Hypertensive diseases in pregnancy

The hypertensive diseases of pregnancy encompass chronic hypertension, with and without pre-eclampsia, and many other types of blood pressure elevation that can occur before, during and after pregnancy. It is important to carry out ongoing risk assessment for pre-eclampsia at antenatal visits through the identification of elevations in blood pressure or new onset proteinuria before symptoms appear. Placental function testing can also be used for risk assessment and diagnosis.
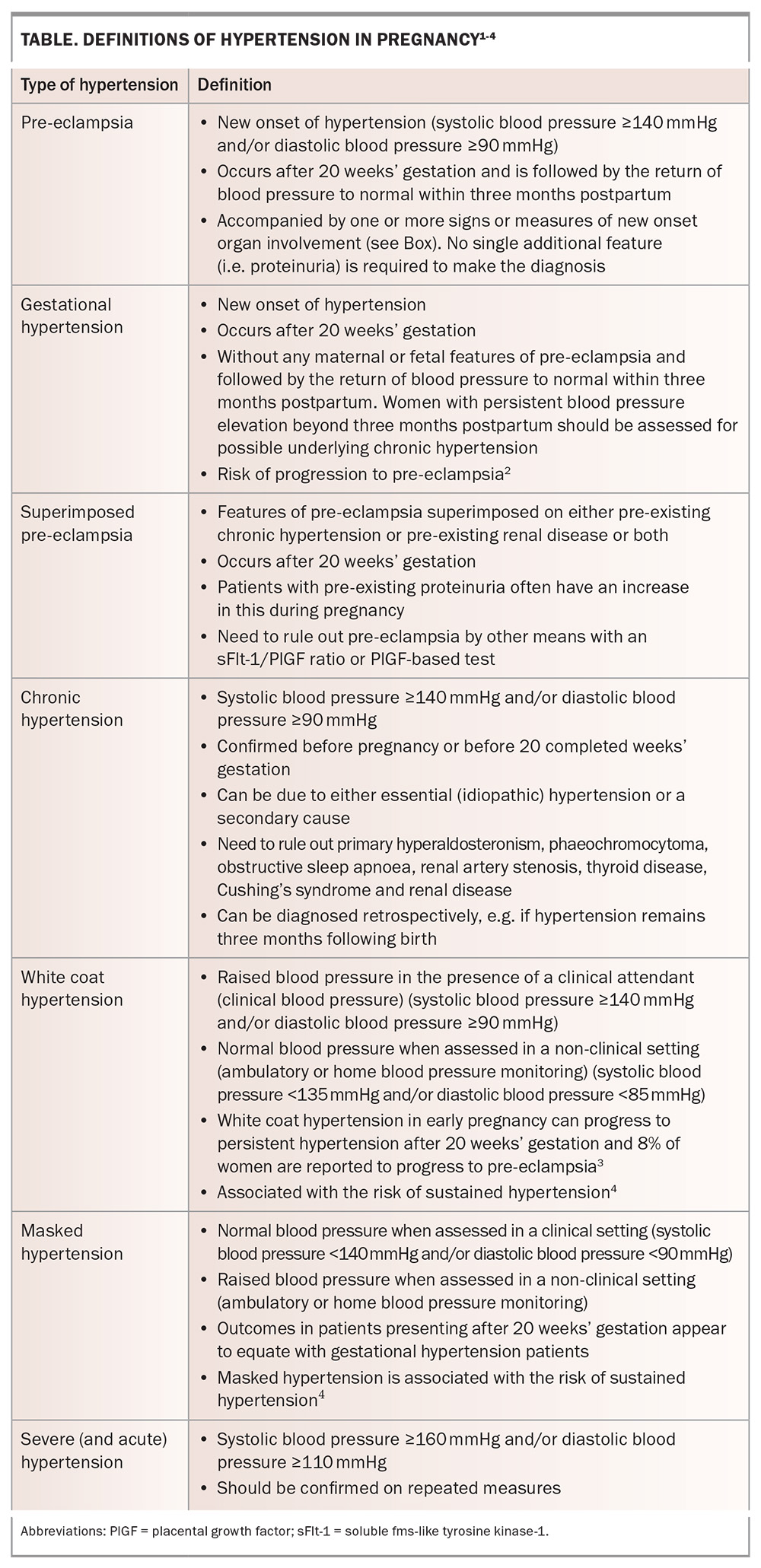
- The types of high blood pressure in pregnancy include pre-eclampsia, chronic hypertension (including secondary, masked and white coat hypertension), superimposed pre-eclampsia (on chronic hypertension), gestational hypertension and severe hypertension.
