Revolutionising RSV infection prevention and control: unveiling new options in the fight against RSV

Respiratory syncytial virus (RSV) poses significant challenges, particularly in infants and older individuals. The advent of new vaccines and monoclonal antibodies in Australia marks a revolutionary shift in RSV infection prevention and control. Understanding these novel prophylactic options, including their efficacy, safety and access issues, is essential for optimising patient care.
- RSV burden: Respiratory syncytial virus (RSV) significantly impacts all age groups, causing severe respiratory illness, particularly in infants and elderly individuals.
- Past challenges: Early vaccine efforts, such as the formalin-inactivated RSV vaccine in the 1960s, failed and led to worse disease outcomes, demonstrating the complexity of RSV immunopathogenesis.
- Innovative monoclonal antibodies: Nirsevimab, a new long-acting monoclonal antibody, has shown high efficacy in preventing RSV-related hospitalisations in infants, marking a major advancement in RSV prophylaxis.
- Breakthrough vaccines: The introduction of the Arexvy and Abrysvo vaccines, targeting the RSV prefusion F glycoprotein, offers strong protection for older adults and pregnant women, ensuring newborn immunity.
- Accessibility and funding: Disparities in access and funding for nirsevimab and RSV vaccines across Australia highlight the need for equitable healthcare solutions to maximise the benefits of these innovations.
- Future prospects: Ongoing development of new antivirals and monoclonal antibodies, combined with addressing vaccine hesitancy and public awareness, is essential for the comprehensive control of RSV infection.
Respiratory syncytial virus (RSV) is a leading cause of respiratory illness across all age groups, causing significant morbidity and mortality in infants and older individuals and, thus, posing substantial challenges in primary care settings. GPs often encounter these cases, especially during the peak of the season. With the introduction of new vaccines and immunoprophylaxis in Australia, there is a pivotal shift in how GPs can help prevent RSV infection. This article details the newly available and soon-to-be available prophylactic options in Australia, and explores current access challenges including funding. This knowledge is crucial for optimising patient care while anticipating future developments in RSV infection management.
Past efforts in combatting RSV
Over the decades, various strategies have been trialled to combat RSV infection, ranging from vaccines to antivirals and immunoglobulin preparations. Early efforts in vaccine development, particularly in the 1960s, faced significant setbacks. The first RSV vaccines, based on formalin-inactivated virus, not only failed to provide adequate protection but also led to enhanced respiratory disease upon natural infection, particularly in infants.1 This tragic outcome, resulting in severe respiratory distress, systemic inflammation and even fatalities, underscored the complexity of RSV immunopathogenesis and the need for safer and more effective vaccines.
Antiviral treatments have also been explored. Ribavirin (a nucleoside analogue) was one antiviral drug used for severe RSV infections in children,2,3 although it was also used off label in other populations.4-6 When administered via inhalation, ribavirin showed variable efficacy in infants and young children with severe RSV bronchiolitis.7 It was also extremely expensive, with a wholesale price of about US$30,000 per day,8 and coupled with its toxicity issues, its widespread use was limited.9 Moreover, its administration required a specific hospital setup; this posed logistical challenges, further restricting its practicality. Therefore, aerosolised ribavirin is no longer recommended for routine use in the UK, USA or Australia. Recently, small case series studies in adult haematopoietic stem cell transplant (HSCT) recipients with RSV infection showed that oral ribavirin was noninferior and substantially less expensive than aerosolised ribavirin, with an associated reduction in morbidity and mortality.10,11 However, given none of these studies included a placebo, the clinical efficacy remains unknown. Despite these limitations, a combination of intravenous and/or oral ribavirin is employed in some Australian solid organ and HSCT units for high-risk, immunocompromised patients with laboratory-confirmed RSV infection.6,12 Neither intravenous nor oral ribavirin are registered for this indication in Australia and are therefore accessed through the TGA Special Access Scheme. The RSV antivirals in development are discussed later in this article.
Immunoglobulin preparations, such as RSV immune globulin intravenous (RSV-IGIV), has been shown to provide passive immunity by delivering antibodies against RSV derived from pooled human sera. Although RSV-IGIV showed some prophylactic efficacy in reducing RSV infection-related hospitalisations,13 it was expensive and required monthly intravenous infusions, making it impractical for large-scale use, particularly in primary care and outpatient settings. Safety concerns regarding hyperviscosity in preterm infants with congenital heart disease who had been administered RSV-IGIV also surfaced.14 With further research showing it was significantly less potent than the new monoclonal antibody palivizumab,15 RSV-IGIV was withdrawn from the market in the early 2000s. Regarding the utility of polyclonal immunoglobulin, a recent Cochrane Review indicated that treatment with these products had minimal impact on reducing the length of stay for infants and young children hospitalised with RSV lung infections, and the effectiveness in reducing mortality remains unclear.16
Palivizumab, a humanised immunoglobulin G1 monoclonal antibody, emerged in 1998 as a more convenient prophylactic option, offering monthly intramuscular injections during the RSV season. In Australia, palivizumab is TGA approved for the prevention of serious lower respiratory tract disease (LRTD) caused by RSV infection in high-risk children, specifically those born prematurely (<36 weeks’ gestation), with haemodynamically significant congenital heart disease or with bronchopulmonary dysplasia.17 Although palivizumab significantly reduces hospitalisations due to RSV infection,18,19 its high cost and limited efficacy prevented its PBS listing; instead, the drug is provided at the discretion of state and territory health authorities and individual hospitals.20
Given there are no approved treatments for most individuals with RSV infection, management remains largely supportive through oral hydration, nutritional support, simple analgesia and antipyretics and, in severe cases, hospitalisation for supplemental oxygen or mechanical ventilation. Furthermore, bronchodilators, corticosteroids and decongestants have not shown a benefit for RSV infections, including in cases of bronchiolitis.21
Monoclonal antibody – nirsevimab for passive immunisation
The landscape of RSV prevention has been revolutionised with the introduction of nirsevimab, an injectable human immunoglobulin G1κ monoclonal antibody containing a mutation in the Fc domain to prolong the serum half-life,22,23 enabling effective prophylaxis for at least five months after a single dose.24 Nirsevimab targets the prefusion F glycoprotein of RSV, a critical component for viral entry into host cells.25 Nirsevimab binds to this glycoprotein to neutralise the virus, preventing subsequent disease.26 This advancement is built on the discovery and detailed description of the prefusion F glycoprotein’s structure and function, which has provided a refined approach compared with the less specific and ineffective strategies of the 1960s. The ability to stabilise the prefusion F glycoprotein has been a game-changer in the fight against RSV, inducing a more potent immune response and providing better protection against RSV infection.
