Resistant hypertension: an approach to management
Resistant hypertension (RH) is a prevalent and significant cause of morbidity and mortality. Adverse health outcomes, including cardiovascular disease, hypertension-mediated organ damage and chronic kidney disease, can be significant. This article describes the means for GPs to identify predisposing and contributing factors to RH and recommends an evidence-based approach to diagnosis and management.
- Resistant hypertension (RH) affects about 10 to 15% of treated patients diagnosed with hypertension and is associated with an increased risk of adverse cardiovascular outcomes and hypertension-mediated organ damage (HMOD).
- True RH must be confirmed by adequate in-office and out-of-office blood pressure (BP) measurements (home or ambulatory). Common causes of apparent RH include white-coat hypertension, nonadherence with prescribed antihypertensive therapy, inadequate antihypertensive combination therapy and the use of interfering concomitant medications.
- Obesity, obstructive sleep apnoea and renal parenchymal disease are among the most common contributing features of RH; affected patients should be screened for secondary causes of hypertension (especially primary aldosteronism) regardless of their age.
- Management of RH relies on lifestyle measures (maintaining a healthy weight through regular physical activity and a healthy diet, salt restriction, limiting alcohol intake and smoking cessation), pharmacotherapy and interventional approaches, where required.
- Pharmacotherapy includes a combination of a renin-angiotensin-system blocker, a long-acting calcium channel blocker and a diuretic at maximally tolerated doses, ideally as a single pill combination.
- Spironolactone is currently recommended as the preferred fourth-line therapy, with alpha blockers, beta blockers, centrally acting sympatholytic agents, or vasodilators as alternatives.
- If BP control cannot be achieved with the above strategies, interventional approaches such as renal denervation and novel therapeutic agents (once available) should be considered.
Hypertension affects nearly one billion individuals worldwide and is a common presentation in general practice. It is one of the most significant modifiable risk factors for disability and death worldwide.1 The global prevalence of resistant hypertension (RH) is 5 to 30%; this wide range is due to variability in the definition and the analysed population among different studies. The prevalence is estimated to be about 10 to 15% in the treated population based on the latest RH definitions (excluding apparent causes).2
Defining resistant hypertension
The European Society of Cardiology/European Society of Hypertension (ESC/ESH) 2018 guidelines defined RH as a diagnosis given to patients who are above the target blood pressure (BP) despite being on maximally tolerated doses of three antihypertensive drug classes, including a diuretic, and preferably a long-acting calcium channel blocker and a renin-angiotensin system blocker (i.e. ACE inhibitor or angiotensin receptor blocker [ARB]).3 Patients who require treatment with four or more classes of antihypertensive medication are also considered to have RH, regardless of their BP.4
The American College of Cardiology/American Heart Association 2018 hypertension guidelines adopt a lower office BP threshold of 130/80 mmHg or higher for the definition of hypertension, compared with most other guidelines (including those for Australia), which propose an office BP threshold of 140/90 mmHg or higher.4
RH is associated with a higher incidence of adverse cardiovascular outcomes, including myocardial infarction, cerebrovascular events and congestive heart failure.5 It is also associated with a higher incidence of hypertension-mediated organ damage (HMOD), chronic kidney disease (CKD) and all-cause mortality.6 Hence, it is crucial to identify and treat RH aggressively to improve outcomes.
Risk factors and pathophysiology
Predictive risk factors for difficult-to-control hypertension include:7
- higher baseline BP (especially systolic)
- older age
- obesity
- African-American heritage
- male sex
- presence of left ventricular hypertrophy
- CKD
- diabetes.
The underlying pathophysiology of RH is a combination of volume and sodium retention, aldosterone excess and sustained increased activity of the sympathetic nervous system and the renin-angiotensin-aldosterone system.8-11 The endothelin-1 pathway is another important contributor to the pathophysiology of RH; blockade of this pathway has recently demonstrated promising outcomes in the management of RH.12
Clinical assessment
The patient should be asked about potential contributing lifestyle and dietary factors, including their salt and alcohol intake, weight and height, level of physical activity and sleep patterns. Details of personal and family history of hypertension and presence of cardiovascular risk factors should be obtained.
