree of symptom severity can vary widely, influenced by factors such as the individual’s age, immune status and pre-existing medical conditions.3,22 Furthermore, complications such as secondary bacterial infections (e.g. pneumococcal pneumonia) can exacerbate the condition and lead to more severe clinical presentations.
Epidemiology
During the 2023 influenza season, there were a total 252,296 laboratory-confirmed cases in Australia, with 376 associated deaths.23 These figures are likely to be an underestimate of the true magnitude of influenza-related disease. Notification rates were the highest in the 5- to 9-year-old age group. Only 32% of the population received an influenza vaccine during the 2023 reporting period.23 The influenza season typically peaks during the winter months from June to August, but seasonal variations in temperate climates and year-round notifications are typical in tropical environments.24 Historically, each season is unpredictable, with the severity and length of outbreaks fluctuating significantly. Australian data from before the COVID-19 pandemic suggested a trend towards more severe seasons, including increased interseasonal influenza notifications during the summer months.25 In recent years, influenza A(H1N1), A(H3N2) and B/Victoria viruses have predominated among the human population in Australia and globally.8 Monitoring current trends in virus strains and transmission rates is crucial for appropriate healthcare planning and sufficient resource allocation. This allows health authorities to anticipate the demand for medical services and vaccines, thus improving patient care responsiveness.
Diagnosis: clinical and laboratory factors
The rapid and accurate diagnosis of influenza allows for effective patient management and enables the collection of vital epidemiological data to inform ongoing population management more accurately. In the clinical landscape, influenza is typically characterised by a constellation of signs and symptoms, including a sudden onset of high fever, chills, cough, sore throat, malaise, headache and myalgia. These manifestations herald the infection’s acute phase, leading to considerable discomfort and debilitation. However, the nonspecific nature of these features poses a diagnostic challenge, as they substantially overlap with those of other acute viral and bacterial respiratory illnesses, such as the common cold, respiratory syncytial virus infection and even early stages of bacterial pneumonia.
This overlap in clinical presentation can complicate the differential diagnosis, especially when relying solely on clinical judgement without the support of laboratory confirmation. The similarity of symptoms among various respiratory pathogens can lead to misdiagnosis, resulting in inappropriate management strategies. For instance, the unwarranted use of antibacterial agents for a viral infection such as influenza is a common consequence of such diagnostic inaccuracies. This not only fails to alleviate the patient’s viral symptoms and contributes to broader public health problems, such as antimicrobial resistance, but further neglects the possible directed use of antivirals in high-risk groups.
Moreover, the reliance on symptomatology alone for diagnosis without laboratory confirmation can hinder the potential effectiveness of antiviral treatments. Antiviral agents, such as neuraminidase inhibitors, are most efficacious when administered within the first 48 hours of symptom onset. Delays in accurate diagnosis thus miss this critical window, diminishing the potential benefits of antiviral therapy and possibly prolonging the course of illness.
In light of these challenges, it is imperative that clinicians consider influenza within the broader differential diagnosis of acute febrile respiratory illnesses and employ rapid diagnostic tests, such as polymerase chain reaction (PCR) assays, when available. PCR testing offers high sensitivity and specificity (e.g. about 94% and 99%, respectively, for influenza A),26 making it an invaluable tool for accurately distinguishing influenza from other viral respiratory pathogens. The rapid turnaround time of PCR testing facilitates prompt and appropriate therapeutic interventions, enabling clinicians to administer targeted treatments efficiently and effectively, thereby improving patient outcomes and preventing the further spread of infection.
In Australia, the landscape of diagnostic testing for influenza and other respiratory pathogens is comprehensive and robust, and incorporates a variety of methodologies including rapid antigen and PCR testing, that facilitate prompt and accurate aetiological identification. This approach is critical not only for clinical diagnosis, but also for guiding treatment strategies and public health interventions such as resource allocation.
The most common specimens for respiratory pathogen testing, including influenza, are nasopharyngeal swabs or nose and throat swabs; aspirate samples, bronchoalveolar lavages and sputum samples may also be utilised. The choice of specimen depends on the clinical setting, suspected aetiology and available testing modality. For most patients, nasopharyngeal swabs are preferred for their higher yield in detecting respiratory viruses because of the concentration of pathogens in the posterior nasopharynx.
The scope of respiratory viral testing in Australia has expanded significantly with the adoption of multiplex PCR assays, which can detect a wide range of respiratory pathogens from a single sample (Box 1). Originally limited to hospitals and their associated laboratories, PCR testing is provided across Australia through a network of private pathology services, offering bulk-billed or minimal out-of-pocket expenses (with a valid referral) for these specialised molecular diagnostic techniques, and ensuring high standards of accuracy and reliability. In recent years, the COVID-19 pandemic has given rise to the availability and convenience of rapid antigen tests. Rapid antigen self-test kits available from pharmacies and supvermarkets currently include SARS-CoV-2 alone, SARS-CoV-2 with influenza A and B, and a more comprehensive option including RSV.