- Ongoing blood pressure measurement should be carried out to identify blood pressure elevations in pregnancy; new onset proteinuria should also be identified.
- Chronic hypertension, masked hypertension and white coat hypertension are all risk factors for pre-eclampsia, which is associated with poorer maternal and neonatal outcomes.
- The risks associated with pre-eclampsia need to be assessed before pregnancy, early in pregnancy and during pregnancy (for complications in late pregnancy), and also post-pregnancy (for future risk of cardiovascular disease).
- Treatment strategies must factor in pregnancy timing and safety, including when considering prescribing aspirin in early pregnancy for the prevention of pre-eclampsia, controlling elevated blood pressure throughout pregnancy, managing ongoing hypertension during breastfeeding, and managing pain after delivery (avoiding NSAIDS).
- The prompt diagnosis and referral to a hospital setting of women with or at increased risk of pre-eclampsia is an important step in any antenatal clinical assessment.
New hypertension is an easily measured critical component of determining the risk associated with a pregnancy and occurs in 10% of pregnancies. Chronic hypertension, masked hypertension and white coat hypertension are all risk factors for pre-eclampsia, which is associated with poorer maternal and neonatal outcomes. Maternal complications can include unexpected caesarean delivery, stroke, acute kidney injury, liver rupture or death. Complications for the baby can include pre-term delivery, stillbirth, small-for-gestational-age neonates, or admission to intensive care for monitoring of respiratory impacts, feeding and prematurity complications (e.g. intraventricular haemorrhage).
The types of high blood pressure in pregnancy, particularly those associated with difficulties in classification and risk determination for pre-eclampsia, are outlined in this article. The use of placental function testing and the complexities of preventative strategies for pre-eclampsia will also be discussed. These complexities commonly concern women with chronic hypertension, masked hypertension and white coat hypertension, with incidences of these conditions of up to 30% in some communities. For women with pre-eclampsia superimposed on established chronic hypertension, or those who develop de novo pre-eclampsia after 20 weeks of pregnancy, new biochemical measures are available that contribute to the precision of diagnosis and the clinical ability to prepare women and their families for an unexpected or poor outcome of the pregnancy. While pre-eclampsia is the most worrying pregnancy complication of hypertension, poor management of chronic hypertension in early pregnancy can be associated with pregnancy loss or fetal anomalies.
From the perspective of general practice, the journey starts with defining pre-pregnancy hypertension and identifying any relevant secondary cause (such as underlying renal disease, primary aldosteronism, phaeochromocytoma or thyroid disease). Both women with and without pre-existing hypertension should have their blood pressure assessed as early as possible in early pregnancy to identify those who are at risk. This can then facilitate risk mitigation strategies, which are essential for improving outcomes.
The prompt diagnosis and referral to a hospital setting of women with or at increased risk of pre-eclampsia is an important step in any antenatal clinical assessment, especially for those in a GP-initiated or GP-shared model of care. It is also important to measure blood pressure with increased frequency after delivery to help manage it within the context of breastfeeding and postpartum depression (to ensure that any antihypertensive management used is safe in breastfeeding and does not have interactions with medications used for postnatal depression). Those without resolution of hypertension (or those with proteinuria) should be identified so that renal disease or other secondary causes can be investigated and excluded. Decisions about pain relief medication, such as the use of NSAIDS, need to take into consideration any ongoing antihypertensive management to be safe.
Classifications of hypertension in pregnancy
The latest (2023) comprehensive guideline on hypertension in pregnancy from the Society of Obstetric Medicine of Australia and New Zealand (SOMANZ) defines the types of hypertension in pregnancy as pre-eclampsia, gestational hypertension, superimposed pre-eclampsia, chronic hypertension, white coat hypertension and masked hypertension, as outlined in the Table and Box.1-5 Hypertension in pregnancy is defined as systolic blood pressure 140 mmHg or higher and/or diastolic blood pressure 90 mmHg or higher, with measurements confirmed by three consecutive readings at least two minutes apart within a minimum of four hours to confirm true hypertension, or elevated measurements on home blood pressure monitoring.1
Blood pressure monitoring and control
Increasingly, remote blood pressure devices are being used to increase patient confidence in the decision to treat and the capacity to detect elevations in blood pressure as early as possible. It is possible to order 24-hour blood pressure monitoring in a general practice setting, especially to detect masked hypertension or white coat hypertension. Both these variants of chronic hypertension can incur risks to pregnancy.
