When worlds collide – connective tissue disease and interstitial lung disease
Connective tissue diseases (CTDs) are a heterogenous group of autoimmune conditions including systemic sclerosis, idiopathic inflammatory myopathies and systemic lupus erythematosus. Patients may present with skin thickening, Raynaud’s phenomenon, photosensitive rash or weakness. Interstitial lung disease (ILD) is a potentially serious complication of CTDs that can develop at any time during the disease course. However, ILD may be the first, and sometimes even the only, presenting feature of an underlying CTD.
- Interstitial lung disease (ILD) is a common complication of connective tissue disease, particularly systemic sclerosis, rheumatoid arthritis and idiopathic inflammatory myopathies.
- Clinicians should be aware of the sometimes nonspecific presentation of ILD, as well as important investigations and screening.
- Connective tissue diseases (CTDs) can present in a multitude of ways; Raynaud’s phenomenon, photosensitive rashes and muscle weakness are particularly suggestive symptoms.
- Optimal management of CTD-ILD involves a specialised multidisciplinary team including physicians, radiologists, pathologists, allied health staff and the patient.
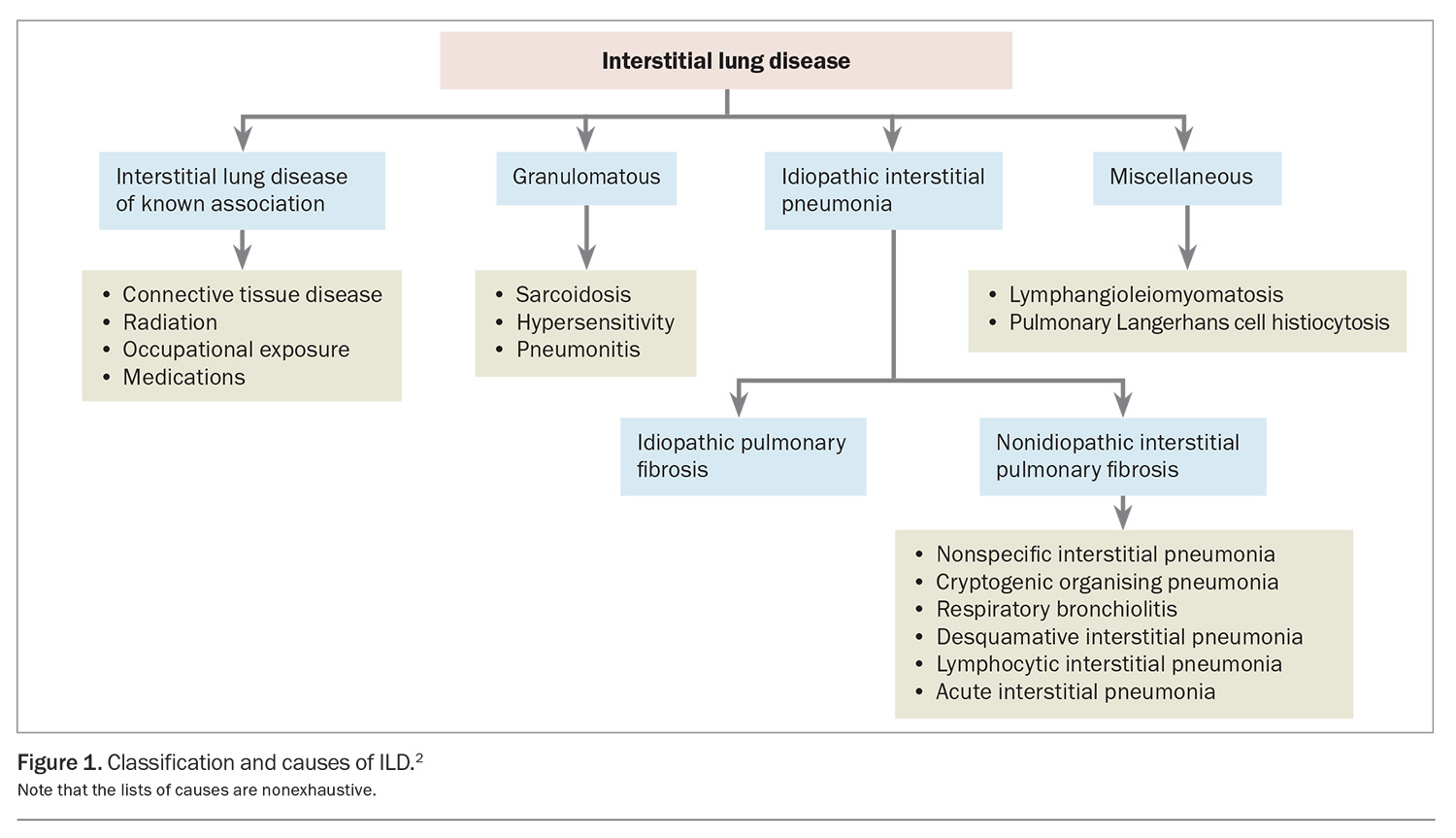
Interstitial lung disease (ILD) is a group of heterogenous disorders that cause fibrosis and inflammation of the lung parenchyma, classified by various clinical, radiological and histopathological features. Causes of ILD include:
- idiopathic, radiation, occupational and environmental exposures (e.g. silicosis, asbestosis)
- drugs
- granulomatous conditions
- connective tissue disease
- allergens (hypersensitivity pneumonitis).
