Renal artery stenosis and hypertension: when to screen and how to treat
Renal artery stenosis (RAS) is a common cause of secondary hypertension. Individuals with RAS are at increased risk of cardiovascular events. Careful assessment of patients with hypertension can help identify those who warrant further investigations for RAS. GPs have a crucial role in the intensive management of cardiovascular risk factors in patients with RAS.
-
Renal artery stenosis (RAS), especially atherosclerotic RAS, is a common cause of secondary hypertension.
-
Cardiovascular morbidity and mortality rates are high in individuals with RAS.
-
Intensive medical therapy to reduce modifiable cardiovascular risk factors is the cornerstone of management of RAS and should be applied to all patients.
-
Patients with stable atherosclerotic RAS are best managed conservatively.
-
Endovascular interventions should be reserved for those with fibromuscular dysplasia and for only a select few of those with atherosclerotic RAS.
Peripheral arterial diseases (PADs) encompass all arterial diseases other than coronary artery disease and diseases of the aorta.1 Renal artery stenosis (RAS) is a subtype of PAD and a common cause of secondary hypertension. The rapid growth of endovascular interventions for coronary artery disease promised superior outcomes for endovascular intervention over medical therapy alone in other vascular territories, such as the renal arteries. However, evidence suggests that renal revascularisation does not generally improve blood pressure, or renal or cardiovascular outcomes in atherosclerotic RAS. There may be select cases when revascularisation is indicated, although this remains a matter of contention. As a result, good medical therapy remains the cornerstone of management for these conditions.
However, it is important to consider the scope of vascular territories affected by PAD and be sensitive to the fact that many individuals have concomitant conditions. When one vascular bed is affected by atherosclerosis, the individual is at risk of end-organ damage in that region, as well as an overall increased cardiovascular risk. This is attributable to the shared risk factors for these atherosclerotic diseases.
This article provides an overview of the current recommendations for identifying patients at risk of atherosclerotic RAS, outlines appropriate imaging modalities to confirm the diagnosis and medical management for all patients, and considers select patients who may warrant specialist review and potential endovascular treatment.
Case study
A 75-year-old man comes to your practice with a blood pressure of 160/70 mmHg. He has been a smoker, with high lipid levels and a 10-year history of poorly controlled hypertension, despite taking four antihypertensive agents.
Clinical examination shows carotid bruits, no renal bruits and a fourth heart sound. His serum creatinine level is 150 mcmol/L (estimated glomerular filtration rate [eGFR] 39 mL/min/1.73 m2) and urinalysis shows 1+ protein.
The ultrasound of his renal tract shows a discrepancy in renal size (right 10 cm; left 7.8 cm) and a suggestion of increased velocity in the renal artery of the smaller kidney; however, there is too much interference to make any significant conclusion about the presence of RAS.
How should this patient be further evaluated and treated?
Epidemiology of renovascular disease
Pathophysiology
Renovascular hypertension is one of the most common causes of secondary hypertension. It occurs when the main renal artery and proximal branches narrow to a sufficient degree to trigger the kidney’s adaptive mechanisms to counteract the fall in pressure beyond the stenosis. Although there is some disagreement over what constitutes a sufficient degree of stenosis to cause such a haemodynamic effect, a reduction in luminal diameter of at least 50% is considered the point at which a lesion is ‘significant’ (based on MRI and direct flow measurements).4 However, for the purpose of determining suitability for endovascular stenting, a cut off of above 70% stenosis is generally applied.
The pathophysiology that results from RAS is mediated by the degree of blood flow impairment and whether the disease is unilateral or bilateral. Acutely unilateral RAS triggers a renin-mediated form of blood pressure elevation that, over time, can progress to chronic hypertension.5 In bilateral renal artery stenoses, more extensive renin-angiotensin-aldosterone system activation triggers salt and fluid retention, inducing extracellular fluid expansion and worsening degrees of hypertension.5 Progressively more severe stenoses also result in renal ischaemia and progressive renal impairment, although the proportion of
end-stage kidney disease due to RAS is difficult to quantify.2
Classification
The three main aetiologies of RAS are atherosclerosis, fibromuscular dysplasia (FMD) and a third group of myriad uncommon vascular pathologies. About 90% of all renovascular lesions are secondary to atherosclerosis as a manifestation of systemic vascular disease. Atherosclerotic RAS is usually ostial in nature. The risk factors associated with atherosclerotic RAS are metabolic risk factors typically associated with most PADs and are outlined in Box 1.