Nirsevimab gained TGA approval in November 2023 for the prevention of RSV LRTD in the following populations:27
- neonates and infants born during or entering their first RSV season
- children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
Efficacy and safety of nirsevimab
The efficacy and safety of nirsevimab in preventing medically attended (MA) RSV lower respiratory tract (LRT) infection in full term and preterm infants during their first RSV season were assessed in two randomised, double-blind, placebo-controlled, multicentre trials (D5290C00003 [phase 2b]28 and MELODY [phase 3]29), both involving Australian sites. In the phase 2b trial, the incidence of MA RSV LRT infection was 70.1% lower with nirsevimab prophylaxis than with placebo, and that of hospitalisation for RSV LRTD was 78.4% lower with nirsevimab than with placebo (0.8% vs 4.1% of infants, respectively).28 In the MELODY trial, the efficacy of nirsevimab was 74.5%, and that against hospitalisation for RSV LRTD was 62.1%, compared with placebo (0.6% vs 1.6% of infants, respectively).28
The safety profile of nirsevimab was shown to be favourable, with similar incidences of adverse events across treatment groups in the phase 2/3 MEDLEY trial, which compared nirsevimab with palivizumab in high-risk infants.30 Common adverse reactions included rash (0.7%), pyrexia (0.5%) and nonserious injection-site reactions (0.3%) occurring shortly after dosing. The HARMONIE study, a phase 3b real-world trial, further supported the efficacy of nirsevimab in a cohort of 8,058 infants, demonstrating an 83.2% efficacy against hospitalisation for RSV LRT infection and 75.7% against very severe RSV LRT infection.31 Results from early implementation programs with inirsevimab, such as in France,32 Luxembourg,33 Spain,34-37 and the USA,38 confirm the efficacy seen in the clinical trials. Furthermore, in a continuation of the MELODY trial, concerns of enhanced disease in infants during the second RSV season following nirsevimab administration were unfounded, with an ongoing low incidence of RSV infection and no increase in disease severity compared with placebo.39
Administration of nirsevimab
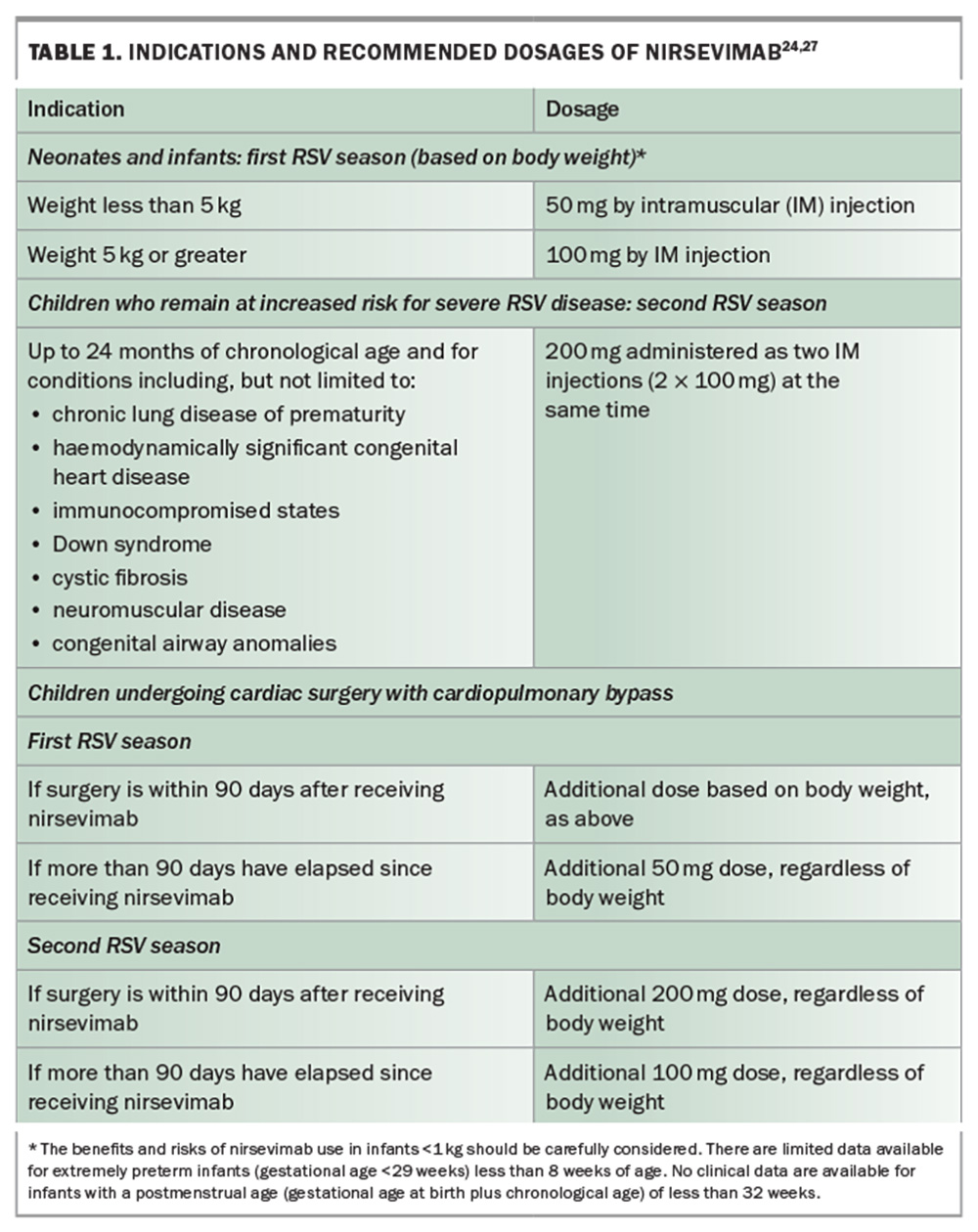
Nirsevimab is available as a 50 mg solution in a 0.5 mL prefilled syringe (purple plunger rod), and as a 100 mg solution in a 1 mL prefilled syringe (light blue plunger rod); needles are not included.27Table 1 lists the indications and recommended dosages of nirsevimab.24,27 The drug must be stored at 2°C to 8°C and protected from light. Once removed from refrigeration, nirsevimab must be used within eight hours or discarded. Administration is via an intramuscular injection, preferably in the anterolateral aspect of the thigh. Injection into the gluteal muscle should be avoided because of the risk of sciatic nerve damage. Coadministration of nirsevimab with other childhood vaccines is permitted.27 Nirsevimab-mediated drug–drug interactions have not been studied, nor is there information regarding coadministration with other immunoglobulin products.20 Palivizumab administration is contraindicated in infants who have received nirsevimab during the same RSV season. Conversely, nirsevimab can be administered prior to or during a second RSV season in children up to 24 months of age who remain at risk of severe RSV disease and have previously received palivizumab during their first RSV season.27 Furthermore, the US Advisory Committee on Immunization Practices40 and the American Academy of Pediatrics41 recommend that infants who initially received fewer than five doses of palivizumab for the season should be given a single dose of nirsevimab. Subsequent doses of palivizumab should not be given. Additionally, there is no required minimum interval between the final dose of palivizumab and the administration of nirsevimab. However, as the protective effect of palivizumab diminishes after 30 days, it is advisable to administer nirsevimab within 30 days following the last dose of palivizumab, whenever feasible.