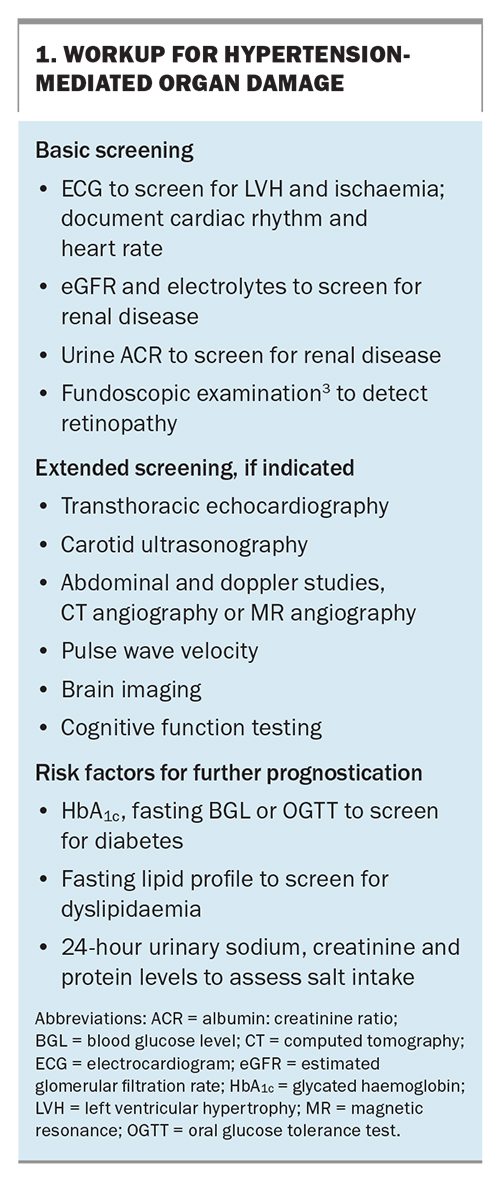
On physical examination, the signs of secondary hypertension as well as evidence of HMOD, including hypertensive retinopathy, left ventricular hypertrophy, microalbuminuria and CKD, should be sought. The basic and extended screening tests for HMOD are detailed in Box 1.
Diagnosis
RH can be true or apparent. Diagnosis of true RH requires the exclusion of undetected secondary causes of hypertension and pseudoresistant hypertension, which is defined as seemingly treatment-resistant hypertension that is primarily due to interfering factors in BP measurement or treatment.3
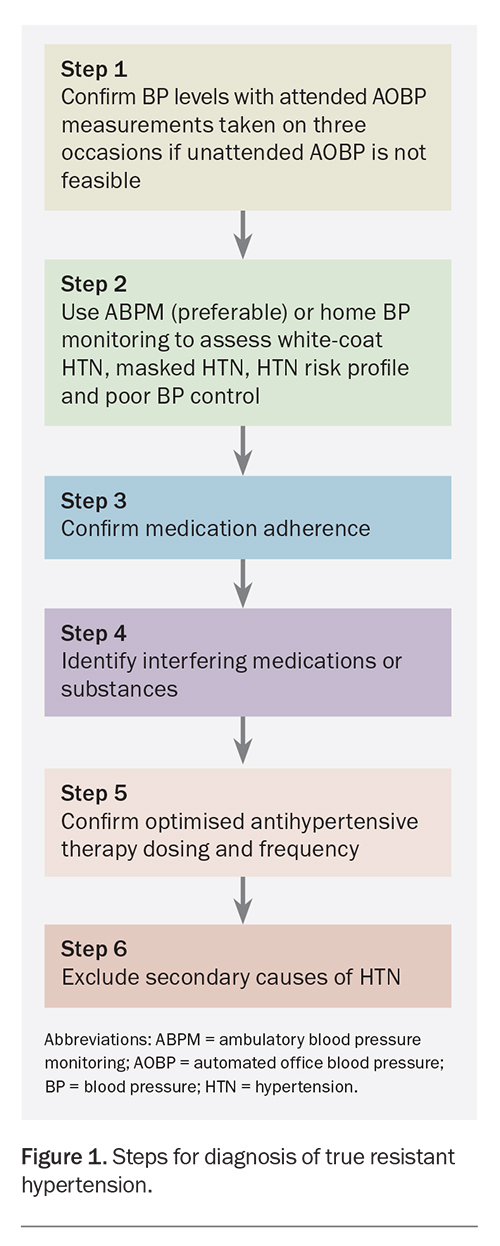
The ESC/ESH 2018 guidelines list five of the most common causes of pseudoresistant hypertension to be excluded in confirming the diagnosis of true RH. These include poor adherence to antihypertensive therapy, poor office BP measurement techniques, suboptimal antihypertensive therapy, white-coat phenomenon, and severe brachial artery calcification.3 Figure 1 summarises the proposed steps in diagnosing true RH.