These causes can determine the classification of ILD, as outlined in Figure 1.1 A consensus diagnosis reached at a multidisciplinary team meeting is considered the gold standard of ILD diagnosis.2
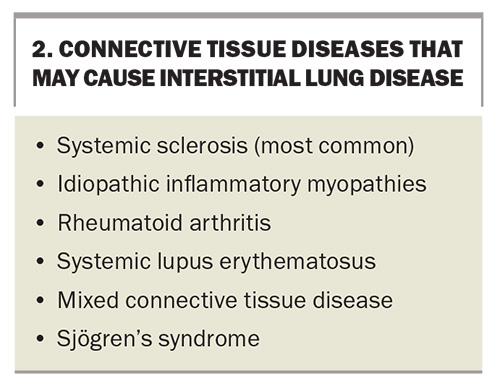
Connective tissue diseases (CTDs) are a group of heterogeneous systemic autoimmune conditions defined by differing but sometimes overlapping clinical features. Taken collectively, they are an important cause of ILD, making up 7 to 35% of all cases in published cohort studies.3 CTDs associated with ILD include systemic sclerosis (SSc), rheumatoid arthritis (RA), idiopathic inflammatory myopathies (IIM) (including the subtypes of dermatomyositis and antisynthetase syndrome [ASyS]), Sjögren’s syndrome, systemic lupus erythematosus (SLE) and mixed connective tissue disease (MCTD).
Clinicians should remain alert to the often subtle symptoms of evolving ILD in a patient with a CTD, as this development portends significantly increased morbidity and mortality and has a substantial impact on management decisions. Conversely, it is well recognised that a proportion of patients (7 to 24%) reported to have idiopathic ILD proceed to develop features of an underlying CTD over time, with the lung manifestations effectively representing a ‘forme fruste’.4,5 Some patients with CTD-ILD continue to show very few or no other organ manifestations (lung-dominant CTD-ILD). A diagnosis of a CTD-ILD confers substantially different prognosis and management compared with a diagnosis of idiopathic ILD. As the symptoms of both CTDs and ILD can be so varied, it is important that all clinicians are aware of the bidirectional relationship. This article summarises an approach to patients with either a CTD or an ILD who are suspected to have developed the other, and reviews the common symptoms, signs and investigations of these two conditions.
Interstitial lung disease in patients with connective tissue disease
When would you suspect ILD in a patient with CTD?
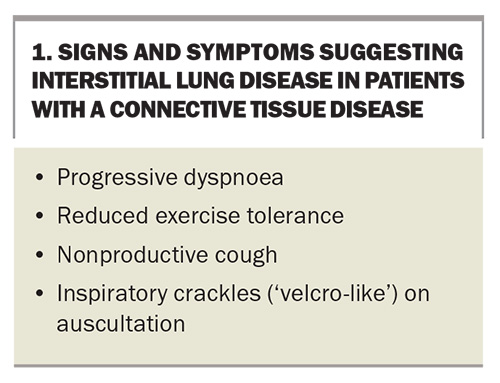
Signs and symptoms that may suggest underlying ILD in patients with CTD are listed in Box 1. Common respiratory symptoms that could suggest underlying ILD include exertional dyspnoea and a nonproductive cough, typically with a time course over a period of months. Dyspnoea can be under-reported because of the functional limitations from the underlying CTD, such as joint and muscle disease or underlying systemic inflammation leading to fatigue.6 A productive cough is more likely to suggest chronic bronchitis or bronchiectasis, although these could coexist with ILD. Overall, symptoms are nonspecific; therefore, other causes such as congestive heart failure, stable angina, lung malignancy, asthma and chronic obstructive pulmonary disease (COPD) must be considered.