Availability of nirsevimab in Australia
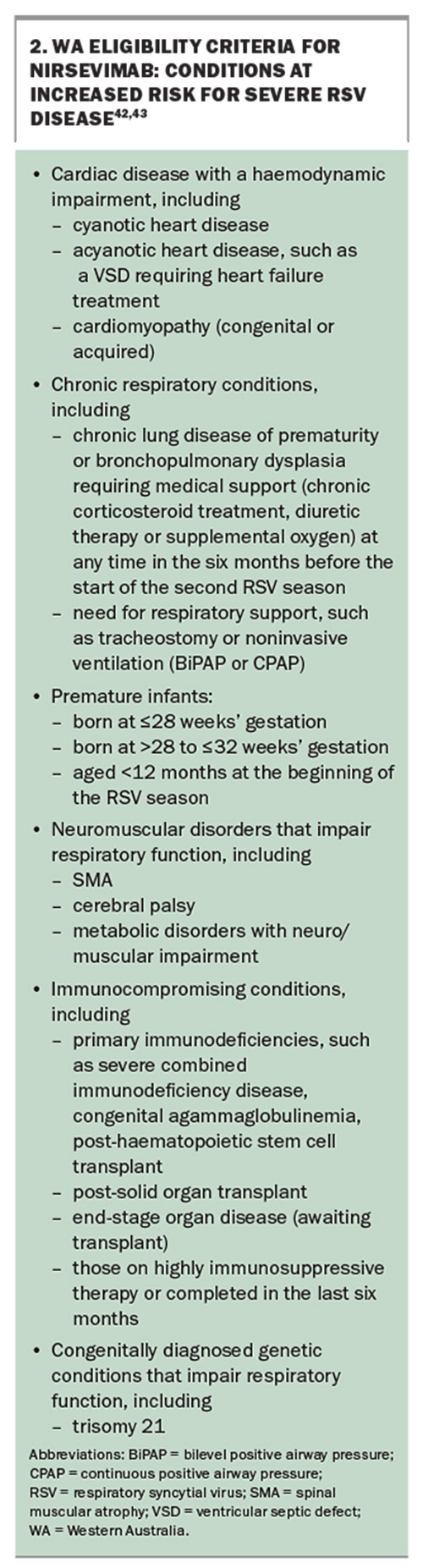
To date, only three Australian states have made nirsevimab available. WA and Queensland offer free, large-scale programs open to all newborns as well as infants at increased risk of severe RSV disease due to complex medical conditions, whereas NSW only offers nirsevimab to certain vulnerable infants. See Box 1, Box 2, Box 3 and Box 4 for the various program details.42-46 In WA and Queensland, nirsevimab is also available through GPs and routine immunisation providers.42-45 In NSW, nirsevimab for eligible infants can only be accessed through treating hospitals.46 Nirsevimab will be considered for General Schedule Restricted Benefit listing on the PBS at the July 2024 meeting of the Pharmaceutical Benefits Advisory Committee.47
New vaccines for active immunisation
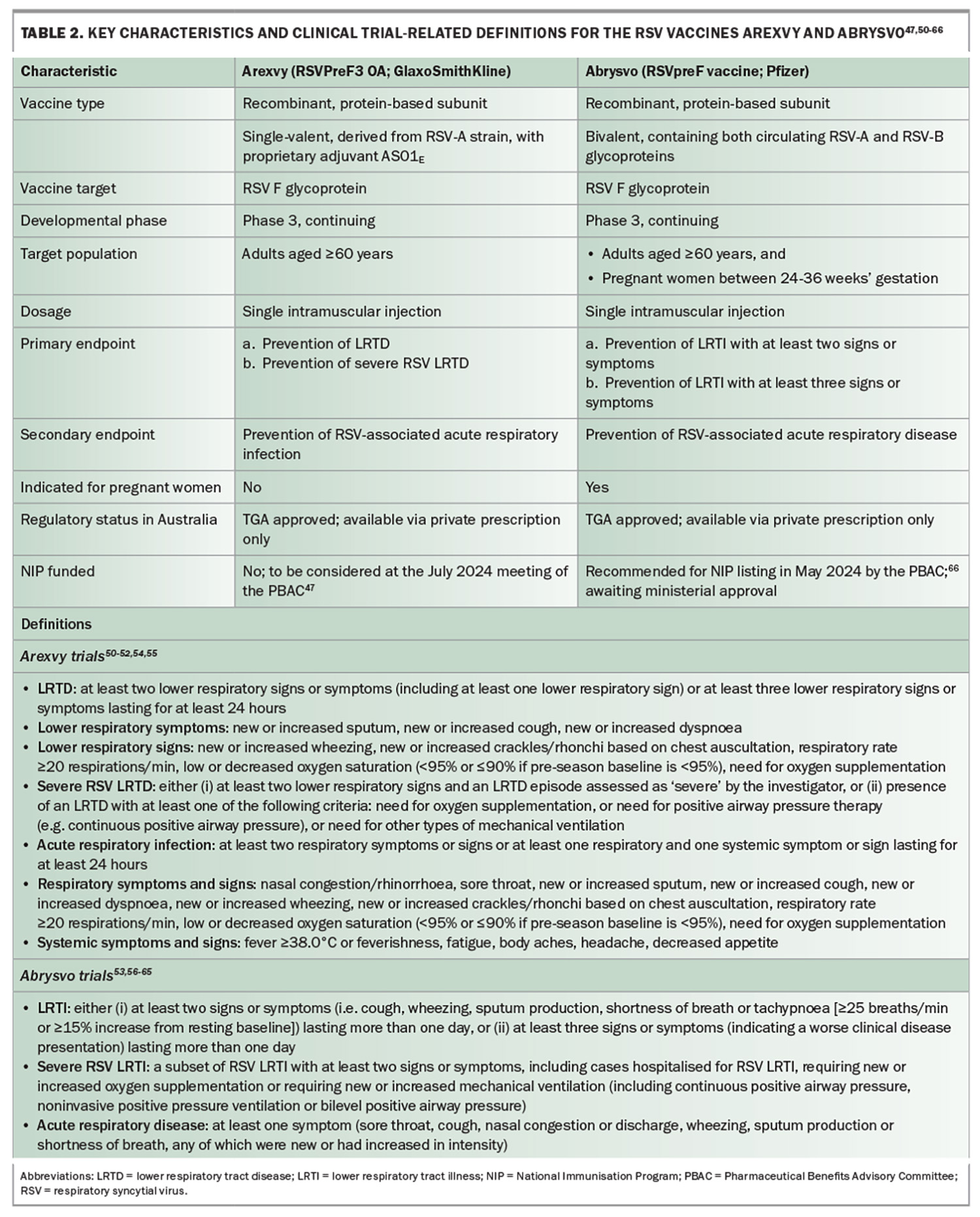
The TGA approval of the RSV vaccines Arexvy48 (RSVPreF3 OA) in January 2024 and Abrysvo49 (RSVpreF vaccine) in April 2024 represents a significant breakthrough for RSV infection prevention in Australia Table 2 summarises the key characteristics and definitions for these vaccines.47,50-66 These vaccines are designed to induce robust immune responses that protect against RSV infection in older adults and, with Abrysvo, in pregnant women with the additional placental transfer of immunity to newborns.67 Both Arexvy and Abrysvo target the prefusion F glycoprotein of RSV, with Arexvy utilising an adjuvant (AS01E) to enhance the immune response in older patients who may have immunosenescence (similar to some influenza vaccines designed for recipients of older age).50
Indications for RSV vaccines
In Australia, Arexvy and Abrysvo are indicated for the active immunisation of individuals aged 60 years and older to prevent LRTD associated with RSV infection.68,69 Abrysvo is also approved for the active immunisation of pregnant women between 24 and 36 weeks’ gestation for the prevention of LRTD caused by RSV in infants from birth through 6 months of age.69 The recently updated Australian Immunisation Handbook recommends RSV vaccination for the following groups:24
- all adults aged 75 years and older
- Aboriginal and Torres Strait Islander people aged 60 years and older
- adults aged 60 years and older with risk factors for severe disease due to RSV (see Box 5)
- pregnant women to protect their newborn infant.