Treatment strategies for hypertension throughout pregnancy are limited by drug safety concerns.6 Control of volatile or inconsistent elevations can be complex and should be managed in conjunction with a specialist obstetric medical or antenatal service.7 The tight control of blood pressure through frequent review is essential for reducing the risk of progression to pre-eclampsia and for decreasing morbidity in pregnancy.
Risk management and prevention of pre-eclampsia in patients with and without chronic hypertension
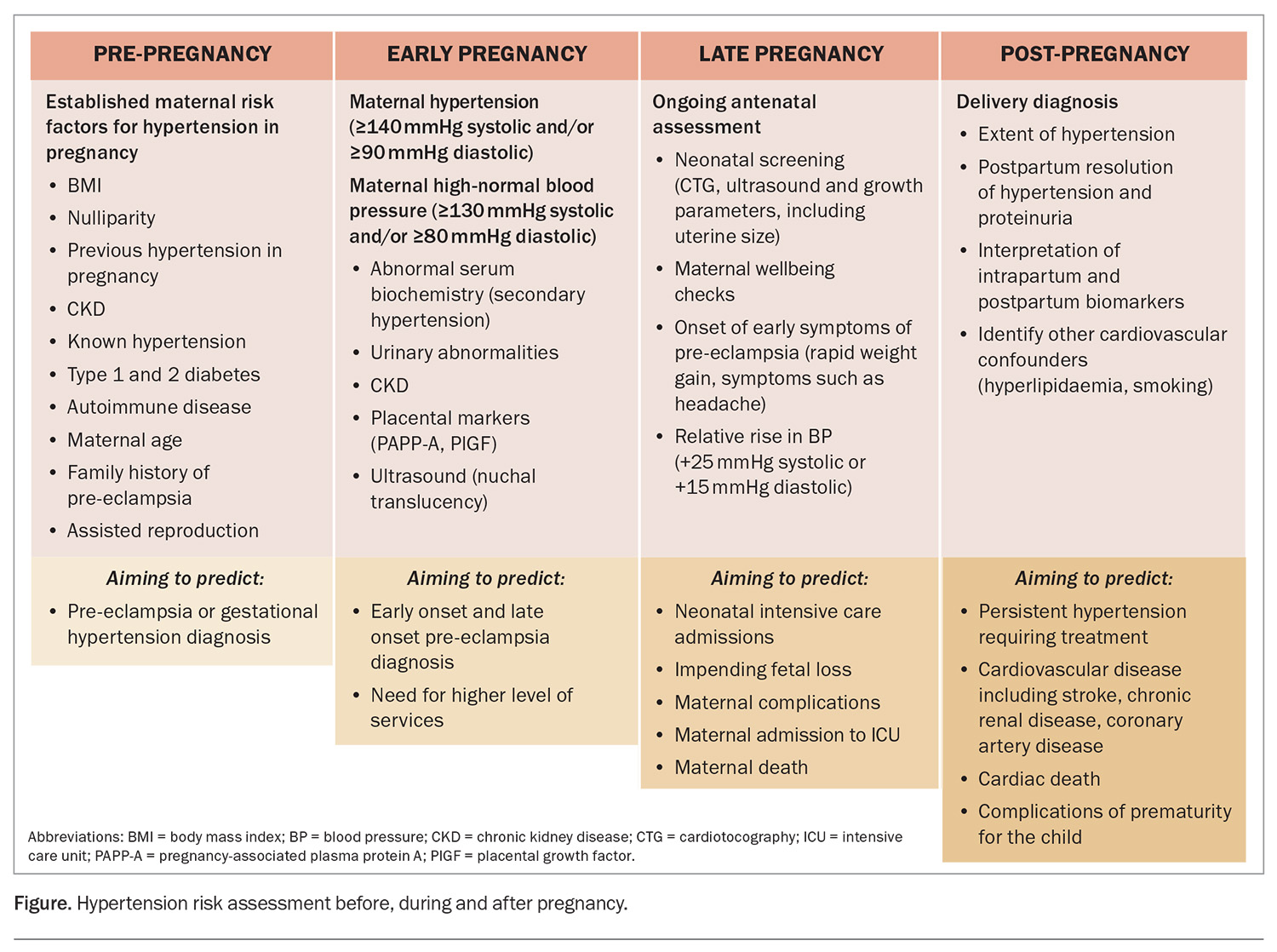
The risk environment for hypertension in pregnancy includes pre-gestation, in gestation and post-delivery phases, with different outcomes in each time period (Figure).