Clinicians are encouraged to consider drug-induced ILD in a patient with CTD, especially if immunosuppressive therapy or other medications have recently changed. Therefore, a detailed medication history including initiation, duration and dose is important. Many medications used for CTDs have been implicated as causes of drug-induced ILD, including methotrexate, leflunomide, sulfasalazine and biologic agents (e.g. tumour necrosis factor inhibitors).7 Although methotrexate can cause acute or subacute pneumonitis, it is important to note that more recent evidence suggests that methotrexate likely does not cause progressive fibrotic ILD, including in patients with RA-ILD.8
Alternative causes of ILD should also be explored, even if less likely in a patient with a known CTD. A careful occupational and exposure history (e.g. to birds, silica, organic matter, moulds) is important. Patients with CTD are often immunosuppressed because of their treatment; therefore, differential diagnosis should include chronic or atypical infections such as Pneumocystis jiroveci pneumonia, which can mimic the radiographical changes of ILD. Chronic aspiration and subsequent pneumonitis are possible, particularly in patients with SSc or IIM, which may also produce confounding appearances. This may occur because of severe reflux associated with SSc or, more rarely, severe oropharyngeal dysphagia in IIM. It is also important to consider a patient’s malignancy history, as exposure to radiation therapy can cause pulmonary fibrosis in around 16 to 28% of individuals and some chemotherapy agents are associated with the onset of ILD.7,9
The most characteristic physical examination finding is fine bibasal ‘velcro-like’ inspiratory crackles on auscultation. Although clubbing is a common sign of many ILDs, such as idiopathic pulmonary fibrosis, asbestosis and chronic hypersensitivity pneumonitis, it is much less common in patients with CTD-ILD. The presence of wheeze is more likely to suggest an alternative diagnosis, such as chronic airways disease or hypersensitivity pneumonitis, but wheeze can occur in some autoimmune conditions causing ILD, such as eosinophilic granulomatosis with polyangiitis (formerly known as Churg–Strauss syndrome).10 It is important to examine patients for signs of pulmonary hypertension and right ventricular failure, which may include a raised jugular venous pressure, loud P2 on auscultation, palpable right ventricular heave and peripheral oedema. These signs are always clinically important and usually indicate advanced pulmonary disease, although they can occur earlier in patients with concomitant pulmonary arterial hypertension.
Which connective tissue diseases cause interstitial lung disease?
CTDs that may cause ILD are listed in Box 2.
Systemic sclerosis
SSc is the CTD that is most commonly complicated by ILD development. Clinically significant disease is estimated in 40 to 75% of patients with SSc, and along with pulmonary hypertension, is a leading cause of mortality (35%).11,12 Risk factors for developing ILD include advanced age, diffuse (rather than limited) skin involvement, long disease duration, the presence of anti-Scl-70 (also called anti-topoisomerase I) antibodies and high erythrocyte sedimentation rate.13,14 Although patients with diffuse disease are more likely to develop ILD compared with patients with limited disease, all patients with SSc should be closely monitored for the development of ILD.
Idiopathic inflammatory myopathies
IIM comprise multiple disease subgroups including dermatomyositis, ASyS and immune-mediated necrotising myopathies. The prevalence of IIM-ILD varies significantly between different subtypes. Overall, it is estimated at 20 to 43% but is particularly high (50 to 100%) in patients with ASyS.15,16 Patients with ASyS usually present with other features such as polyarthritis, Raynaud’s phenomenon, mechanic’s hands or hiker’s feet (a pathognomonic rash), gastrooesophageal reflux disease and oesophageal dysmotility. However, some patients have very few or no extrapulmonary manifestations of IIM, which makes their diagnosis particularly challenging. In fact, ILD has been found to be the presenting symptom of ASyS in 7 to 38% of cases.15 Clinicians should be aware of a rare subtype of IIM associated with the antimelanoma differentiation-associated protein 5 antibody, which can cause rapidly progressive ILD and very high mortality if treatment is delayed. Patients typically develop a rash that resembles dermatomyositis and relatively minor elevations in levels of muscle enzymes such as creatine kinase (a presentation often called clinically amyopathic dermatomyositis).17
Rheumatoid arthritis
Between 8 and 68% of patients with RA have been described to develop ILD.18 The wide variation stems from a substantial proportion of asymptomatic patients with ‘subclinical’ RA-ILD that is incidentally detected. Clinically significant ILD is present in about 10% of all patients with RA.19 Risk factors for the development of RA-ILD include male sex, a history of COPD, smoking, high disease activity and a high titre of anticyclic citrinullated peptide antibodies.20 A large cohort study showed the mean time of ILD onset being about three years post-RA diagnosis.20 A small but important subgroup of patients with RA-ILD present with ILD before any apparent arthritis – the only clue to the underlying aetiology may be autoantibody positivity.
Other connective tissue diseases
Pulmonary involvement in patients with SLE is common and occurs in up to 50% of patients. However, ILD as a complication is relatively rare, occurring in around 1 to 15% of patients.21 It very rarely results in lung fibrosis. Risk factors for the development of ILD include advanced age, longstanding disease, Raynaud’s phenomenon, presence of anti-U1-ribonucleoprotein antibodies, sclerodactyly and abnormal findings on nailfold capillaroscopy examination.22-24 The latter three factors suggest the presence of an overlapping syndrome and, in part, reflect the issue with evolving and overlapping nomenclature among CTDs. In particular, the presence of anti-U1-ribonucleoprotein antibodies is a key feature of MCTD, a condition with overlapping features of multiple CTDs and in which the prevalence of ILD has been found to be more than 50% in patients undergoing high-resolution CT scans.25
Respiratory complications of Sjögren’s syndrome occur in about 9 to 20% of individuals, commonly presenting as ILD.26 Sjögren’s syndrome-related ILD typically presents late in the disease course and is associated with a characteristic cystic radiological pattern called lymphocytic interstitial pneumonia. Although vasculitis is not always considered a CTD, it can cause ILD in up to 23% and 45% of patients with granulomatosis with polyangiitis (formerly known as Wegener’s granulomatosis) and microscopic polyangiitis, respectively.27
Which investigations and screening tests should be performed for suspected ILD in a patient with CTD?