Other adults aged 60 to 74 years may consider vaccination, bearing in mind the benefits may be reduced.
Administration of RSV vaccines
A single-dose Arexvy vaccine consists of two components: a lyophilised, freeze-dried powder in a 3 mL glass vial with a stopper, containing RSV glycoprotein prefusion F stabilised in the prefusion conformation, and a liquid suspension in a 3 mL glass vial with a stopper, containing the GSK proprietary AS01E liposome-based adjuvant system. After reconstitution, one 0.5 mL dose contains 120 micrograms of RSVPreF3 antigen adjuvanted with AS01E. Prior to reconstitution, it must be stored at 2°C to 8°C and protected from light. Once removed from refrigeration, the vaccine should be reconstituted and used within four hours or discarded. Administration is via an intramuscular injection, preferably in the deltoid muscle. Arexvy may be given concomitantly with other vaccines in older adults, including SARS-CoV-2, seasonal influenza, pneumococcal and recombinant zoster (Shingrix) vaccines.24
Each single-dose Abrysvo vaccine consists of three components: sterile water diluent in a 1 mL prefilled type 1 glass syringe with a stopper and a tip cap, lyophilised RSVpreF vaccine in a 2 mL type 1 glass or equivalent vial with a stopper, and a vial adapter. Following reconstitution, one 0.5 mL dose contains 60 micrograms of RSV subgroup A stabilised prefusion F glycoprotein, and 60 micrograms of RSV subgroup B stabilised prefusion F glycoprotein. Prior to reconstitution, it must be stored in its original packaging in a refrigerator at 2°C to 8°C. After reconstitution, Abrysvo should be administered within four hours (maximum room temperature <30°C) and must not be re-stored in a refrigerator (2°C to 8°C) or allowed to freeze. Administration is via an intramuscular injection, preferably in the deltoid muscle.
Efficacy and safety of RSV vaccines
In an ongoing phase 3 clinical trial, a single dose of Arexvy in adults aged 60 years and older (n=24,966 at interim analysis) demonstrated 82.6% efficacy in preventing symptomatic, laboratory-confirmed RSV LRTD (primary endpoint a), 94.1% efficacy against severe RSV LRTD (primary endpoint b) and 71.7% efficacy against RSV-associated acute respiratory infection (secondary endpoint), compared with placebo.51 The vaccine maintained its protective effects throughout the first RSV season (median follow-up: 6.7 months) and was well tolerated, with the most common side effects being mild to moderate injection-site reactions and systemic symptoms, such as fatigue and headache. Recent results analysing one Arexvy dose through a second RSV season (up to 22 months following vaccination) showed a modest, although reduced, efficacy of 67.2% against laboratory-confirmed RSV LRTD, and 78.8% against severe RSV LRTD.52 Revaccination at the beginning of the second season showed no change in efficacy.
Abrysvo demonstrated similar efficacy and safety results in adults aged 60 years and older in the continuing phase 3 RENOIR trial.53 Interim analysis (n=34,284) after the first RSV season (mean follow-up, seven months) showed a 66.7% efficacy in preventing laboratory-confirmed RSV LRTI with two or more signs or symptoms (primary endpoint a), an 85.7% efficacy against RSV LRTI with three or more signs or symptoms (primary endpoint b) and a 62.1% efficacy against RSV-associated acute respiratory disease (secondary endpoint), compared with placebo. The Abrysvo vaccine was also well tolerated, with common side effects including mild to moderate injection-site reactions and fatigue. Results through a second RSV season included in a recent Pfizer press release,70 although not yet peer-reviewed and published, revealed a vaccine efficacy of 65.1% against two or more signs or symptoms after the first season, and 55.7% following the completion of the second season. Even more notable is an efficacy of 88.9% after the first and 77.8% at the conclusion of the second season against three or more signs or symptoms.
The side effects with both vaccines are generally mild, and include soreness at the injection site, fatigue and low-grade fever, with serious adverse reactions being rare.68,69
Agents on the horizon
Several promising medicines for treating RSV infection are in development. These include next-generation antivirals, such as RSV fusion inhibitors and RNA polymerase inhibitors, designed to target critical steps in the viral replication cycle.
In a collaborative study involving Australian researchers, sisunatovir (RV521), an oral inhibitor of RSV fusion to host cells, demonstrated oral bioavailability ranging from 42% to 100% and efficient penetration into lung tissue.71 Furthermore, in healthy adult volunteers experimentally infected with RSV, the use of sisunatovir had a potent antiviral effect, significantly reducing both the viral load and symptoms.71 Another potent, orally bioavailable protein inhibitor, ziresovir (AK0529), in a recently published phase 2 study involving hospitalised infants aged 1 to 24 month(s) with RSV infection (including 20 from subtropical regions of Australia), showed a reduction in viral load with 2 mg/kg twice-daily dosing compared with placebo.72 Disease remission by day 5 was also achieved in 73% of patients compared with 31% receiving placebo (p=0.0412). Rilematovir (JNJ-53718678), another oral RSV fusion inhibitor, significantly reduced the viral load and clinical disease severity in a phase 2 trial.73 A subsequent double-blind, phase 2a trial showed that individuals receiving rilematovir experienced greater reductions in RSV RNA viral loads and faster resolution of key RSV symptoms compared with placebo.74 However, among the subset of high-risk adults (36% of participants), no significant efficacy was observed and, given the small sample size, data interpretation is problematic.