The turnaround time for test results can vary based on the test method, healthcare setting and distance to the pathology facility. For rapid antigen tests, including point-of-care testing, results can be available in as little as 15 to 30 minutes, providing a quick assessment of the patient’s infectious status. These are particularly useful in clinical settings where immediate decisions about patient management and infection control are necessary. More comprehensive PCR-based tests, which are more sensitive and can detect a broader array of pathogens, typically have a turnaround time ranging from several hours to a day. During outbreaks or peak seasons, when laboratory services are in high demand, turnaround times may be influenced by the increased volume of samples.
During a seasonal influenza epidemic, when viral prevalence within the community is high, clinicians are often able to make a presumptive diagnosis based on characteristic signs and symptoms alone. This clinical diagnosis, termed ‘influenza-like illness’, does not necessarily require laboratory confirmation. The predictive value of such a clinical diagnosis is considerably enhanced during the peak of the influenza season; however, it is noteworthy that clinical prediction models, which incorporate a range of symptoms, have demonstrated varying degrees of diagnostic accuracy, with sensitivities reported between 36% and 80%, and specificities ranging from 78% to 98%.27,28 Although these models can be helpful, they are not infallible and should be used judiciously. Nevertheless, this approach may be especially useful in managing and initiating treatment promptly, particularly in settings where rapid diagnostic tools are not readily available.
Influenza in vaccinated individuals
It is vital not to dismiss the possibility of influenza in patients who have been vaccinated for the current influenza season. There are a couple of important reasons for this consideration.
First, there may be the presence of non-vaccine-related strains. Influenza viruses are highly mutable, occasionally leading to the emergence of strains not covered by the current vaccine. These strains may circulate concurrently with vaccine-included strains, causing infections, even in vaccinated individuals. This scenario is particularly likely when there has been antigenic drift.
Second, suboptimal seroconversion may occur. The immune response elicited by the vaccine may vary significantly among different populations. In older people and those with compromised immune systems, the response to the vaccine may not be sufficiently robust to prevent the disease. This reduced immunogenicity results in lower levels of antibody production, which may not be sufficient to provide protective immunity against the virus. Factors such as age-related immunosenescence and immunosuppression due to disease or treatment may diminish the effectiveness of the vaccine. Consequently, vaccines specifically designed to enhance the immune response (e.g. the adjuvanted quadrivalent inactivated influenza vaccine [surface antigen] and the high-dose quadrivalent inactivated influenza vaccine [split virion]) are now available and preferentially recommended over standard influenza vaccines for adults aged 65 years and older.29 Furthermore, people of any age receiving the influenza vaccine for the first time following haematopoietic stem cell or solid organ transplant or chimeric antigen receptor T-cell therapy should receive two doses of the standard influenza vaccine, four weeks apart.29 These strategies aim to boost immunogenicity and provide better protection in these vulnerable groups.
For these reasons, clinicians must consider influenza as a potential diagnosis even in vaccinated patients presenting with influenza-like symptoms, particularly during an epidemic. Adequate assessment and, if indicated, laboratory confirmation should be pursued to ensure accurate and appropriate management of all patients.
Complications of influenza
Influenza can lead to numerous complications, particularly in high-risk groups, such as adults aged 65 years and older, children younger than 6 years of age, pregnant women, Indigenous people and those with certain chronic health conditions.3 These complications range from moderate, such as sinus and ear infections, to severe, including pneumonia, myocarditis, acute myocardial infarction, Guillain–Barré syndrome and encephalitis.6,30 Prompt recognition and management of these complications are imperative to prevent prolonged morbidity and potential mortality.
Antiviral treatments
Antiviral medications may play a critical role in the management of influenza in individuals at higher risk of poor outcomes. The neuraminidase inhibitors oseltamivir (capsule or oral suspension) and zanamivir (powder for inhalation) are commonly prescribed in Australia, with peramivir reserved for severe cases requiring intravenous administration. These drugs are most effective when administered within 48 hours of symptom onset.6 Treatment guidelines recommend dosages based on the patient’s age, weight and renal function.31 Antivirals may not only reduce the duration of symptoms but also decrease the risk of complications and hospitalisation.32
Baloxavir marboxil represents a novel class of antivirals approved for the treatment of influenza and has garnered attention for its unique mechanism of action and clinical efficacy. As a cap-dependent endonuclease inhibitor, baloxavir disrupts the viral replication process by inhibiting the activity of the polymerase acidic protein, a component of the viral RNA polymerase complex essential for viral gene transcription. Baloxavir is TGA approved but is not yet widely available in Australia.33,34 Current clinical trial data demonstrate its effectiveness in reducing the duration of influenza symptoms and viral shedding when administered within 48 hours of symptom onset.35 Notably, the drug has shown a rapid reduction in viral load, often within one day post-administration, which is considerably faster compared with traditional neuraminidase inhibitors such as oseltamivir.36 Moreover, baloxavir’s single-dose regimen offers a significant advantage in terms of ease of administration and adherence over the multidose regimens required for other antivirals. However, concerns have been raised regarding the emergence of viral resistance, particularly mutations in the polymerase acidic protein that confer resistance to baloxavir.37 Such mutations have been observed both in clinical trials and in the real-world setting, suggesting a potential limitation in its effectiveness over time. The introduction of baloxavir into the influenza treatment landscape may offer an important alternative to existing antivirals, particularly for patients who require a simple and fast-acting treatment option. Ongoing surveillance for resistance patterns and further research into the long-term efficacy of baloxavir are essential to fully understand its role in managing seasonal and pandemic influenza.