Diagnostic steps
Step 1. Measure BP accurately
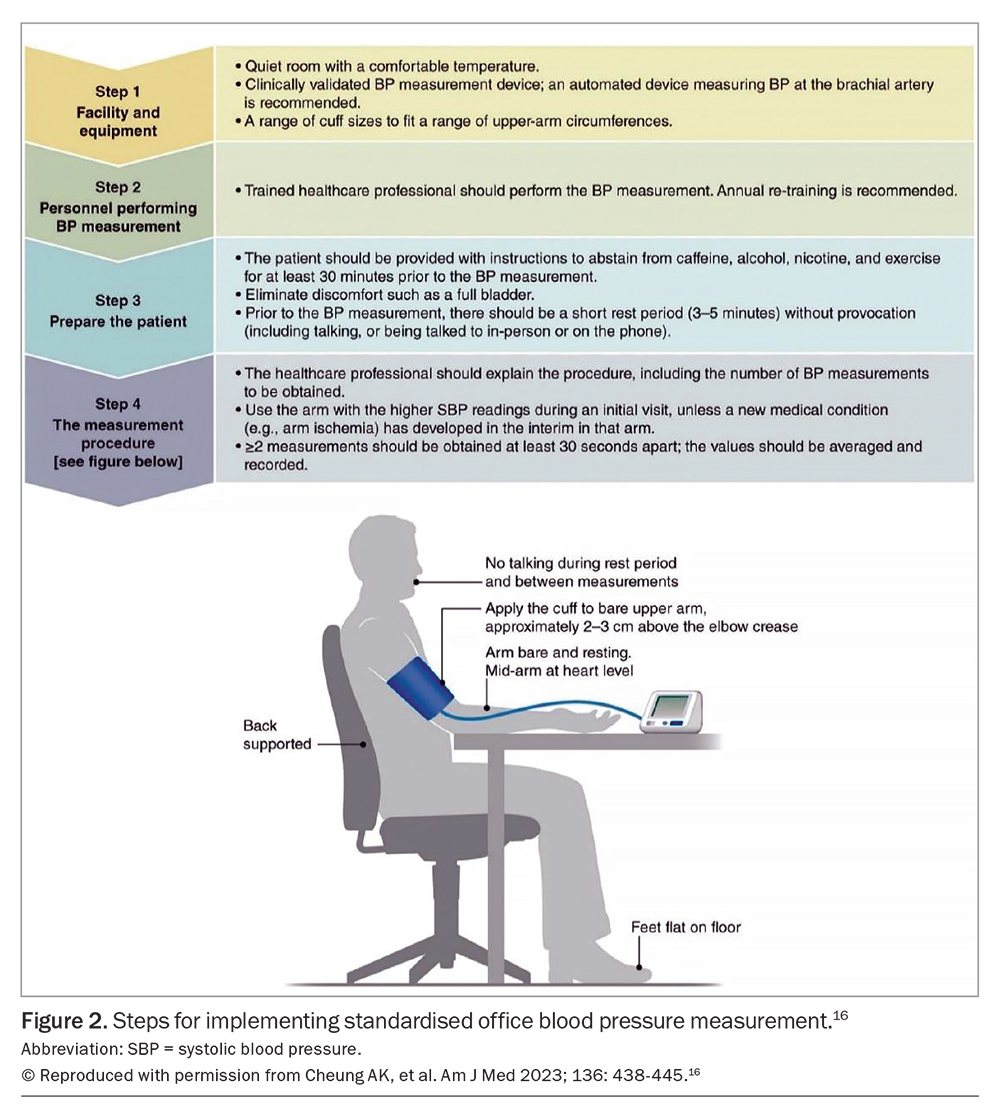
Office BP measurement remains the basis of diagnosing and managing hypertension. Automated office BP (AOBP), with the attending clinician absent from the room, is the most reliable method of office BP measurement, and was used in The Systolic BP Intervention Trial (SPRINT) and other studies.13,14 However, the triplicate standardised attended AOBP is the most feasible method in primary care. If attended AOBP is performed on three or more occasions under standardised conditions, the result is comparable in reliability to unattended AOBP.15 Figure 2 displays the international consensus on standardised AOBP.16
Step 2. Exclude white-coat hypertension, masked hypertension and poor BP control
White-coat hypertension is defined as elevated office BP, but normal out-of-office BP. It is estimated that white-coat hypertension might be present in 28 to 39% of individuals with apparent RH.2 The white-coat phenomenon can be significantly reduced or eliminated by AOBP that is unattended.17 However, relying solely on office BP measurements can result in missed diagnoses of RH in patients with masked hypertension; such patients may have BP exceeding the recommended targets during ambulatory blood pressure monitoring (ABPM), but not during office BP measurement. Additionally, the initial reason for a patient’s clinic visit, such as pain or other medical issues, could influence the office BP readings.18
Patients whose RH was confirmed by ABPM have a higher prevalence of HMOD and cardiovascular disease.19 ABPM recordings provide additional relevant indices, such as 24-hour BP variability, morning BP surge, and ambulatory arterial stiffness index, which have some predictive value. Overall, there is clear evidence that office BP measurements alone are inadequate in identifying RH. ABPM with or without home BP monitoring must be used to confirm the diagnosis.3
Step 3. Confirm adherence to medication
Partial medication adherence and complete nonadherence occurs in 17 to 46% and 9 to 35% of patients with RH, respectively.19 The high prevalence of nonadherence in this patient group is in part attributable to polypharmacy, complex medication regimens, high incidence of medication intolerance and adverse effects and poor patient–clinician relationship.13 Some of the practical methods to assess medication adherence include direct questioning about adherence in a nonjudgemental manner, medication adherence questionnaires, pill counts, and electronic pill boxes. The less feasible (but more reliable) methods of assessing adherence include biochemical assay of medications or their metabolites in blood or urine, as well as observed pill intake when patients’ medication is administered in the clinic with their BP measured before and several hours after medication consumption.19