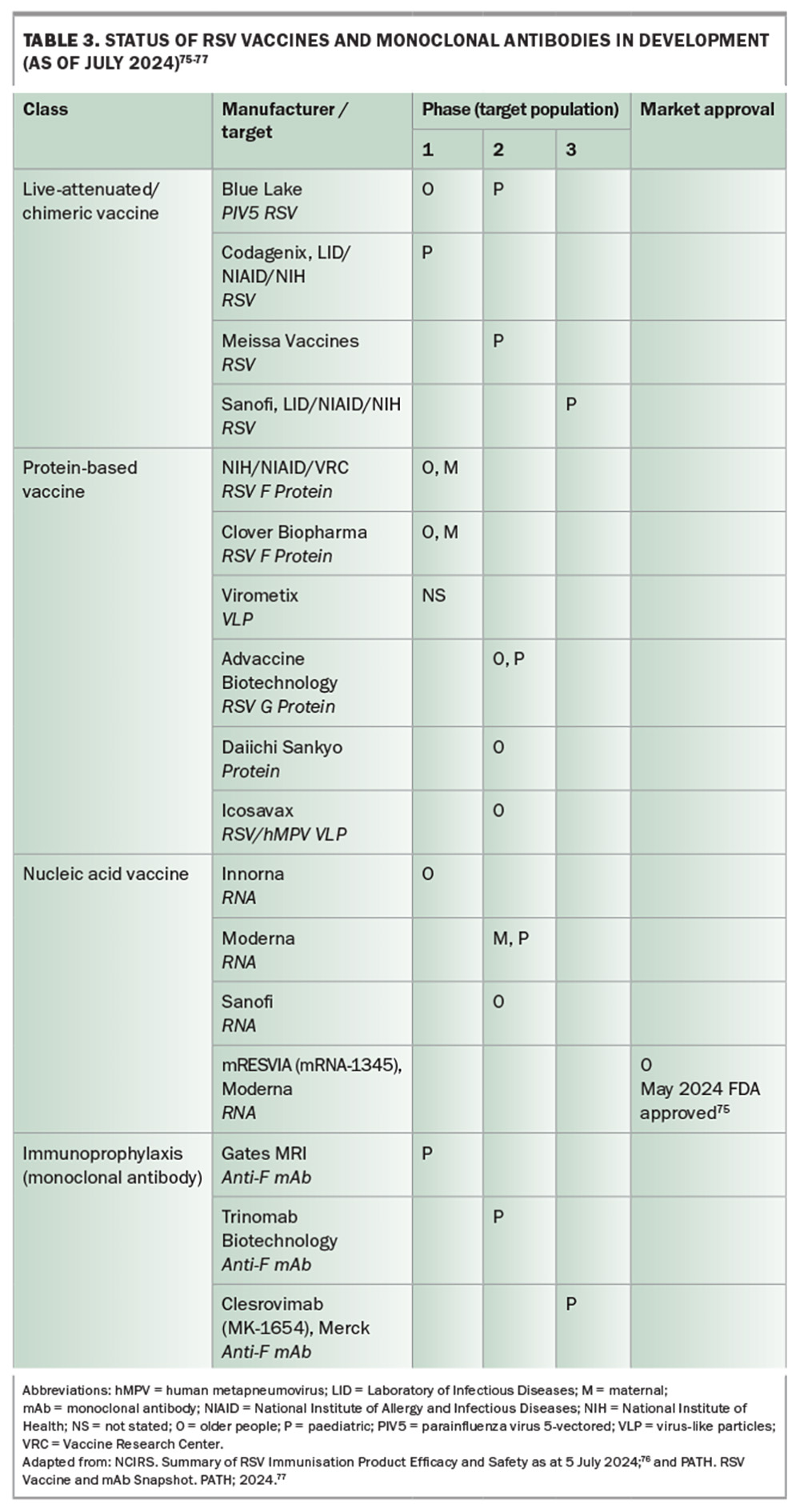
Although these new agents aim to provide more effective treatment options with improved safety profiles compared with existing therapies, such as ribavirin, none of these novel therapies appear close to regulatory approval at the time of writing this article. Additionally, many vaccines and monoclonal antibodies are in various stages of development (see Table 3),75-77 with some targeting different viral epitopes and alternative pathways for controlling RSV infection.
In late May 2024, the US FDA approved mRESVIA (mRNA-1345, Moderna), an mRNA vaccine to protect adults aged 60 years and older from RSV LRTD.75 This approval was based, in part, on an initial analysis (median follow up, 3.3 months) of the phase 2/3 ConquerRSV trial , which showed that the efficacy of mRESVIA was 83.7% against RSV-associated LRTD with at least two signs or symptoms and 82.4% with at least three signs or symptoms, compared with placebo.78,79 No serious safety concerns emerged, with the most frequent adverse effects being injection site pain and fatigue. However, at a meeting of the US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices on 26 June 2024, Moderna presented updated mRESVIA vaccine data (median follow-up, 18.8 months) demonstrating a reduction in efficacy to just 50.3% for RSV-associated LRTD with at least two signs or symptoms, and 49.9% in those with at least three signs or symptoms.79 Despite the lower than expected efficacy, Moderna claims the single-dose regimen in a convenient prefilled syringe containing 50 micrograms of modified mRNA sequence encoding the stabilised prefusion F glycoprotein will save vaccinators’ time and reduce the risk of administration error.79 Following the recent European Medicines Agency’s Committee for Medicinal Products for Human Use positive opinion recommending the granting of marketing authorisation for mRESVIA in the EU (now pending European Commission approval), Moderna is currently preparing further approval applications for multiple countries, including Australia.80
Current challenges of available preventives
The implementation of nirsevimab and RSV vaccines in Australia faces several significant challenges that must be addressed to optimise the prevention and control of RSV infection.
Access and funding
One of the primary challenges in the prevention and control of RSV infection in Australia is the varied access to nirsevimab across different regions. Currently, only two states – WA and Queensland – have made nirsevimab freely accessible to all infants, as well as several paediatric groups at increased risk of severe RSV disease, whereas NSW provides limited access to infants with a narrow range of paediatric medical conditions. This lack of a national approach results in unequal protection against RSV infection, leaving children in other states without access to this vital prophylactic measure and exacerbating disparities in healthcare provision and outcomes.
Additionally, funding issues pose a significant barrier, particularly with the RSV vaccines not included in the National Immunisation Program (NIP) for older individuals. The absence of NIP funding means they may only be available via private prescription, limiting their accessibility to those who can afford about $320 per dose. This financial barrier prevents widespread uptake and diminishes the potential public health impact of the vaccine. Advocacy for NIP inclusion of RSV preventives and interim funding solutions are necessary to broaden access and ensure equitable protection against RSV infection.
Timing challenges related to regional seasonality
Australia’s diverse climate results in different RSV infection seasonality patterns across regions, complicating the timing of immunisation campaigns. For example, RSV activity peaks during the winter months in temperate regions, whereas tropical areas experience year-round circulation with seasonal spikes. This variability makes it challenging to implement a uniform timing strategy for nirsevimab administration and RSV vaccination, potentially impacting the effectiveness of these interventions. In contrast to nirsevimab administration targeted to cover the RSV season only, the UK is considering a year-round immunisation strategy for all infants at birth.81 This approach aims to simplify implementation and address the unpredictability of RSV season onset and duration, although if adopted in Australia, it may offer limited benefit to infants born in temperate regions during spring or early summer. Data from countries using nirsevimab may provide valuable insights for optimising and implementing this strategy.