FMD is a noninflammatory systemic disorder with five main variants. The most common variant is medial fibroplasia, identified histologically in about 80% of cases. It produces a characteristic ‘string of pearls’ appearance on angiographic assessment. The middle and distal two-thirds of the main renal arteries are affected in 75% of cases.6 FMD typically presents with hypertension and accounts for 8 to 10% of RAS. The classic patient is female (9-to-1 female-to-male ratio) aged 15 to 50 years.1,6 Other miscellaneous causes of renovascular disease contribute less than 1% to the aetiology of RAS and include renal artery aneurysms, trauma, radiation-induced injuries, embolic events and neurofibromatosis.2
Diagnosis and assessment
Who to screen
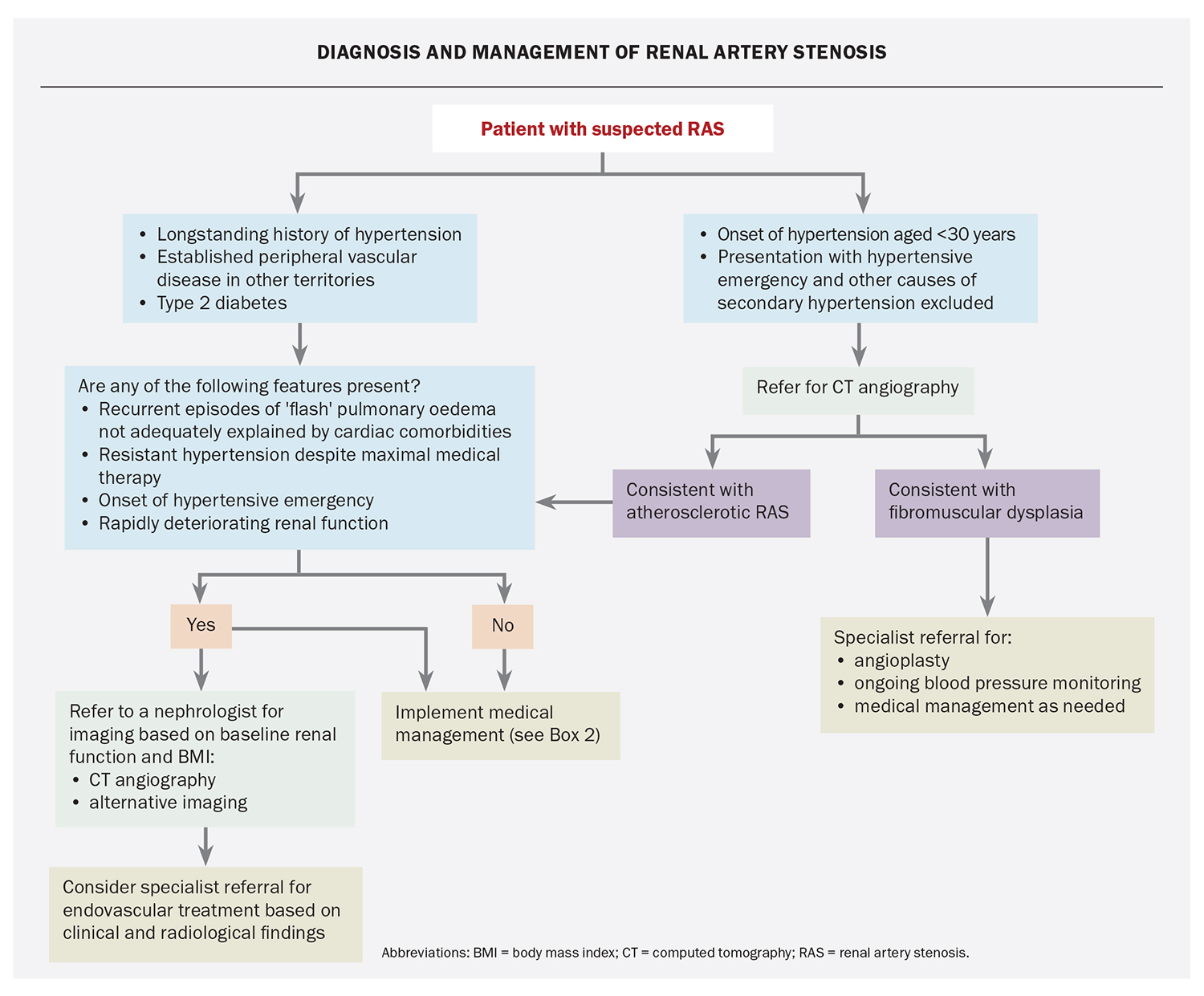
The presence of hypertension should trigger clinicians to consider the possibility of underlying RAS. However, as not all individuals with hypertension warrant investigation, careful patient assessment to determine a high pre-test probability of RAS is important to avoid unnecessary testing that carries cost, inconvenience and the potential for harm. International guidelines support screening in the following clinical presentations:1,2
- resistant hypertension (blood pressure above 140/90 mmHg despite the use of three antihypertensive drug classes, including a diuretic)7
- recurrent episodes of ‘flash’ pulmonary oedema that are not adequately explained by cardiac comorbidities
- onset of hypertension before the age of 30 years
- hypertension and the presence of an abdominal bruit in a younger patient
- rapid and persistent worsening of previously controlled hypertension
- onset of hypertensive emergency
- hypertension and the presence of unexplained unilateral kidney atrophy (renal size less than 7 to 8 cm) or discrepancy in kidney size of 1.5 cm or larger
- onset of severe acute kidney injury with incident use of renin-angiotensin system inhibitor drug classes.
A simple algorithm to help in the diagnosis and management of RAS is presented in the Flowchart.
The patient has had treatment-resistant hypertension for many years, and investigations reveal that the renal vascular bed is affected with a shrunken kidney (likely from ischaemia) and proteinuria of a level consistent with vascular disease (urine albumin-to-creatinine ratio 44 mg/mmol; reference range <2.5 for men and <3.5 for women). Albuminuria in this situation usually indicates glomerular scarring and capillary leak – glomerulosclerosis.