Spirometry or, ideally, more extensive pulmonary function testing (PFT) is indicated as the initial investigation for suspected ILD, typically showing a restrictive pattern with a reduced forced vital capacity (FVC) and a normal or high forced expiratory volume (FEV)/FVC ratio. A baseline FVC less than 70% predicted for age- and sex-matched healthy controls is a strong predictor of mortality.28 Bronchodilator reversibility is not normally seen unless there is a mixed obstructive and restrictive process. A mixed pattern or obstructive- only pattern can occur with coexistent COPD or in more rare causes of ILD such as sarcoidosis, Langerhans cell histiocytosis and hypersensitivity pneumonitis.
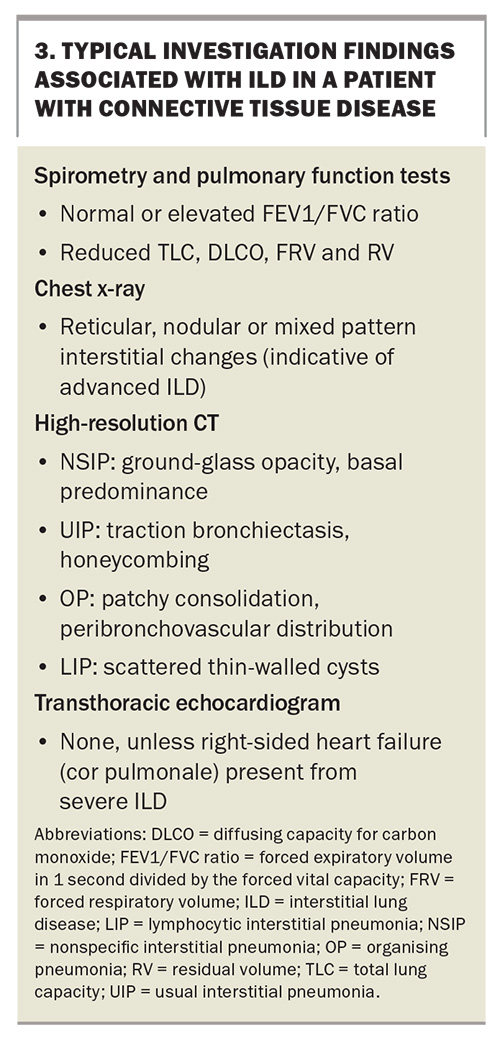
Restrictive lung disease cannot be formally diagnosed on spirometry alone and formal PFTs are required to assess lung volumes and diffusion capacity for carbon monoxide (DLCO). Confirmatory findings in patients with ILD are a reduction in lung volumes with a low total lung capacity, functional residual volume and residual volume, as well as a reduction in the DLCO. Lung volumes and DLCO are important not only for diagnosis but also in assessing prognosis and monitoring response to therapy. A decline in the FVC of less than 10% or a reduction in the DLCO of greater than 15% is predictive of mortality in patients with ILD.29 All patients with SSc should undergo PFTs at diagnosis to screen for ILD; however, there is no consensus on the frequency of subsequent screening, and many clinicians repeat PFTs at annual intervals. For all other CTDs, there are no official guidelines or consensus for ILD screening, which is therefore performed based on the clinical situation and individual patient factors.