Before pregnancy, risk assessment focuses on factors that may categorise any future pregnancy as high risk. This information is important for the future mother and her family to know, and for determining if prevention strategies are warranted. In the context of blood pressure, any prior hypertension, including as a reaction to the oral contraceptive pill, is classified as chronic hypertension for the purpose of monitoring in pregnancy. For those with chronic hypertension, consideration of medication and a transition to medications that are safer for the baby (i.e. stopping ACE inhibitors) should be planned where possible.
All pregnant women should be assessed in their first trimester for the risk of developing pre-eclampsia. Blood pressure should be measured and proteinuria should be identified early in pregnancy. Screening using biochemical testing and ultrasound for the risk of early onset and late onset pre-eclampsia can help in the choice of level of care for antenatal visits (high risk vs low risk medical clinics, or midwifery-based care). The easiest clinical algorithm uses the UK’s National Institute for Health and Care Excellence (NICE) guideline indicators, which include history and early blood pressure assessment in the mother.8
Asymptomatic renal disease (identified by the presence of proteinuria or the elevation of serum creatinine concentration) may coincidentally be first diagnosed in early pregnancy. Screening ultimately aims to define the risk of pre-eclampsia without presupposing that the events of the pregnancy can be predicted.9,10
Where available, combined first trimester screening is now offered in early pregnancy to predict the risk of hypertensive disease. The screening includes measurement of blood pressure on the initial antenatal visit, the clinical history, a standardised uterine artery pulsatility index test (a highly specialised ultrasound) and serum markers (pregnancy- associated plasma protein A [PAPP-A] and PlGF). These tests attribute a score (rate) for the pregnancy and allow adequate counselling and the development of the appropriate clinic or shared care arrangement for that individual.11,12
The risk assessment score also allows for the consideration of using antenatal low-dose aspirin (150 mg daily, taken at night) before 16 weeks of gestation, which has been shown to decrease the rate of pre-term pre-eclampsia in the ASPRE trial (Combined Multimarker Screening and Randomized Patient Treatment with Aspirin for Evidence-Based Preeclampsia Prevention) and other trials.13 Low-dose aspirin in combination with supplemental calcium (where assessed dietary calcium intake is less than 1 g/day), as well as good blood pressure control, are potential mitigating measures to reduce the rate of superimposed pre-eclampsia in some circumstances.14
The purpose of antenatal visits, particularly with increasing frequency in later pregnancy, is to identify an elevation in blood pressure or new onset proteinuria before the mother is symptomatic. As well as moving towards a delivery plan with the mother, there are opportunities to monitor fetal growth as a marker of placental function. Progressive loss of fetal growth velocity, although not universal in pre-eclampsia, can be an early marker of placental failure.
Increasingly, placental angiogenic or antiangiogenic marker testing is being used to rule out a diagnosis of placental dysfunction. This testing is of most value when there is diagnostic uncertainty, when blood pressure has not reached a level to make a firm diagnosis or in the presence of emerging symptoms. The soluble fms-like tyrosine kinase-1/placental growth factor (PlGF) ratio or a PlGF measure alone, either in the laboratory or at the bedside, are useful emerging measures of placental ischaemia and have been shown to predate the presence of clinical symptoms and signs by up to five weeks.15
Post-pregnancy management of hypertension
Finally, after the pregnancy, it is now realised that ongoing cardiovascular risk is determined by the diagnosis and the extent of the hypertension. Classically, in hypertensive disorders of pregnancy, blood pressure will return to normal by three months postpartum. Any persistent hypertension is either unmasking of chronic hypertension or a return to a previous hypertensive state; this should be treated as per usual hypertension guidelines.