Although it is possible that the patient might experience side effects from imaging, he has a high pre-test probability of having RAS and should therefore be further investigated.
What imaging test to use
Choosing an appropriate diagnostic test depends on the local expertise of imaging providers, patient factors (e.g. central adiposity, degree of renal impairment) and the available resources based on geographical location.
Intra-arterial digital subtraction angiography (IA-DSA) is regarded as the gold-standard modality for diagnosing RAS. IA-DSA not only measures lumen size, it also estimates the pressure gradient across a stenosis, which is an estimate of functional significance. The main limitation of this technique is its invasive nature, with associated risks of embolic events, bleeding at puncture sites and contrast exposure.
Duplex ultrasonography is often recommended as the
first-line method for evaluating RAS. However, its diagnostic accuracy is highly dependent on the sonographer’s experience and the patient’s body mass index, and is prone to false-positive results, with patients often requiring subsequent testing. It is therefore best suited to rule out RAS in a person of lower body mass index and a low pre-test probability (e.g. a young woman), accepting its weaknesses.
Computed tomography angiography (CTA) affords rapid, noninvasive acquisition of an extended vascular field that is useful both diagnostically and in planning interventional strategies. It is particularly sensitive for fibromuscular dysplasia. Although low-iodinated preparations and low-contrast technologies continue to develop, the main limitation for the use of this technique is in individuals with advanced chronic kidney disease who are at risk of contrast-induced nephropathy. Some of these risks can be managed with adequate hydration around the time of the procedure, and most cases of contrast nephropathy are mild, self-limiting and reversible.
Magnetic resonance angiography with gadolinium enhancement provides the most accurate results; however, it can be performed without contrast enhancement, with inferior resolution. The use of gadolinium is not recommended in individuals with an eGFR less than 30 mL/min/1.73 m2, given the rare but significant risk of nephrogenic systemic fibrosis. Captopril renal scintigraphy and renal vein renin measurements are no longer recommended in the investigation of RAS.
If the resources were available, the specialist would most likely consider spiral CTA for this patient or move directly to IA-DSA because he has poorly controlled hypertension.