Influenza prevention
Influenza vaccination remains the primary preventive strategy against the disease, constituting a fundamental component of public health initiatives. Recent research provides compelling evidence supporting the efficacy of influenza vaccination; data from a recent study indicate that, during the 2022-2023 influenza season, vaccination was associated with a substantial reduction in the incidence of influenza-related complications among adults in the USA.38 Specifically, the study found influenza vaccinations decreased the risk of visits to emergency departments and urgent care facilities by nearly 50% and reduced hospitalisations by over one-third. These data underscore the critical role of influenza vaccination in mitigating the burden of the disease on healthcare systems and highlight its effectiveness in protecting populations from severe influenza outcomes. In addition to vaccination, other measures including good hand hygiene, mask-wearing and the judicious use of antiviral medication (including postexposure prophylaxis) play a vital role in preventing the spread of influenza, as shown through the significant reduction of influenza during COVID-19 lockdowns.39,40 The prevention of influenza is particularly important for individuals at higher risk of poor disease outcomes and in healthcare settings.
Influenza vaccines and vaccination programs
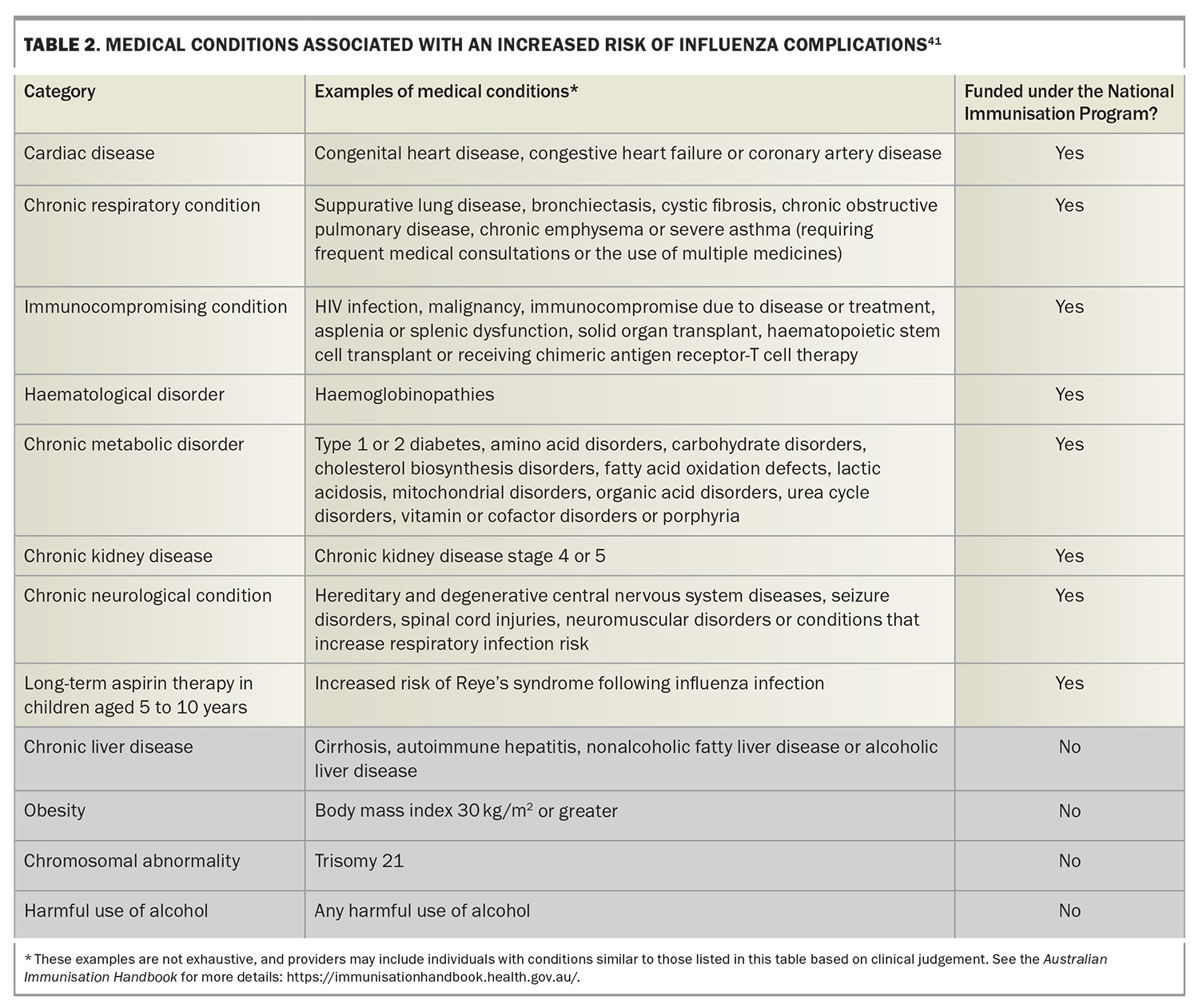
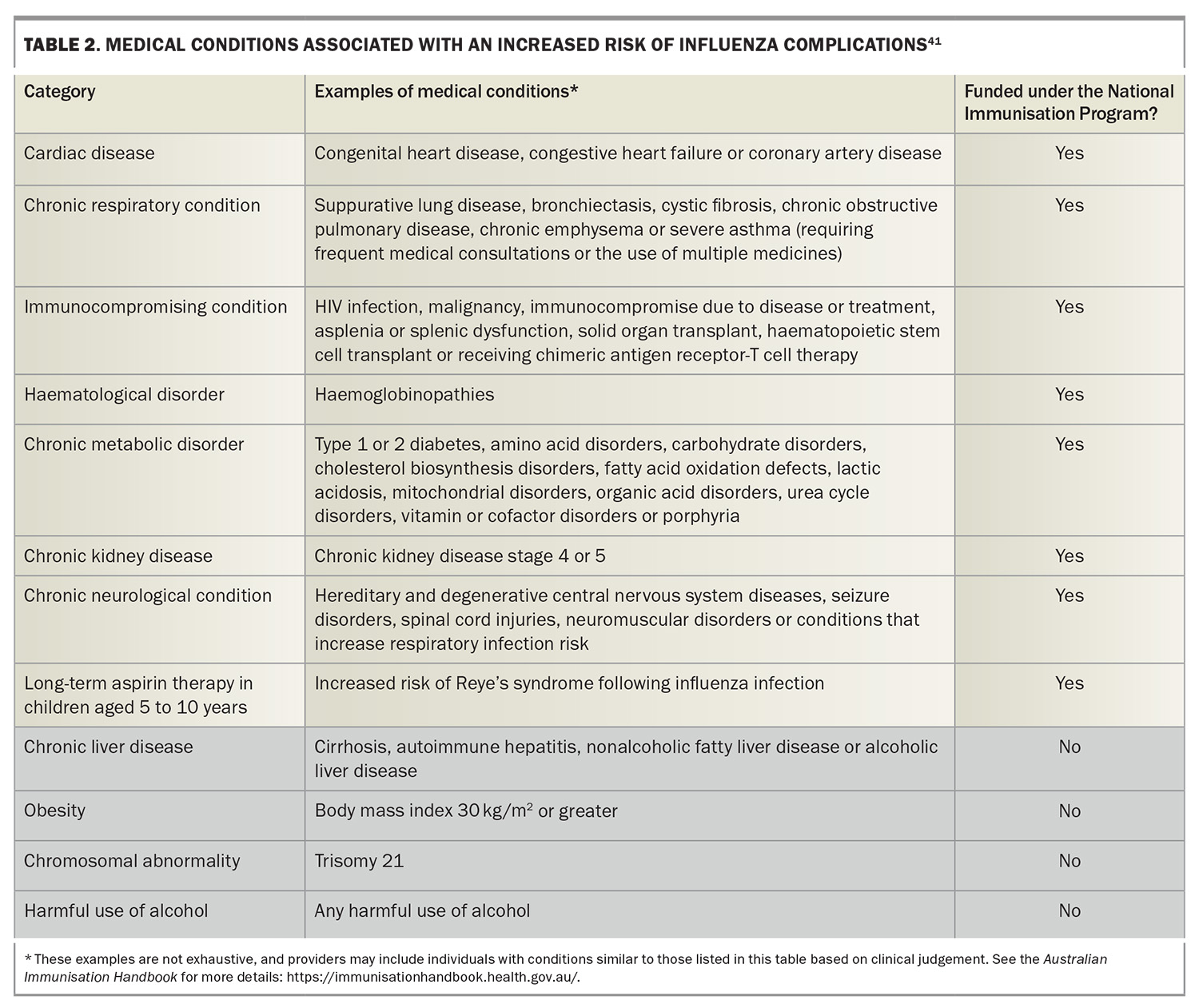
In Australia, annual influenza vaccination is recommended for all individuals older than 6 months of age. The Australian Immunisation Handbook specifies several groups for which influenza vaccination is particularly recommended (Box 2), in addition to highlighting people with certain medical conditions who have a higher risk of severe influenza (Box 3).29 The Australian Government’s National Immunisation Program (NIP) provides free influenza vaccination for certain people at increased risk of complications related to influenza (Box 4), including specific populations aged 5 to 65 years (Table 2).41 In addition, some state and territory governments offer free vaccines administered to residents older than the age of 6 months not eligible under the NIP.