In the event of persistent hypertension, there is the opportunity to consider cardiovascular risk factors, including hyperlipidaemia, smoking and new-onset diabetes (especially after gestational diabetes), and to provide advice and intervention to minimise these risk factors in the longer term.16-18 Taking reproductive histories and recognising the difference in risk and outcomes for women’s cardiovascular health that result from pregnancy are the best ways in which rates of cardiovascular death can be reduced for women.19
Conclusion
Hypertension, including chronic hypertension, masked hypertension and white coat hypertension, is a vital component of determining the risks associated with a pregnancy. Persistent hypertension after delivery signals chronic hypertension and is a major contributor to future cardiovascular risk. Hypertension is a readily treated risk factor and a diagnostic characteristic of pre-eclampsia, the careful treatment of which can reduce the complications of the pregnancy for the mother and baby, and hypertension later in life. MT
COMPETING INTERESTS: Professor Hennessy has received support for attending meetings and travel from Western Sydney University. Dr Rajkumar: None.
References
1. Shanmugalingam R, Barrett HL, Beech A, et al. A summary of Society of Obstetric Medicine Australia and New Zealand (SOMANZ) Hypertension in Pregnancy Guideline (2023). MJA 2024; 220: 582-591.
2. Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol 1998; 105: 1177-1184.
3. Brown MA, Mangos G, Davis G, Homer C. The natural history of white coat hypertension during pregnancy. BJOG 2005; 112: 601-606.
4. Mancia G, Bombelli M, Facchetti R, et al. Long-term risk of sustained hypertension in white-coat or masked hypertension. Hypertension 2009; 54: 226-232.
5. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004; 350: 672-683.
6. Beech A, Mangos G. Management of hypertension in pregnancy. Aust Prescr 2021; 44: 148-152.
7. Mancia G, Bombelli M, Seravalle G, Grassi G. Diagnosis and management of patients with white-coat and masked hypertension. Nat Rev Cardiol 2011; 8: 686-693.
8. Tan MY, Wright D, Syngelaki A, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol 2018; 51: 743-750.
9. Poon LC, Kametas NA, Pandeva I, Valencia C, Nicolaides KH. Mean arterial pressure at 11(+0) to 13(+6) weeks in the prediction of preeclampsia. Hypertension 2008; 51: 1027-1033.
10. Sonek J, Krantz D, Carmichael J, et al. First-trimester screening for early and late preeclampsia using maternal characteristics, biomarkers, and estimated placental volume. Am J Obstet Gynecol 2018; 218: 126.e1-.e13.
11. Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther 2013; 33: 8-15.
12. Chaemsaithong P, Pooh RK, Zheng M, et al. Prospective evaluation of screening performance of first-trimester prediction models for preterm preeclampsia in an Asian population. Am J Obstet Gynecol 2019; 221: 650.e1-.e16.
13. Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017; 377: 613-622.
14. Wright D, Wright A, Magee LA, Von Dadelszen P, Nicolaides KH. Calcium supplementation for the prevention of pre-eclampsia: challenging the evidence from meta-analyses. BJOG 2024; 131: 1524-1529.
15. Barton JR, Woelkers DA, Newman RB, et al. Placental growth factor predicts time to delivery in women with signs or symptoms of early preterm preeclampsia: a prospective multicenter study. Am J Obstet Gynecol 2020; 222: 259.e1-.e11.
16. Hermes W, Ket JC, van Pampus MG, et al. Biochemical cardiovascular risk factors after hypertensive pregnancy disorders: a systematic review and meta-analysis. Obstet Gynecol Surv 2012; 67: 793-809.
17. Henry A, Arnott C, Makris A, et al. Blood pressure postpartum (BP2) RCT protocol: follow-up and lifestyle behaviour change strategies in the first 12 months after hypertensive pregnancy. Pregnancy Hypertens 2020; 22: 1-6.
18. Vogel B, Acevedo M, Appelman Y, et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet 2021; 397: 2385-2438.
19. Hasdarngkul A, Hennessy A, Vignarajan S. Short communication: where is women’s cardiovascular health taught in Australian and New Zealand medical schools? Aust N Z J Obstet Gynaecol 2024; 64: 165-167.