Step 4. Identify interfering substances or medications
Concurrent use of a variety of medications (e.g. over-the-counter medications, substances and homeopathic medications) can raise BP or interfere with the effects of antihypertensives by increasing sympathetic activity, increasing intravascular volume or decreasing sodium excretion.2 NSAIDs are possibly the most common drugs to raise BP or interfere with effects of antihypertensives.3 Some of the other common agents that affect BP include homeopathic medications, decongestants, stimulants used for weight loss (e.g. phentermine), oral contraceptives, recreational drugs (e.g. cocaine, anabolic steroids), ciclosporin and corticosteroids, through volume retention.2
Step 5. Confirm optimised antihypertensive therapy dosing and frequency
Failure of treatment intensification is a common cause of high BP.20,21 It is crucial to optimise the dose of all three recommended antihypertensive medications in RH to the maximum tolerated dose.
Step 6. Exclude secondary causes of hypertension
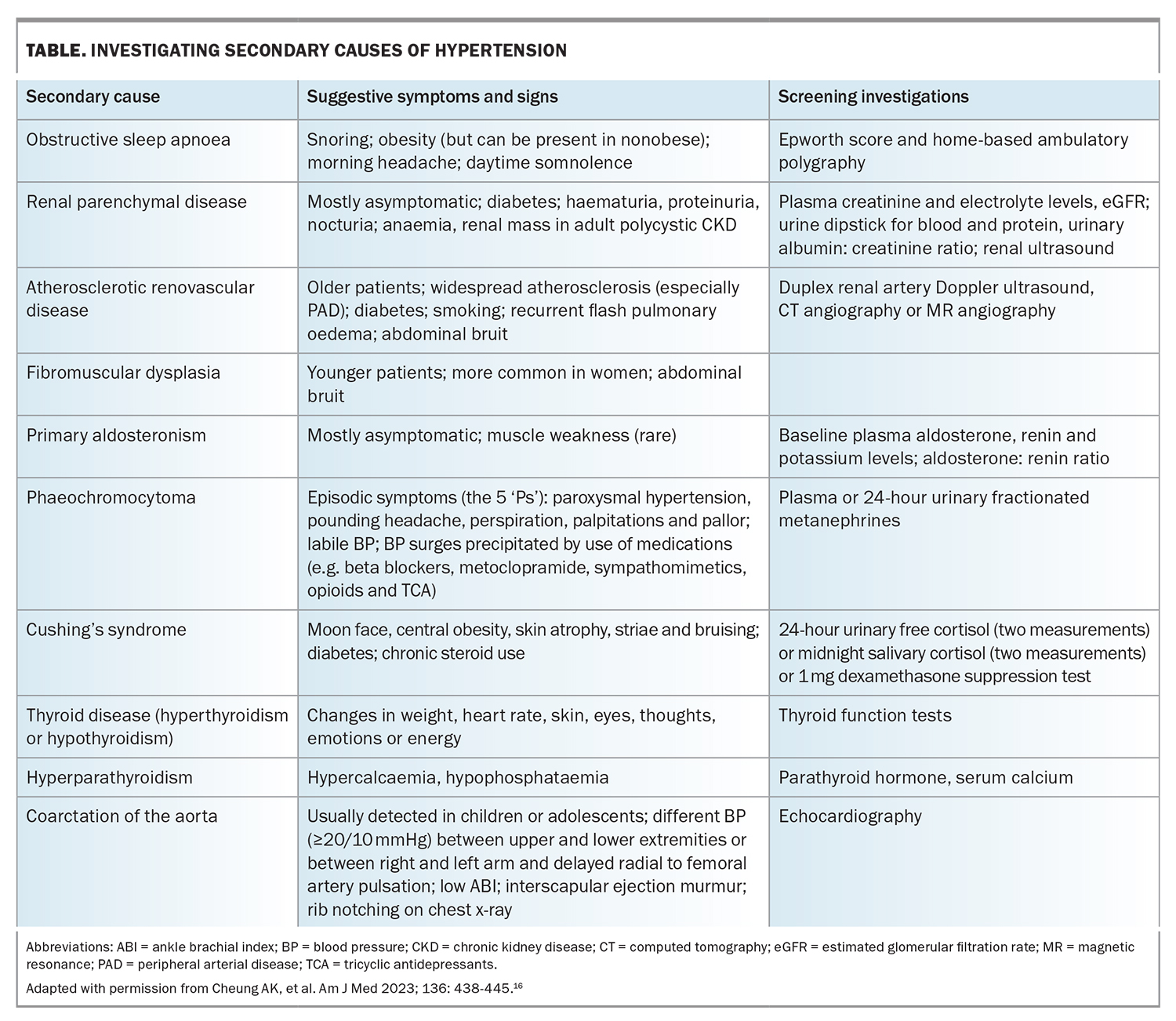
The prevalence of secondary causes of hypertension is approximately 10% in adult populations.22 Obstructive sleep apnoea, primary aldosteronism and renovascular and renal parenchymal diseases are among the most prevalent secondary causes of hypertension. It is important to remember that secondary causes of hypertension can also occur in the elderly.22,23
RH is a common indication for secondary hypertension screening, in addition to grade 3 hypertension, grade 2 hypertension in adults younger than 40 years of age, acute worsening in previously well-controlled cases of hypertension, evidence of extensive HMOD, clinical or biochemical signs of secondary causes and hypertension in children and adolescents.3 A list of secondary causes of hypertension and the initial screening is outlined in the Table.
Interpreting some initial screening tests for RH (e.g. aldosterone-renin ratio to exclude primary hyperaldosteronism) may pose challenges if patients are taking antihypertensive medications that could interfere with the accuracy of these tests.24 GPs should seek the opinion of relevant specialists, particularly if the initial screening tests yield results that are inconclusive or suggestive of secondary causes.
Management
The general principles of treatment for RH are the same as those for milder forms of hypertension, including:
- the modification of contributory lifestyle factors
- the cessation of interfering substances/medications, where possible
- the sequential addition of guideline-recommended antihypertensive agents with different modes of action, ideally in the form of single pill combinations to improve adherence
- using the full armamentarium of antihypertensive drugs, where required.
Promising novel drugs for RH, such as the dual endothelin antagonist, aprocitentan, and the aldosterone synthase inhibitor, baxdrostat, are in the advanced stages of clinical development and may facilitate improved BP control in RH when they become available.12,25 Interventional approaches, such as renal denervation, should be considered a safe and effective means of achieving sustained BP reduction at various stages of the patient’s management.26
Lifestyle modification
Patients with RH are more likely to be nonadherent to lifestyle and dietary measures, such as limiting alcohol intake, reducing sodium intake, maintaining a healthy weight and performing regular physical activity.3
Regular light to moderate physical activity (including long-term aerobic exercise and heated pool exercise) showed favourable outcomes as a successful BP lowering intervention.27,28
Smoking cessation and minimal alcohol intake should be recommended in individuals with RH, as with all other patients diagnosed with hypertension.29
Overweight and obese individuals with RH should aim for a 6 to 8% decrease in body weight to achieve a 5 and 4 mmHg reduction in systolic and diastolic BP, respectively.29
High dietary sodium intake significantly contributes to RH and is a well-known factor in antihypertensive medication resistance. A reduced sodium intake of less than 1500 mg daily decreases office systolic and diastolic BP readings by 22.7 and 9.1 mmHg, respectively.30 The BP-lowering effect of sodium restriction is similarly profound in patients with CKD and RH.31 Diets, such as the DASH and Mediterranean diets, can help with BP control, but have not been well studied in the management of RH (Box 2).
Pharmacotherapy
The antihypertensive regimen for the management of RH is usually based on comorbidities, underlying secondary causes, previous medication intolerances and financial constraints.
A common and effective combination includes an ACE inhibitor or ARB, a long-acting calcium channel blocker and a thiazide or thiazide-like diuretic. This combination should be prescribed at maximally tolerated doses at an appropriate frequency, with reference to the half-life of the medications involved.