Management
Atherosclerotic RAS is frequently associated with disease in other vascular beds and, therefore, affected patients should be considered to be at high risk for progressive chronic kidney disease, stroke, myocardial infarction and vascular death, and managed accordingly. Optimal care should be based on good medical management of patients’ risk factors for cardiovascular and kidney disease progression. This should include a combination of diet and lifestyle strategies and pharmacological measures as outlined in Box 2.
Involvement of a renal specialist (or similar physician) may be indicated if blood pressure management proves difficult or there is rapid deterioration in kidney function. Renin-angiotensin system inhibitors, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), are important in the management of these patients, as they improve survival by reducing cardiovascular events and are often crucial in blood pressure control. The lowering in eGFR associated with the use of this class of medications is uncommon but acceptable (up to 30% drop) and reversible. These agents should be introduced at a low dose, then increased over a period of two to three months as needed, with monitoring of eGFR and serum potassium level. It is recommended to use either class, not both, in the same patient, as using both increases the risk of hyperkalaemia.
When to consider endovascular treatment
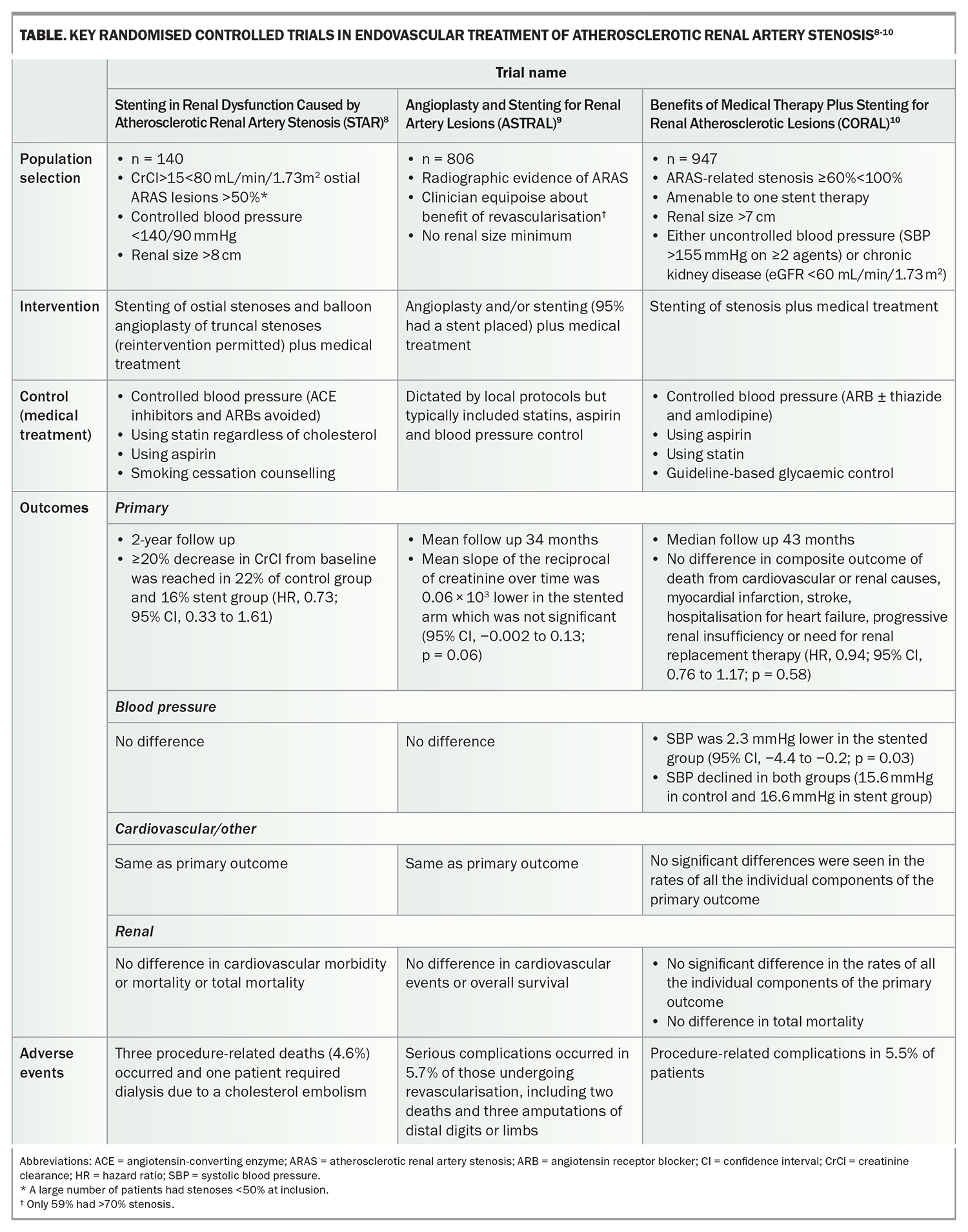
Many nonrandomised studies suggest that revascularisation in atherosclerotic RAS improves renal function and blood pressure; however, the potential biases of a nonrandomised design are well known. Three subsequent randomised studies comparing medical treatment with add-on stenting showed no benefit across the renal, cardiovascular and blood pressure domains (Table).8-10 Moreover, the rate of serious adverse events across these studies add to the argument against ready solicitation of endovascular treatment. FMD can be treated with balloon angioplasty alone, with stenting reserved for recurrent restenosis or difficultto-treat lesions.1
Since their publication, however, the design and patient selection of these randomised trials have been questioned, and many feel that the universal restriction of endovascular treatment from individuals with RAS needs a more nuanced approach. As a result, a restricted subset of individuals with atherosclerotic RAS is still considered suitable for endovascular treatment, including individuals with:1-3,11
- haemodynamically significant (≥70% stenosis) RAS causing resistant hypertension despite maximal medical therapy
- recurrent cardiac instability, such as pulmonary oedema or hypertensive crises
- rapidly deteriorating renal function with evidence of sufficient residual renal function (renal size >7 cm).