Influenza vaccine hesitancy in Australia, an issue predating the COVID-19 pandemic, has notably worsened in recent years. Despite longstanding public health efforts, national influenza vaccination rates fell by 17.7% in 2023 and have continued to decline, with a further 17.9% reduction in vaccinations observed in the first quarter of 2024.42 This trend highlights a growing reluctance among the population to receive influenza vaccinations, potentially because of vaccine fatigue or misconceptions about vaccine effectiveness and safety. Interestingly, Queensland stands as an exception, where influenza vaccine uptake surged by 29.8% over the same period in 2023.42 This increase is largely attributed to the state government's initiative to fund influenza vaccines for individuals not covered under the NIP, demonstrating that targeted government interventions can effectively counteract vaccine hesitancy and improve public health outcomes.
Annual influenza vaccination is recommended throughout the influenza season; however, specific populations, such as pregnant women and residents of northern Australia, are advised to receive immunisation promptly upon the availability of the new seasonal vaccine formulation. This recommendation underscores the importance of early vaccination in these groups to maximise the protective benefits against influenza, considering their increased vulnerability to severe outcomes and the distinct epidemiological patterns observed in northern regions of Australia. This strategy ensures timely protection and aligns with public health objectives to mitigate the impact of influenza across susceptible demographics.
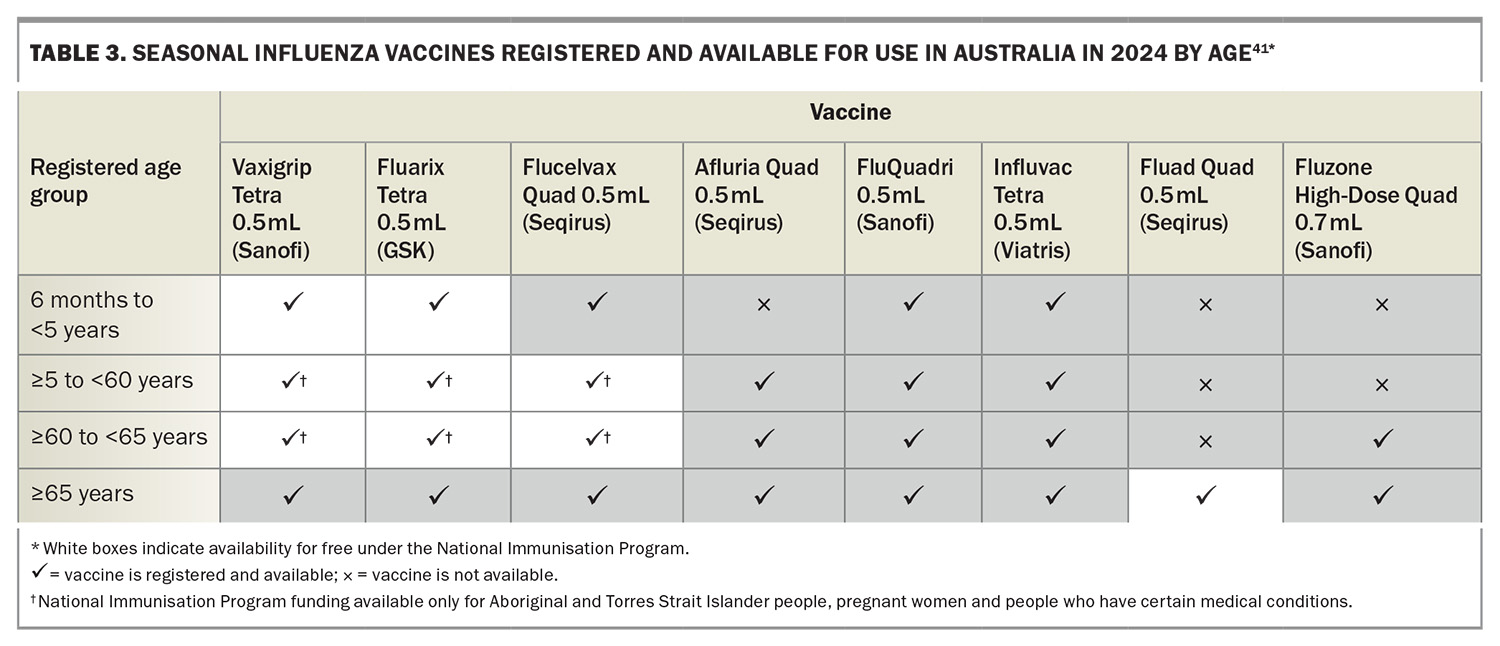
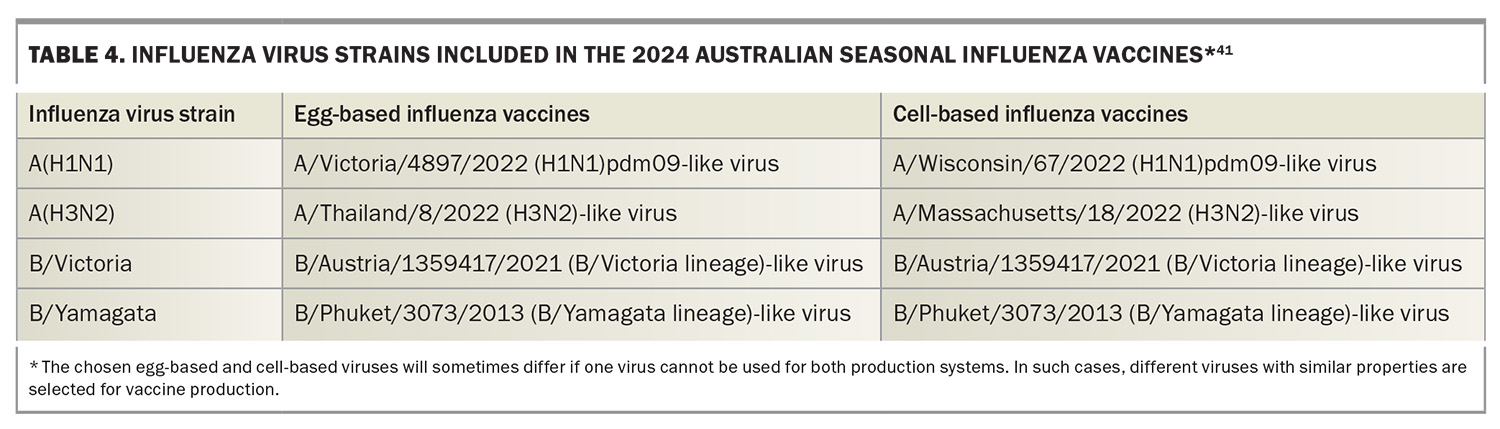
The 2024 seasonal influenza vaccines available for use in Australia include the usual chicken egg-based formulations (i.e. standard-dose, high-dose and adjuvated influenza vaccine) and the relatively new cell-based formulations (propagated in Madin-Darby canine kidney cells) (Table 3). All vaccines are quadrivalent, containing two influenza A subtypes and two influenza B lineages (Table 4). Among these, the cell-based influenza vaccine is currently funded under the NIP for at-risk populations aged 5 to 65 years (Table 2). Importantly, the Australian Technical Advisory Group on Immunisation (ATAGI) states there is no preference for use between cell-based and standard-dose, egg-based influenza vaccines.41 The cell-based influenza vaccine offers an enhanced match to recommended vaccine strains compared with traditional egg-based vaccines, primarily because of the elimination of viral adaptation that can occur during cultivation in eggs. This adaptation often results in antigenic changes that may compromise the vaccine’s effectiveness against circulating strains. By utilising cell culture technologies for vaccine production, this issue is mitigated, potentially improving the immunogenicity and efficacy of the vaccine.