Maximised diuretic therapy is essential for the treatment of RH, as volume retention and sodium excess are common causes of RH, even though obvious signs of hypervolaemia (such as lower limb oedema) may be absent.30-32 An important consideration for the management of ongoing residual hypertension is the switching of thiazide therapy to a more potent diuretic (e.g. indapamide or chlorthalidone) if the estimated glomerular filtration rate (eGFR) is 30 mL/min/1.73 m2 or greater. Chlorthalidone is frequently used in the USA, but rarely prescribed in Australia, despite being a more potent diuretic with a longer half-life than hydrochlorothiazide.35
The recently published results from the Diuretic Comparison Project suggested no significant difference in cardiovascular outcomes and noncancer-related deaths between chlorthalidone and hydrochlorothiazide, but found more frequent hypokalaemia with the use of chlorthalidone.36 Given that single pill combinations are associated with improved adherence, hydrochlorothiazide and indapamide are likely to remain the preferred choices, as, unlike chlorthalidone, they are both available in combination with renin-angiotensin system blockers as single pill combinations. If a patient’s eGFR is 30 mL/min/1.73 m2 or lower, the benefit of thiazide-like therapy is less, and loop diuretics (e.g. frusemide or bumetanide) can be added to the regimen or used as a substitute.3
The mineralocorticoid receptor antagonist (MRA) spironolactone is currently considered the preferred fourth-line agent, to be used in addition to previously maximised combination therapy.8,37 If spironolactone is not tolerated, eplerenone can be used as an alternative.38 The potassium-sparing diuretic amiloride is no longer available in Australia. Common adverse effects of MRAs include hyperkalaemia and reduction in eGFR, which require close monitoring. With particular reference to spironolactone, high doses of MRAs can cause gynaecomastia and erectile dysfunction and preferably should not be prescribed at doses greater than 50 mg daily in people with primary RH. Such adverse effects are minimal with eplerenone, which is less potent and requires twice daily dosing due to its shorter half-life.
There are limited data to guide the choice of a fifth-line antihypertensive agent, if uncontrolled hypertension persists despite the recommended regimens. A reasonable approach is to add further agents based on comorbidities and the patient’s preference.
Patients with a baseline heart rate above 70 beats per minute and those with a diagnosis of coronary artery disease or heart failure would benefit from the addition of beta blockers. Labetalol, carvedilol and nebivolol have vasodilatory effects and are more effective in lowering BP compared with other commonly used beta blockers.39
Centrally acting agents (e.g. methyldopa or clonidine) can be used to suppress sympathetic nervous activity, which is usually elevated in RH. In the Resistant Hypertension Optimal Treatment (ReHOT) trial, clonidine was shown to have similar efficacy to spironolactone in lowering BP; however, common adverse effects of clonidine include somnolence, dry mouth sensation and rebound phenomena.40 More advanced and better tolerated centrally acting sympatholytic agents, such as the selective imidazoline receptor agonist moxonidine, are a useful alternative.
Other available medications for RH management include alpha blockers (e.g. prazosin) or direct vasodilators (e.g. hydralazine or minoxidil). The latter group should generally be used with a loop diuretic to address the common adverse effect of hypervolaemia and lower limb oedema.3
Promising novel drugs
New classes of medications with substantial BP-lowering efficacy are under investigation. Baxdrostat, an aldosterone synthase inhibitor, resulted in reduced office systolic BP readings over 12 weeks of 20.3, 17.5 and 12.1 mmHg at 2 mg, 1 mg and 0.5 mg doses, respectively, confirming the significant dose-related BP-lowering effect when added to stable doses of three antihypertensive medications in the management of RH.25 Aprocitentan, a dual endothelin antagonist, was recently shown to have a significant dose-dependent reduction in office and 24-hour ABPM after four weeks of therapy in individuals with RH on three background antihypertensive medications. The greatest BP-lowering effect was evident in elderly patients and those with macroalbuminuria and stage 3 to 4 CKD.12