Based on his poorly controlled hypertension and evidence of renal damage, including a shrunken kidney, proteinuria, and reduced eGFR, the patient was referred to a nephrologist. A minimal contrast angiogram was performed using prior nephroprotective strategies and showed a near-occluded RAS on the side of the shrunken kidney but only a 50% stenosis on the 10 cm kidney side. Because of the patient’s age and these findings, angioplasty was not carried out. The nephrologist focused on aggressive medical control of the patient’s refractory hypertension and high, long-term cardiovascular risk.
Conclusion
The rising incidence of risk factors for atherosclerosis and PAD and an ageing Australian population are likely to result in higher rates of RAS. Identifying patients with RAS can help stratify those who are at high risk of cardiovascular morbidity and mortality, and aggressively maximise the medical treatment of their modifiable risk factors. Overall, patients who are stable, with controlled hypertension and stable kidney function, do not need to be tested for RAS. Imaging modalities to screen for RAS should be selected with careful consideration of the pros and cons of each technique. Judicious selection of individuals with progressive complications of their RAS may warrant referral for endovascular intervention. Further research is needed to provide a higher level of evidence to better inform guidelines on which individuals should progress to invasive management. MT
COMPETING INTERESTS: None.
References
1. Aboyans V, Ricco J-B, Bartelink M-L, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European Stroke Organization (ESO) The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018; 39: 763-816.
2. Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113: e463-e654.
3. MacGinley R, Kumar SK, Mantha M, Mount P, Roberts M, Mangos G. Endovascular treatment. Nephrology 2010; 15: S210-S217.
4. Mantha M, Kumar SK, MacGinley R, Mount P, Roberts M, Mangos G. Screening tests for diagnosis of renal artery stenosis. Nephrology 2010; 15: S218-S26.
5. Martinez-Maldonado M. Pathophysiology of renovascular hypertension. Hypertension 1991; 17: 707-719.
6. Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med 2004; 350: 1862-1871.
7. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension 2018; 72: e53-e90.
8. Bax L, Woittiez A-JJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150: 840-848.
9. ASTRAL Investigators; Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361: 1953-1962.
10. Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370: 13-22.
11. Prince M, Tafur JD, White CJ. When and how should we revascularize patients with atherosclerotic renal artery stenosis? JACC Cardiovasc Interv 2019; 12: 505-517.