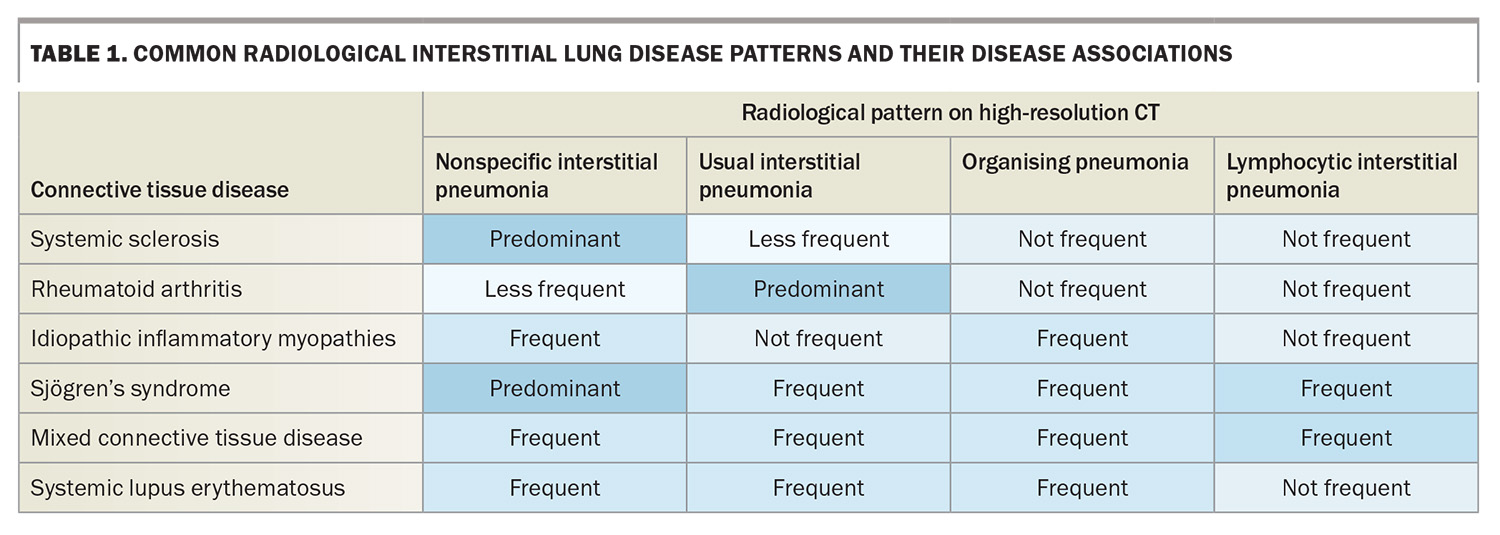
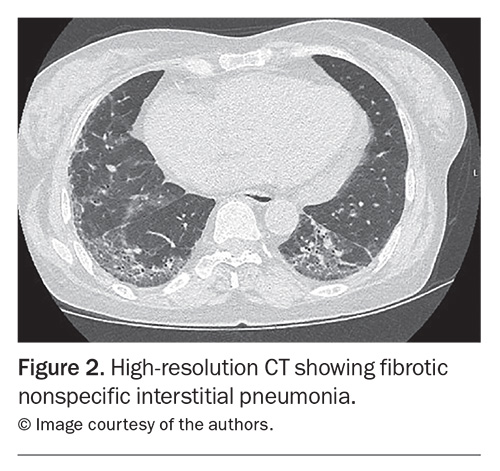
A chest x-ray can be performed to identify a reticular, nodular or mixed pattern with increased interstitial markings, which can indicate ILD. It is not sensitive enough to exclude ILD alone, and if there is any clinical suspicion, cross-sectional imaging is indicated. High-resolution CT is the imaging modality of choice for the investigation of ILD. Common patterns that clinicians may encounter in radiological reports and their disease associations are listed in Table 1. Across CTDs, nonspecific interstitial pneumonia is the most common pattern (Figure 2), with the exception of RA where usual interstitial penumonia is more prevalent.30
Finally, a transthoracic echocardiogram (TTE) should be arranged. Although it cannot be used to identify ILD, it can help screen for pulmonary hypertension, which is an important diagnostic differential for dyspnoea in patients with CTD. Investigations and typical findings for ILD are listed in Box 3.
Connective tissue disease in patients with interstitial lung disease
Which clinical symptoms and signs suggest CTD in a patient with ILD?
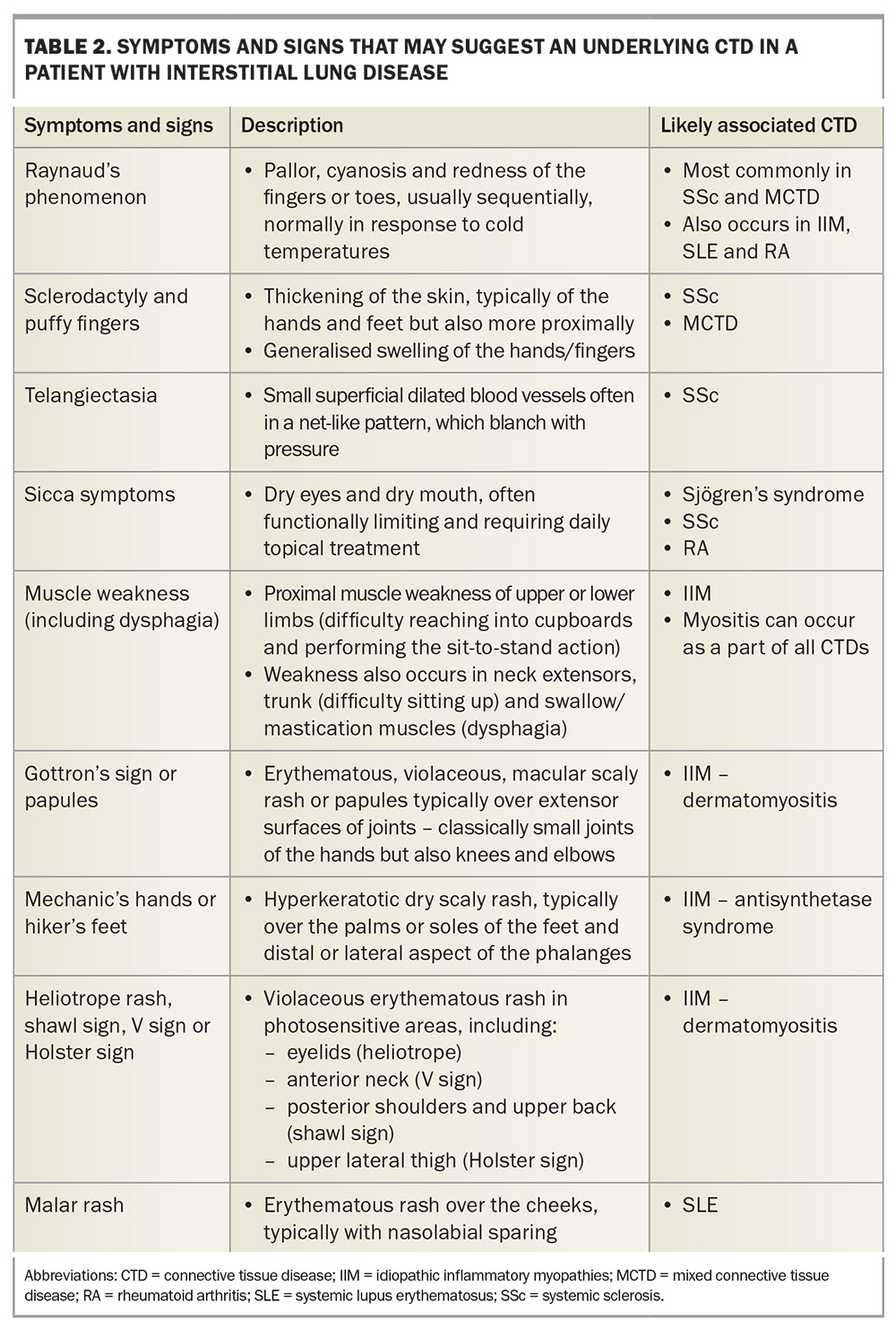
Signs and symptoms that may suggest underlying CTD in patients with ILD are listed in Table 2. The assessment for occult CTD in a patient with ILD of unknown cause requires meticulous history taking and examination. Clinicians should be on high alert for an autoimmune cause of ILD if the patient is young, female and a nonsmoker, as the converse (a patient who is older, male and a smoker) is more suggestive of idiopathic pulmonary fibrosis, the most common cause of ILD.