Interventional therapies
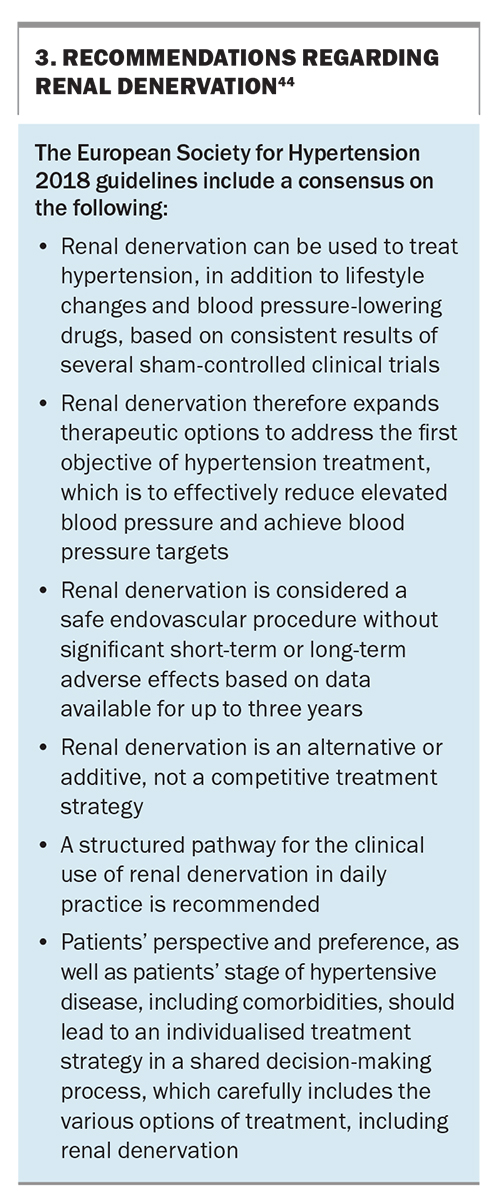
Interventional approaches (e.g. renal denervation) have been shown in multiple sham-controlled trials to exert substantial BP-lowering effects in various forms of hypertension, including RH. The catheter- based procedure has a favourable safety profile with long-term BP-lowering efficacy demonstrated to nine years postprocedure.41,42 This method aims to inhibit renal sympathetic efferent nerve activity, which contributes to reduced renal blood flow, decreased urinary salt, reduced water excretion and high renin release in the kidneys.43 The ESH recently issued a position paper on renal denervation, summarising the available evidence and providing recommendations for its use in routine clinical practice (Box 3).44 A position paper on the use of renal denervation in an Australian context is in preparation.
Conclusion
RH remains challenging to manage and is a modifiable risk factor for adverse health outcomes. Recommended management of RH includes: excluding pseudoresistant hypertension; identifying relevant contributing factors, focusing on lifestyle modification; using simple medication regimens (ideally, in the form of single pill combinations of guideline-recommended drugs with different modes of action), adding further drug classes as required (based on comorbidities); and considering renal denervation as an evidence-based option to safely and effectively lower BP. Appropriate management of RH results in improved cardiovascular outcomes for this high-risk patient cohort. MT
COMPETING INTERESTS: Professor Schlaich has received consulting fees, and travel and research support from Medtronic, Abbott, Novartis, Servier, Pfizer, and Boehringer-Ingelheim. Dr Nejad and Dr Azzam: None.
References
1. Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1345-1422.
2. Doumas M, Imprialos KP, Kallistratos MS, Manolis AJ. Recent advances in understanding and managing resistant/refractory hypertension. F1000Res 2020; 9.
3. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). G Ital Cardiol 2018; 19 (11 Suppl 1) :3S-73S.
4. Carey RM, Calhoun DA, Bakris GL, et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertens 2018; 72: e53-e90.
5. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circ 2012; 125: 1635-2112.
6. Sim JJ, Bhandari SK, Shi J, et al. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int 2015; 88: 622-632.
7. Chia R, Pandey A, Vongpatanasin W. Resistant hypertension-defining the scope of the problem. Prog Cardiovasc Dis 2020; 63: 46-50.
8. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386: 2059-2068.
9. Gaddam KK, Nishizaka MK, Pratt-Ubunama MN, et al. Characterization of resistant hypertension - Association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Arch Intern Med 2008; 168: 1159-1164.
10. Wilson AL, Gandhi J, Suh YJ, Joshi G, Smith NL, Khan SA. Renal innervation in resistant hypertension: a review of pathophysiology and renal denervation as potential treatment. Curr Hypertens Rev 2020; 16: 115-127.
11. Hwang AY, Dietrich E, Pepine CJ, Smith SM. Resistant hypertension: mechanisms and treatment. Curr Hypertens Rep 2017; 19: 56.
12. Schlaich MP, Bellet M, Weber MA, et al. Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial. Lancet 2022; 400: 1927-1937.
13. Johnson KC, Whelton PK, Cushman WC, et al. Blood pressure measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension 2018; 71: 848-857.
14. Stergiou G, Kollias A, Parati G, O’Brien E. Office blood pressure measurement the weak cornerstone of hypertension diagnosis. Hypertension 2018; 71: 813-815.
15. Stergiou GS, Kyriakoulis KG, Kollias A. Office blood pressure measurement types: different methodology-different clinical conclusions. J Clin Hypertens 2018; 20: 1683-1685.