Nonpharmaceutical interventions
Public health campaigns play a pivotal role in controlling influenza spread through emphasising education and behavioural strategies, particularly in environments susceptible to outbreaks. Viral shedding of the influenza virus can begin 24 hours before the onset of symptoms and typically persist for up to seven days in adults, extending to 10 days or more in children and potentially several weeks in immunocompromised individuals. Shedding is most pronounced within the first two days after symptoms appear in adults and significantly decreases by the fifth day. Although not all patients with influenza present with fever, its presence, especially when accompanied by respiratory symptoms, typically signals active viral shedding.
To mitigate influenza transmission in community settings, such as workplaces, residential aged care facilities, schools and childcare centres, Australian public health guidelines stipulate adults diagnosed with influenza should remain isolated until they are fever-free for at least 24 hours (without the use of antipyretics).43 This isolation is contingent upon either 72 hours passing since the initiation of antiviral therapy, or five days from the onset of respiratory symptoms. Consequently, this typically necessitates exclusion from these settings for about five to seven days, depending on the speed of recovery and symptom persistence. Extended precautions are recommended for children and immunocompromised individuals because of their longer periods of viral shedding.
General practice and other outpatient settings must adhere to stringent infection control guidelines to manage patients presenting with suspected influenza, ensuring the safety of healthcare workers, including administration staff, and other patients. The recommended personal protective equipment includes surgical masks, which should be worn by both staff and patients to prevent the spread of respiratory particles. Additionally, when performing procedures that generate aerosols, clinicians should use P2/N95 respirators to offer enhanced protection against airborne particles.
In terms of practice strategies, it is advised that patients with symptoms suggestive of influenza be triaged at the point of entry to the facility. Practices should have clearly marked areas for symptomatic patients, maintaining physical distancing and minimising waiting times in shared spaces. Where possible, dedicated consultation rooms should be used for examining patients with respiratory symptoms, and these rooms should be well ventilated and cleaned regularly following established protocols. Furthermore, ensuring the availability of alcohol-based hand sanitisers for both healthcare workers and patients throughout the practice is important, as it promotes consistent and effective hand hygiene practices for safeguarding public health. Overall, adhering to these guidelines is essential for effective infection prevention and control across the community.