Vascular manifestations such as Raynaud’s phenomenon, digital ulcers and telangiectasia are normally suggestive of SSc or an overlap syndrome. Raynaud’s phenomenon is characterised by sudden episodes of digital vasospasm, associated with a triphasic colour change from white (low perfusion) to blue (cyanosis) to red (reactive hyperaemia) that usually progresses over a number of minutes and can be painful. Individual episodes are triggered by changes in ambient temperature, as well as many other potential factors, but can occur spontaneously. Raynaud’s phenomenon can be present without an underlying CTD when it is more accurately termed primary Raynaud’s phenomenon. In such patients, it is usually present from adolescence and there is often a family history of the same symptoms. New development of Raynaud’s symptoms in an adult should always raise suspicion of a CTD and should never be disregarded without careful consideration. When present with a relevant CTD, this is termed secondary Raynaud’s phenomenon. Secondary Raynaud’s phenomenon is present in more than 95% of patients with SSc or MCTD and up to half of patients with SLE or ASyS.31-34
About 50% of patients with SSc develop digital ulcers, an often extremely painful manifestation associated with poor quality of life.31 The ulcers are commonly found on the pulps of the fingers and can be complicated by infection. Telangiectasias are visibly dilated cutaneous blood vessels associated with SSc, normally distributed on the face (including the periorbital and gingival areas), neck, trunk and distal extremities (particularly the periungal areas).