16. Cheung AK, Whelton PK, Muntner P, et al. International Consensus on Standardized Clinic Blood Pressure Measurement - A Call to Action. Am J Med 2023; 136: 438-445.
17. Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens 2009; 27: 280-286.
18. Tang O, Juraschek SP, Appel LJ, et al. Comparison of automated clinical and research blood pressure measurements: Implications for clinical practice and trial design. J Clin Hypertens 2018; 20: 1676-1682.
19. Lamirault G, Artifoni M, Daniel M, Barber-Chamoux N, Nantes University Hospital Working Group On Resistant Hypertension: Novel Insights. Curr Hypertens Rev 2020; 16: 61-72.
20. Egan BM, Zhao Y, Li J, Brzezinski WA, et al. Prevalence of optimal treatment regimens in patients with apparent treatment-resistant hypertension based on office blood pressure in a community-based practice network. Hypertension 2013; 62: 691-697.
21. Acelajado MC, Hughes ZH, Oparil S, Calhoun DA. Treatment of resistant and refractory hypertension. Circ Res 2019; 124: 1061-1070.
22. Siru R, Conradie JH, Gillett MJ, Page MM. Approach to the diagnosis of secondary hypertension in adults. Aust Prescr 2021; 44: 165-169.
23. Charles L, Triscott J, Dobbs B. Secondary hypertension: discovering the underlying cause. Am Fam Physician 2017; 96: 453-461.
24. Reincke M, Bancos I, Mulatero P, Scholl UI, Stowasser M, Williams TA. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endo 2021; 9: 876-892.
25. Freeman MW, Halvorsen YD, Marshall W, et al. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med 2022; 388: 395-405.
26. Weber MA, Mahfoud F, Schmieder RE, et al. Renal denervation for treating hypertension current scientific and clinical evidence. JACC-Cardiovasc Interv 2019; 12: 1095-1105.
27. Santos LP, Moraes RS, Vieira PJC, et al. Effects of aerobic exercise intensity on ambulatory blood pressure and vascular responses in resistant hypertension: a crossover trial. J Hypertens 2016; 34: 1317-1324.
28. Guimaraes GV, Cruz LGD, Fernandes-Silva MM, Dorea EL, Bocchi EA. Heated water-based exercise training reduces 24-hour ambulatory blood pressure levels in resistant hypertensive patients: A randomized controlled trial (HEx trial). Int J Cardiol 2014; 172: 434-441.
29. Weiss EP, Albert SG, Reeds DN, et al. Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: a randomized intervention trial. Am J Clin Nutr 2016; 104: 576-586.
30. Pimenta E, Gaddam KK, Oparil S, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension results from a randomized trial. Hypertension 2009; 54: 475-481.
31. McMahon EJ, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol 2013; 24: 2096-2103.
32. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 2001; 344: 3-10.
33. Teres S, Barcelo-Coblijn G, Benet M, et al. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc Natl Acad Sci USA 2008; 105: 13811-13816.
34. Guasch-Ferre M, Willett WC. The Mediterranean diet and health: a comprehensive overview. J Intern Med 2021; 290: 549-566.
35. Ingelfinger JR. Thiazide-like versus Thiazide Diuretics - Finally, an Answer? N Engl J Med 2022; 387: 2462-2463.
36. Ishani A, Cushman WC, Leatherman SM, et al. Chlorthalidone vs. hydrochlorothiazide for hypertension-cardiovascular events. N Engl J Med 2022; 387: 2401-2410.
37. Oparil S, Schmieder RE. New Approaches in the Treatment of Hypertension. Circ Res 2015; 116: 1074-1095.
38. Bazoukis G, Thomopoulos C, Tsioufis C. Effect of mineralocorticoid antagonists on blood pressure lowering: overview and meta-analysis of randomized controlled trials in hypertension. J Hypertens 2018; 36: 987-994.
39. Aronow WS. approaches for the management of resistant hypertension in 2020. Curr Hypertens Rep 2020; 22: 3.
40. Krieger EM, Drager LF, Giorgi DMA, et al. Spironolactone versus clonidine as a fourth-drug therapy for resistant hypertension: The ReHOT Randomized Study (Resistant Hypertension Optimal Treatment). Hypertension 2018; 71: 681-690.
41. Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 2017; 390: 2160-2170.
42. Kandzari DE, Bohm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018; 391: 2346-2355.
43. Osborn JW, Foss JD. Renal nerves and long-term control of arterial pressure. Compr Physiol 2017; 7: 263-320.
44. Schmieder RE, Mahfoud F, Mancia G, et al. Members of the ESH Working Group on Device-Based Treatment of Hypertension. European Society of Hypertension position paper on renal denervation 2021. J Hypertens 2021; 39: 1733-1741.