Gaps in current clinical management
Despite advancements, substantial gaps remain in the clinical management of influenza. These include variations in vaccine uptake, delays in accessing antiviral medications and inconsistencies in the use of preventative measures across different demographics (e.g. pregnant women). There is also a need for better surveillance to track the effectiveness of the vaccine against current circulating strains and to monitor resistance patterns to antiviral medications.
Conclusion
As we brace for 2024’s influenza season, being well prepared is key. The integration of epidemiological insights, effective diagnostic practices and timely treatment interventions can mitigate the impact of influenza significantly. Understanding the virus’s behaviour through its virology and pathogenesis will further enhance our preventive strategies. This structured approach provides a comprehensive blueprint for GPs and specialists alike, ensuring readiness and efficacy in managing the upcoming influenza season with confidence, enhancing patient care and improving overall health outcomes. MT
COMPETING INTERESTS: Professor Griffin has received speaker honoraria from Seqirus. Dr Armstrong and Dr Spence: None.
ACKNOWLEDGEMENT: The authors would like to thank Lydia McLennan for reviewing the manuscript.
References
1. World Health Organization (WHO). Guidelines for the clinical management of severe illness from influenza virus infections. Geneva: WHO; 2022. Available online at: https://www.who.int/publications/i/item/9789240040816 (accessed April 2024).
2. International Committee on Taxonomy of Viruses (ICTV). Current ICTV Taxonomy Release. ICTV; 2022. Available online at: https://ictv.global/taxonomy (accessed April 2024).
3. Uyeki TM, Hui DS, Zambon M, Wentworth DE, Monto AS. Influenza. Lancet 2022; 400: 693-706.
4. Sederdahl BK, Williams JV. Epidemiology and clinical characteristics of influenza C virus. Viruses 2020; 12: 89.
5. Matsuzaki Y, Ohmiya S, Ota R, et al. Epidemiologic, clinical, and genetic characteristics of influenza C virus infections among outpatients and inpatients in Sendai, Japan from 2006 to 2020. J Clin Virol 2023; 162: 105429.
6. Treanor JJ. Influenza viruses, including avian influenza and swine influenza. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Amsterdam: Elsevier; 2020.
7. World Health Organization (WHO). Sixteenth Biregional Meeting of National Influenza Centres and Influenza Surveillance in the WHO South-East Asia and Western Pacific Regions, 1–3 August 2023, Dhaka, Bangladesh. Regional Office for South-East Asia: WHO; 2024. Available online at: https://iris.who.int/bitstream/handle/10665/376340/SEA-WHE-21-eng%20.pdf?sequence=1 (accessed April 2024).
8. Australian Government Department of Health and Aged Care (doHAC), Interim Australian Centre for Disease Control. Australian Respiratory Surveillance Report 1 – 25 March to 7 April 2024. Canberra: Australian Government DoHAC; 2024. Available online at: https://www.health.gov.au/sites/default/files/2024-04/australian-respiratory-surveillance-report-1-25-march-to-7-april-2024_0.pdf (accessed April 2024).
9. Agriculture Victoria. Avian Influenza (Bird Flu). Melbourne: Victorian Government Department of Energy, Environment and Climate Action; 2024. Available online at: https://agriculture.vic.gov.au/biosecurity/animal-diseases/poultry-diseases/avian-influenza-bird-flu#:~:text=Nine%20outbreaks%20of%20HPAI%20have,number%20of%20farms%20were%20affected (accessed April 2024).
10. Koutsakos M, Nguyen TH, Barclay WS, Kedzierska K. Knowns and unknowns of influenza B viruses. Future Microbiol 2016; 11: 119-135.
11. Monto AS, Zambon M, Weir JP. The end of B/Yamagata influenza transmission - transitioning from quadrivalent vaccines. N Engl J Med 2024; 390: 1256-1258.
12. Zheng L, Lin Y, Yang J, Fang K, Wu J, Zheng M. Global variability of influenza activity and virus subtype circulation from 2011 to 2023. BMJ Open Respir Res 2023; 10: e001638.
13. Krammer F, Smith GJD, Fouchier RAM, et al. Influenza. Nat Rev Dis Primers 2018; 4: 3.
14. Liang Y. Pathogenicity and virulence of influenza. Virulence 2023; 14: 2223057.
15. Leung NHL. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol 2021; 19: 528-545.
16. Asadi S, Gaaloul Ben Hnia N, Barre RS, Wexler AS, Ristenpart WD, Bouvier NM. Influenza A virus is transmissible via aerosolized fomites. Nat Commun 2020; 11: 4062.
17. Fong MW, Leung NHL, Xiao J, et al. Presence of influenza virus on touch surfaces in kindergartens and primary schools. J Infect Dis 2020; 222: 1329-1333.
18. Biggerstaff M, Cauchemez S, Reed C, Gambhir M, Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis 2014; 14: 480.
19. Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med 2000; 160: 3243-3247.