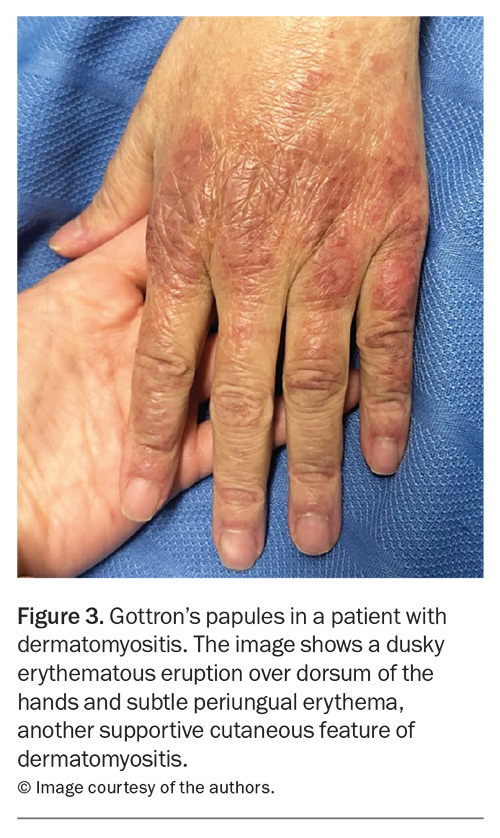
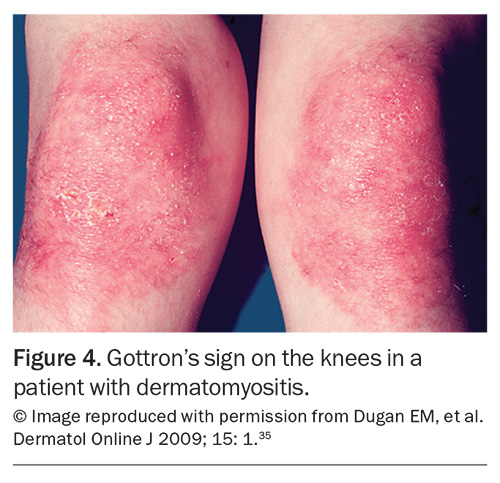
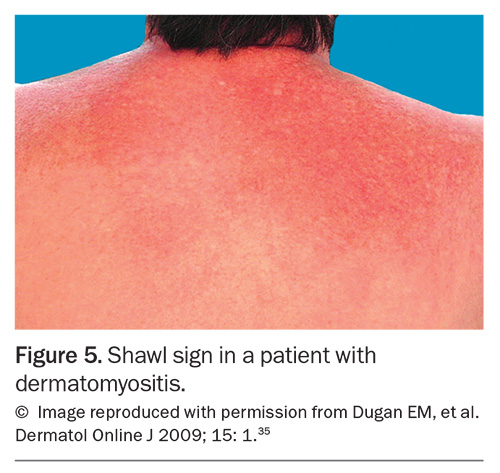
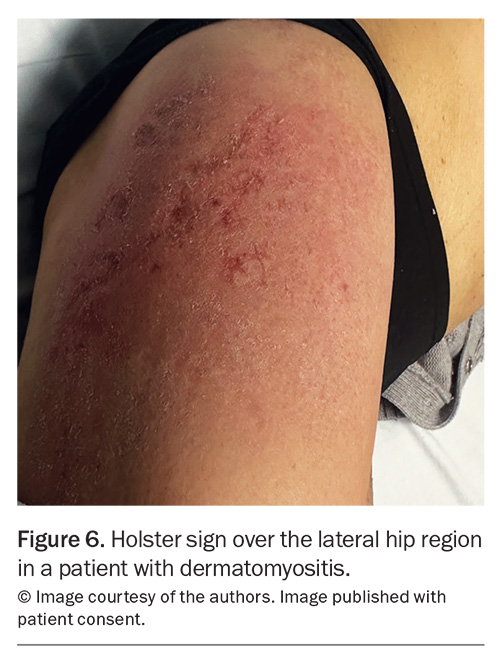
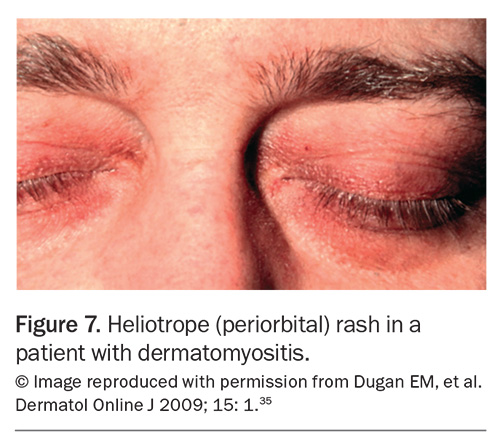
Rashes are particularly common in SLE and IIM. In SLE, the most common skin manifestation is a malar or ‘butterfly’ rash on the cheeks and nose with nasolabial sparing. Many patients with SLE also experience photosensitivity, as well as many other potential cutaneous manifestations. Dermatomyositis is associated with a number of characteristic rashes, such as Gottron’s papules (Figure 3), Gottron’s sign (Figure 4), shawl sign (Figure 5), Holster sign (Figure 6) and heliotrope sign (Figure 7).35 Specifically, patients with ASys can develop mechanic’s hands (Figure 8) and hiker’s feet.35
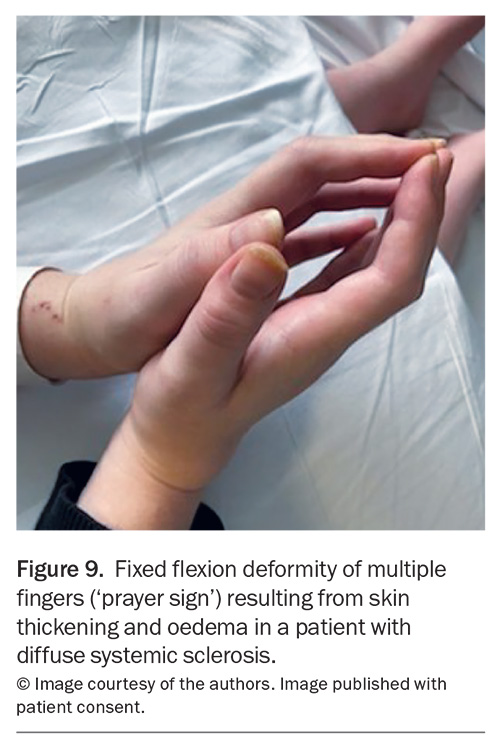
Other more common cutaneous manifestations of CTDs to be aware of include sclerodactyly, distal extremity oedema (puffy hands) and calcinosis, which are characteristic of SSc. Sclerodactyly is a hallmark finding of SSc, where skin becomes thickened, tight and loses elasticity because of underlying fibrosis. An example of fixed flexion deformity of the fingers (a positive ‘prayer sign’) related to distal skin thickening and oedema in a patient with diffuse SSc is shown in Figure 9. Skin thickening can progress proximally and, when present proximal to the elbows or knees, helps differentiate diffuse from limited SSc. ‘Puffy hands’ are a nonspecific finding usually found in early disease or in overlapping syndrome, such as MCTD. Calcinosis cutis occurs in 18 to 49% and 30% of patients with SSc and adult dermatomyositis, respectively.36 In patients with SSc, calcinosis lesions are typically found distally on the digits, whereas they can be much more extensive and proximal in dermatomyositis. Calcinosis can be painful and progress to ulcerations and associated infection.
Muscle weakness is the most common feature of IIM and is usually painless, proximal and progressive. Patients may not voluntarily report or recognise weakness but instead may describe the functional limitations that result from it. Useful screening questions include asking about the ability to perform a sit-to-stand exercise from a chair without upper limb assistance and the ability to reach above one’s head (e.g. reaching into cupboards and getting dressed). Axial muscle weakness is often underappreciated but can result in significant respiratory compromise. A clue to this may be a disproportionate reduction in PFT values compared with the radiological extent of ILD.
Arthralgia and arthritis can occur in a range of different CTDs. Patients should be asked specifically about the presence of stiffness (especially early morning stiffness >30 minutes) and swelling. Examination should focus on identifying sites of swelling or tenderness, as well as the distribution of joint activity, which can vary.