Vaccine hesitancy and public trust
The landscape of vaccine uptake has been significantly affected by the COVID-19 pandemic, leading to increased vaccine hesitancy, misinformation and a general lack of trust in public health initiatives. This hesitancy extends to new vaccines and, coupled with low public awareness of RSV infection, poses substantial additional barriers to achieving high coverage rates. Public health campaigns must address these concerns directly, providing clear, evidence-based information to rebuild trust and encourage vaccine acceptance.
Confusion over maternal vaccination and nirsevimab
There is considerable confusion among healthcare providers and the public regarding the roles of maternal vaccination and nirsevimab. Maternal vaccination aims to confer passive immunity to newborns through the transplacental transfer of antibodies, whereas nirsevimab provides direct passive immunisation to infants. The simultaneous availability of these two prophylactic options may lead to uncertainty about the best approach for protecting infants, particularly concerning the timing and co-ordination of these interventions. Clear guidelines and education are required to delineate the complementary roles of these preventive measures.
Conclusion
The recent introduction of new vaccines and monoclonal antibodies marks a pivotal shift in the prevention and management of RSV infection. Arexvy, Abrysvo and nirsevimab offer significant improvements over previous interventions, promising to reduce the burden of RSV-related illness, particularly in high-risk populations. However, challenges remain in ensuring broad access and addressing gaps in protection across all age groups. Ongoing research and development are essential to build on these advancements and achieve comprehensive control of RSV. MT
COMPETING INTERESTS: Professor Griffin has received speaker honoraria from Seqirus. Dr Armstrong and Dr Spence: None.
References
1. Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89: 422-434.
2. Woolnough E, Wade A, Sasadeusz J. Ribavirin. In: Grayson ML, Cosgrove SE, Crowe SM, et al., eds. Kucers’ The Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs. 7th ed. Boca Raton, FL: Taylor & Francis Group; 2017. p. 4367-4408.
3. Ison MG. Antiviral treatments. Clin Chest Med 2017; 38: 139-153.
4. Marcelin JR, Wilson JW, Razonable RR; Mayo Clinic Hematology/Oncology and Transplant Infectious Diseases Services. Oral ribavirin therapy for respiratory syncytial virus infections in moderately to severely immunocompromised patients. Transpl Infect Dis 2014; 16: 242-250.
5. Wongsurakiat P, Sunhapanit S, Muangman N. Respiratory syncytial virus-associated acute respiratory illness in adult non-immunocompromised patients: outcomes, determinants of outcomes, and the effect of oral ribavirin treatment. Influenza Other Respir Viruses 2022; 16: 767-779.
6. de Zwart A, Riezebos-Brilman A, Lunter G, et al. Respiratory syncytial virus, human metapneumovirus, and parainfluenza virus infections in lung transplant recipients: a systematic review of outcomes and treatment strategies. Clin Infect Dis 2022; 74: 2252-2260.
7. Ventre K, Randolph AG. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst Rev 2007; (1): CD000181.
8. Chemaly RF, Aitken SL, Wolfe CR, Jain R, Boeckh MJ. Aerosolized ribavirin: the most expensive drug for pneumonia. Transpl Infect Dis 2016; 18: 634-636.
9. Gatt D, Martin I, AlFouzan R, Moraes TJ. Prevention and treatment strategies for respiratory syncytial virus (RSV). Pathogens 2023; 12: 154.
10. Trang TP, Whalen M, Hilts-Horeczko A, Doernberg SB, Liu C. Comparative effectiveness of aerosolized versus oral ribavirin for the treatment of respiratory syncytial virus infections: a single-center retrospective cohort study and review of the literature. Transpl Infect Dis 2018; 20: e12844.
11. Foolad F, Aitken SL, Shigle TL, et al. Oral versus aerosolized ribavirin for the treatment of respiratory syncytial virus infections in hematopoietic cell transplant recipients. Clin Infect Dis 2019; 68: 1641-1649.
12. Burrows FS, Carlos LM, Benzimra M, et al. Oral ribavirin for respiratory syncytial virus infection after lung transplantation: efficacy and cost-efficiency. J Heart Lung Transplant 2015; 34: 958-962.
13. The PREVENT Study Group. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 1997; 99: 93-99.
14. Simoes EA, Sondheimer HM, Top FH, Jr et al. Respiratory syncytial virus immune globulin for prophylaxis against respiratory syncytial virus disease in infants and children with congenital heart disease. The Cardiac Study Group. J Pediatr 1998; 133: 492-499.
15. Johnson S, Oliver C, Prince GA, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis 1997; 176: 1215-1224.
16. Sanders SL, Agwan S, Hassan M, Bont LJ, Venekamp RP. Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection. Cochrane Database Syst Rev 2023; 10: CD009417.
17. Therapeutic Goods Administration. SYNAGIS palivizumab (rmc) 100 mg / 1 mL solution for injection vial (231139). Australian Register of Therapeutic Goods. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://www.tga.gov.au/resources/artg/231139 (accessed July 2024).
18. The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102: 531-537.
19. Anderson EJ, Carosone-Link P, Yogev R, Yi J, Simões EAF. Effectiveness of palivizumab in high-risk infants and children. Pediatr Infect Dis J 2017; 36: 699-704.
20. Therapeutic Goods Administration. Australian public assessment report for Beyfortus. Canberra: Australian Government Department of Health and Aged Care; 2024. 38p. Available online at: https://www.tga.gov.au/sites/default/files/2024-04/auspar-beyfortus-240412.pdf (accessed July 2024).
21. Walsh EE, Englund JA. Respiratory syncytial virus. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 9th ed. Elsevier; 2020. p. 2093-2103.e6.
22. Robbie GJ, Criste R, Dall’acqua WF, et al. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother 2013; 57: 6147-6153.
23. Griffin MP, Khan AA, Esser MT, et al. Safety, tolerability, and pharmacokinetics of MEDI8897, the respiratory syncytial virus prefusion F-targeting monoclonal antibody with an extended half-life, in healthy adults. Antimicrob Agents Chemother 2017; 61: e01714-16.
24. Australian Immunisation Handbook. Respiratory syncytial virus (RSV). Canberra: Australian Government Department of Health and Aged Care; 2024. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/respiratory-syncytial-virus-rsv (accessed July 2024).
25. McLellan JS, Chen M, Leung S, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013; 340: 1113-1117.
26. Zhu Q, McLellan JS, Kallewaard NL, et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med 2017; 9: eaaj1928.
27. Therapeutic Goods Administration. Australian Product Information – Beyfortus (Nirsevimab) Solution for Injection (Sanofi-Aventis Australia Pty Ltd). Australian Register of Therapeutic Goods. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://www.tga.gov.au/resources/artg/397898 (accessed July 2024).
28. Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med 2020; 383: 415-425.
29. Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med 2022; 386: 837-846.
30. Domachowske J, Madhi SA, Simoes EAF, et al. Safety of nirsevimab for RSV in infants with heart or lung disease or prematurity. N Engl J Med 2022; 386: 892-894.
31. Drysdale SB, Cathie K, Flamein F, et al. Nirsevimab for prevention of hospitalizations due to RSV in infants. N Engl J Med 2023; 389: 2425-2435.
32. Paireau J, Durand C, Raimbault S, et al. Nirsevimab effectiveness against cases of respiratory syncytial virus bronchiolitis hospitalised in paediatric intensive care units in France, September 2023 – January 2024. Influenza Other Respir Viruses 2024; 18: e13311.
33. Ernst C, Bejko D, Gaasch L, et al. Impact of nirsevimab prophylaxis on paediatric respiratory syncytial virus (RSV)-related hospitalisations during the initial 2023/24 season in Luxembourg. Euro Surveill 2024; 29: 2400033.
34. Estrella-Porter P, Blanco-Calvo C, Lameiras-Azevedo AS, et al. Effectiveness of nirsevimab introduction against respiratory syncytial virus in the Valencian Community: a preliminary assessment. Vaccine 2024 Jun 3; e-pub (https://doi.org/10.1016/j.vaccine.2024.05.078).
35. Ezpeleta G, Navascues A, Viguria N, et al. Effectiveness of nirsevimab immunoprophylaxis administered at birth to prevent infant hospitalisation for respiratory syncytial virus infection: a population-based cohort study. Vaccines (Basel) 2024; 12: 383.
36. Lopez-Lacort M, Munoz-Quiles C, Mira-Iglesias A, et al. Early estimates of nirsevimab immunoprophylaxis effectiveness against hospital admission for respiratory syncytial virus lower respiratory tract infections in infants, Spain, October 2023 to January 2024. Euro Surveill 2024; 29: 2400046.
37. Mazagatos C, Mendioroz J, Rumayor MB, et al. Estimated impact of nirsevimab on the incidence of respiratory syncytial virus infections requiring hospital admission in children <1 year, weeks 40, 2023, to 8, 2024, Spain. Influenza Other Respir Viruses 2024; 18: e13294.
38. Moline HL, Tannis A, Toepfer AP, et al. Early estimate of nirsevimab effectiveness for prevention of respiratory syncytial virus-associated hospitalization among infants entering their first respiratory syncytial virus season - new vaccine surveillance network, October 2023 – February 2024. MMWR Morb Mortal Wkly Rep 2024; 73: 209-214.
39. Dagan R, Hammitt LL, Seoane Nunez B, et al. Infants receiving a single dose of nirsevimab to prevent RSV do not have evidence of enhanced disease in their second RSV season. J Pediatric Infect Dis Soc 2024; 13: 144-147.
40. Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the advisory committee on immunization practices – United States, 2023. MMWR Morb Mortal Wkly Rep 2023; 72: 920-925.
41. American Academy of Pediatrics (AAP). AAP recommendations for the prevention of RSV disease in infants and children. Itasca, IL: AAP; 2024. Available online at: https://publications.aap.org/redbook/resources/25379/AAP-Recommendations-for-the-Prevention-of-RSV (accessed July 2024).
42. Respiratory syncytial virus (RSV) infant immunisation program: quick reference guide. Perth: Western Australia Government Department of Health; 2024. 2p. Available online at: https://www.health.wa.gov.au/~/media/Corp/Documents/Health-for/Immunisation/RSV/RSV-fact-sheet-quick-reference.pdf (accessed July 2024).
43. Respiratory Syncytial Virus (Rsv) infant immunisation program: fact sheet for providers. Perth: Western Australia Government Department of Health; 2024. 3p. Available online at: https://www.health.wa.gov.au/~/media/Corp/Documents/Health-for/Immunisation/RSV/RSV-Fact-Sheet-Providers.pdf (accessed July 2024).
44. Queensland Paediatric Respiratory Syncytial Virus Prevention Program. Brisbane: Queensland Health; 2024. Available online at: https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/diseases-infection/immunisation/paediatric-rsv-prevention-program (accessed July 2024).
45. Queensland Paediatric Respiratory Syncytial Virus Prevention Program: clinical guidance for immunisation service providers 2024, Version 1.4. Brisbane: Queensland Health; 2024. 17p. Available online at: https://www.health.qld.gov.au/__data/assets/pdf_file/0017/1322144/rsv-prevention-program-clinical-guide.pdf (accessed July 2024).
46. Frequently asked questions about Beyfortus™ (nirsevimab) for health professionals. Sydney: New South Wales Ministry of Health; 2024. Available online at: https://www.health.nsw.gov.au/immunisation/Pages/nirsevimab-professionals.aspx#who (accessed July 2024).
47. Pharmaceutical Benefits Advisory Committee (PBAC) Meeting Agenda – July 2024 PBAC Meeting (Version 4). Canberra: Australian Government Department of Health and Aged Care; 2024. 31p. Available online at: https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/agenda/pdf/2024/PBAC-meeting-agenda-July-2024-v4.pdf (accessed July 2024).
48. Therapeutic Goods Administration. Australian Public Assessment Report for Arexvy. Canberra: Australian Government Department of Health and Aged Care; 2024. 34p. Available online at: https://www.tga.gov.au/sites/default/files/2024-05/auspar-arexvy-240503.pdf (accessed July 2024).
49. Therapeutic Goods Administration. Australian Public Assessment Report for Abrysvo. Canberra: Australian Government Department of Health and Aged Care; 2024. 59p. Available online at: https://www.tga.gov.au/sites/default/files/2024-05/auspar-abrysvo-240502.pdf (accessed July 2024).
50. Leroux-Roels I, Davis MG, Steenackers K, et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F (RSVPreF3) candidate vaccine in older adults: phase 1/2 randomized clinical trial. J Infect Dis 2023; 227: 761-772.
51. Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med 2023; 388: 595-608.
52. Ison MG, Papi A, Athan E, et al. Efficacy and safety of respiratory syncytial virus (RSV) prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons. Clin Infect Dis 2024; 78: 1732-1744.
53. Walsh EE, Perez Marc G, Zareba AM, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med 2023; 388: 1465-1477.
54. Sacconnay L, De Smedt J, Rocha-Perugini V, et al. The RSVPreF3-AS01 vaccine elicits broad neutralization of contemporary and antigenically distant respiratory syncytial virus strains. Sci Transl Med 2023; 15: eadg6050.
55. Dieussaert I, Hyung Kim J, Luik S, et al. RSV prefusion F protein-based maternal vaccine – preterm birth and other outcomes. N Engl J Med 2024; 390: 1009-1021.
56. Schmoele-Thoma B, Zareba AM, Jiang Q, et al. Vaccine efficacy in adults in a respiratory syncytial virus challenge study. N Engl J Med 2022; 386: 2377-2386.