20. Ip DK, Lau LL, Leung NH, et al. Viral shedding and transmission potential of asymptomatic and paucisymptomatic influenza virus infections in the community. Clin Infect Dis 2017; 64: 736-742.
21. Cohen C, Kleynhans J, Moyes J, et al. Asymptomatic transmission and high community burden of seasonal influenza in an urban and a rural community in South Africa, 2017-18 (PHIRST): a population cohort study. Lancet Glob Health 2021; 9: e863-e874.
22. Gounder AP, Boon ACM. Influenza pathogenesis: the effect of host factors on severity of disease. J Immunol 2019; 202: 341-350.
23. Australian Government Department of Health and Aged Care (DoHAC). Australian Influenza Surveillance Report – 2023 End of Season Summary. Canberra: Australian Government DoHAC: 2023. Available online at: https://www.health.gov.au/sites/default/files/2023-12/aisr-2023-national-influenza-season-summary.pdf (accessed April 2024).
24. Costantino V, Trent M, MacIntyre CR. Modelling of optimal timing for influenza vaccination as a function of intraseasonal waning of immunity and vaccine coverage. Vaccine 2019; 37: 6768-6775.
25. Moa AM, Adam DC, MacIntyre CR. Inter-seasonality of influenza in Australia. Influenza Other Respir Viruses 2019; 13: 459-464.
26. Huang HS, Tsai CL, Chang J, Hsu TC, Lin S, Lee CC. Multiplex PCR system for the rapid diagnosis of respiratory virus infection: systematic review and meta-analysis. Clin Microbiol Infect 2018; 24: 1055-1063.
27. Hung SK, Wu CC, Singh A, et al. Developing and validating clinical features-based machine learning algorithms to predict influenza infection in influenza-like illness patients. Biomed J 2023; 46: 100561.
28. Dugas AF, Valsamakis A, Atreya MR, et al. Clinical diagnosis of influenza in the ED. Am J Emerg Med 2015; 33: 770-775.
29. Australian Immunisation Handbook (AIH). Influenza (flu). Melbourne: Australian Immunisation Handbook; 2024. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/influenza-flu#people-at-risk-of-severe-disease-from-influenza (accessed April 2024).
30. Chow EJ, Doyle JD, Uyeki TM. Influenza virus-related critical illness: prevention, diagnosis, treatment. Crit Care 2019; 23: 214.
31. Therapeutic Guidelines (TG). Influenza. Melbourne: TG; 2024. Available online at: https://www.tg.org.au (accessed April 2024).
32. Louie JK, Yang S, Acosta M, et al. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin Infect Dis 2012; 55: 1198-1204.
33. Therapeutic Goods Administration (TGA). Australian Public Assessment Report for Baloxavir marboxil. Canberra: Australian Goverment Department of Health; 2020. Available online at: https://www.tga.gov.au/sites/default/files/auspar-baloxavir-marboxil-200604.pdf (accessed April 2024).
34. Australian Register of Therapeutic Goods (ARTG). Australian Product Information: Xofluza (baloxavir marboxil) tablets. Sydney: Roche Products Pty Limited; 2023. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2020-PI-01260-1 (accessed April 2024).
35. Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379: 913-923.
36. Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis 2020; 20: 1204-1214.
37. Hickerson BT, Petrovskaya SN, Dickensheets H, Donnelly RP, Ince WL, Ilyushina NA. Impact of baloxavir resistance-associated substitutions on influenza virus growth and drug susceptibility. J Virol 2023; 97: e0015423.
38. Tenforde MW, Weber ZA, Yang DH, et al. Influenza vaccine effectiveness against influenza-A-associated emergency department, urgent care, and hospitalization encounters among U.S. adults, 2022-2023. J Infect Dis 2023 Dec 2; e-pub (https://doi.org/10.1093/infdis/jiad542).
39. Qi Y, Shaman J, Pei S. Quantifying the impact of COVID-19 nonpharmaceutical interventions on influenza transmission in the United States. J Infect Dis 2021; 224: 1500-1508.
40. Huang QS, Wood T, Jelley L, et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat Commun 2021; 12: 1001.
41. Australian Technical Advisory Group on Immunisation (ATAGI). ATAGI statement on the administration of seasonal influenza vaccines in 2024. Canberra: Australian Government Department of Health and Aged Care; 2024. Available online at: https://www.health.gov.au/resources/publications/atagi-statement-on-the-administration-of-seasonal-influenza-vaccines-in-2024 (accessed April 2024).
42. Australian Immunisation Register – Influenza Data – 1 March to 14 April. Canberra: Australian Government of Department of Health and Aged Care; 2024. Available online at: https://www.health.gov.au/resources/publications/influenza-flu-immunisation-data-1-march-to-14-april-2021-2024?language=en (accessed April 2024).
43. Seasonal Influenza Infection: CDNA National Guidelines for Public Health Units. Canberra: Australian Government Department of Health; 2019. Available online at: https://www.health.gov.au/sites/default/files/documents/2022/06/influenza-infection-flu-cdna-national-guidelines-for-public-health-units.pdf (accessed April 2024).