57. Baber J, Arya M, Moodley Y, et al. A phase 1/2 study of a respiratory syncytial virus prefusion F vaccine with and without adjuvant in healthy older adults. J Infect Dis 2022; 226: 2054-2063.
58. Simoes EAF, Center KJ, Tita ATN, et al. Prefusion F protein-based respiratory syncytial virus immunization in pregnancy. N Engl J Med 2022; 386: 1615-1626.
59. Walsh EE, Falsey AR, Scott DA, et al. A randomized phase 1/2 study of a respiratory syncytial virus prefusion F vaccine. J Infect Dis 2022; 225: 1357-1366.
60. Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med 2023; 388: 1451-1464.
61. Alvarez Aldean J, Rivero Calle I, Rodriguez Fernandez R, et al. Cost-effectiveness analysis of maternal immunization with RSVpreF vaccine for the prevention of respiratory syncytial virus among infants in Spain. Infect Dis Ther 2024; 13: 1315-1331.
62. Athan E, Baber J, Quan K, et al. Safety and immunogenicity of bivalent RSVpreF vaccine coadministered with seasonal inactivated influenza vaccine in older adults. Clin Infect Dis 2024; 78: 1360-1368.
63. Baker J, Aliabadi N, Munjal I, et al. Equivalent immunogenicity across three RSVpreF vaccine lots in healthy adults 18-49 years of age: results of a randomized phase 3 study. Vaccine 2024; 42: 3172-3179.
64. Otsuki T, Akada S, Anami A, et al. Efficacy and safety of bivalent RSVpreF maternal vaccination to prevent RSV illness in Japanese infants: subset analysis from the pivotal randomized phase 3 MATISSE trial. Vaccine 2024 Jun 8; e-pub (https://doi.org/10.1016/j.vaccine.2024.06.009).
65. Walsh EE, Falsey AR, Zareba AM, et al. Respiratory syncytial virus prefusion F vaccination: antibody persistence and revaccination. J Infect Dis 2024 Apr 12; e-pub (https://doi.org/10.1093/infdis/jiae185).
66. Pharmaceutical Benefits Advisory Committee (PBAC) meeting outcomes – May 2024 PBAC Meeting. Canberra: Australian Government Department of Health and Aged Care; 2024. 8p. Available online at: https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/pbac-outcomes/2024-05/pbac-web-outcomes-05-2024.pdf (accessed July 2024).
67. Ruckwardt TJ. The road to approved vaccines for respiratory syncytial virus. NPJ Vaccines 2023; 8: 138.
68. Therapeutic Goods Administration. Australian Product Information – Arexvy (Recombinant Respiratory Syncytial Virus Pre-Fusion F Protein) Powder and Suspension for Injection (GlaxoSmithKline Australia Pty Ltd). Australian Register of Therapeutic Goods. Canberra: Australian Government Department of Health and Aged Care; 2024. Available online at: https://www.tga.gov.au/sites/default/files/2024-05/auspar-arexvy-240503-pi.pdf (accessed July 2024).
69. Therapeutic Goods Administration. Australian Product Information – Abrysvo (Recombinant Respiratory Syncytial Virus Pre-Fusion F Protein) Vaccine (Pfizer Australia Pty Ltd). Australian Register of Therapeutic Goods. Canberra: Australian Government Department of Health and Aged Care; 2024. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2024-PI-01489-1&d=20240528172310101 (accessed July 2024).
70. Pfizer announces positive top-line data for full season two efficacy of ABRYSVO for RSV in older adults. [Press release]. New York, NY: Pfizer; 29 February 2024. Available online at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two (accessed July 2024).
71. Cockerill GS, Angell RM, Bedernjak A, et al. Discovery of sisunatovir (RV521), an inhibitor of respiratory syncytial virus fusion. J Med Chem 2021; 64: 3658-3676.
72. Huang LM, Schibler A, Huang YC, et al. Safety and efficacy of AK0529 in respiratory syncytial virus-infected infant patients: a phase 2 proof-of-concept trial. Influenza Other Respir Viruses 2023; 17: e13176.
73. Stevens M, Rusch S, DeVincenzo J, et al. Antiviral activity of oral JNJ-53718678 in healthy adult volunteers challenged with respiratory syncytial virus: a placebo-controlled study. J Infect Dis 2018; 218: 748-756.
74. Nilsson AC, Pullman J, Napora P, et al. A pilot phase 2a, randomized, double-blind, placebo-controlled study to explore the antiviral activity, clinical outcomes, safety, and tolerability of rilematovir at two dose levels in non-hospitalized adults with respiratory syncytial virus infection. Clin Microbiol Infect 2023; 29: 1320-1327.
75. Moderna receives US FDA approval for RSV vaccine mRESVIAR. [Press release]. Cambridge, MA: Moderna; 2024. Available online at: https://investors.modernatx.com/news/news-details/2024/Moderna-Receives-U.S.-FDA-Approval-for-RSV- Vaccine-mRESVIAR/default.aspx (accessed July 2024).
76. National Centre for Immunisation Research and Surveillance (NCIRS). Summary of RSV Immunisation Product Efficacy and Safety as at 5 July 2024. Sydney: NCIRS; 2024. 17p. Available online at: https://ncirs.org.au/sites/default/files/202407/Summary%20of%20RSV%20immunisation%20product%20
efficacy%20and%20safety%20as%20at%204%20July%202024.pdf (accessed July 2024).
77. PATH. RSV Vaccine and mAb Snapshot. PATH; 2024. Available online at: https://www.path.org/our-impact/resources/rsv-vaccine-and-mab-snapshot/ (accessed July 2024).
78. Wilson E, Goswami J, Baqui AH, et al. Efficacy and safety of an mRNA-based RSV preF vaccine in older adults. N Engl J Med 2023; 389: 2233-2244.
79. Das R. Overview of Moderna’s investigational RSV vaccine (mRNA-1345) in adults ≥ 60 years of age. Paper presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC); June 26-28, 2024; Atlanta, GA, USA; 2024. Available online at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2024-02-28-29/02-RSV-Adults-Das-508.pdf (accessed July 2024).
80. European Medicines Agency (EMA). Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 24-27 June 2024. Amsterdam: EMA; 2024. Available online at: https://www.ema.europa.eu/en/news/meeting-highlights-committee-medicinal-products-human-use-chmp-24-27-june-2024 (accessed July 2024).
81. Joint Committee on Vaccination and Immunisation (JCVI). Respiratory Syncytial Virus (RSV) Immunisation Programme for Infants and Older Adults: JCVI Full Statement, 11 September 2023. London, UK: United Kingdom Department of Health and Social Care; 2023. Available online at: https://www.gov.uk/government/publications/rsv-immunisation-programme-jcvi-advice-7-june-2023/respiratory-syncytial-virus-rsv-immunisation-programme-for-infants-and-older-adults-jcvi-full-statement-11-september-2023 (accessed July 2024).