Dry eyes and mouth (sicca symptoms) are the dominant symptoms in patients with Sjögren’s syndrome but are very common in the general population as well as in patients with other CTDs. To be clinically relevant, symptoms are usually severe (requiring regular topical symptomatic treatment) and involve both the eyes and mouth without an alternative explanation (e.g. medications). Glandular swelling (including submandibular, parotid or cervical lymph nodes) can occur early in Sjögren’s syndrome and is easily palpable on examination. A new or enlarging swelling in a patient with suspected Sjögren’s syndrome should always raise suspicion for lymphoma, which is more common than in the general population.
Dysphagia and gastro-oesophageal reflux disease is common in patients with SSc because of the distal oesophageal and gastric involvement. Dysphagia is also commonly reported in patients with IIM because of the associated oropharyngeal muscle weakness and can be experienced by patients with Sjögren’s syndrome owing to xerostomia. Although dysphagia is nonspecific and relatively common in the general population, new or severe dysphagia, especially if combined with other symptoms, raises the suspicion of an underlying CTD. Finally, it is important to enquire about constitutional symptoms, such as fever, weight loss and fatigue, which are often present because of persistent systemic inflammation.
Which investigations should be performed to investigate for a CTD in a patient with ILD?
Cytopenia, kidney injury, transaminitis and systemic inflammation can all occur with CTD; therefore, a basic panel of blood tests should be arranged, including a full blood count; measurement of electrolytes, urea and creatinine; liver function tests and measurements of C-reactive protein levels and erythrocyte sedimentation rate (Box 4). Measurement of the creatine kinase level should be requested if there is concern about muscle weakness.
Immunopathology testing including autoantibodies should also be arranged depending on the degree of clinical suspicion and differential diagnosis (Box 5).
There are more dedicated tests that are used by rheumatologists and immunologists to investigate for CTD but are less frequently requested in the primary care setting. These include skin, lymph node and muscle biopsies; nailfold capillaroscopy; positron emission tomography/CT scans; and nerve conduction studies. All of these may play a role in the diagnosis of CTD and exclusion of other differentials, depending on the clinical situation.
Management of CTD-ILD: the role of the GP
Patients with either CTD or ILD will often present to their GP first with new symptoms; thus, early recognition is important. Referral should be made early to the relevant physician given the complex interplay between CTD and ILD. Clinicians in primary care play an important role in the initial investigation by arranging immunopathology or chest imaging, in addition to referring patients to specialists. This is important because symptoms can occasionally rapidly progress such that prompt investigation is required.
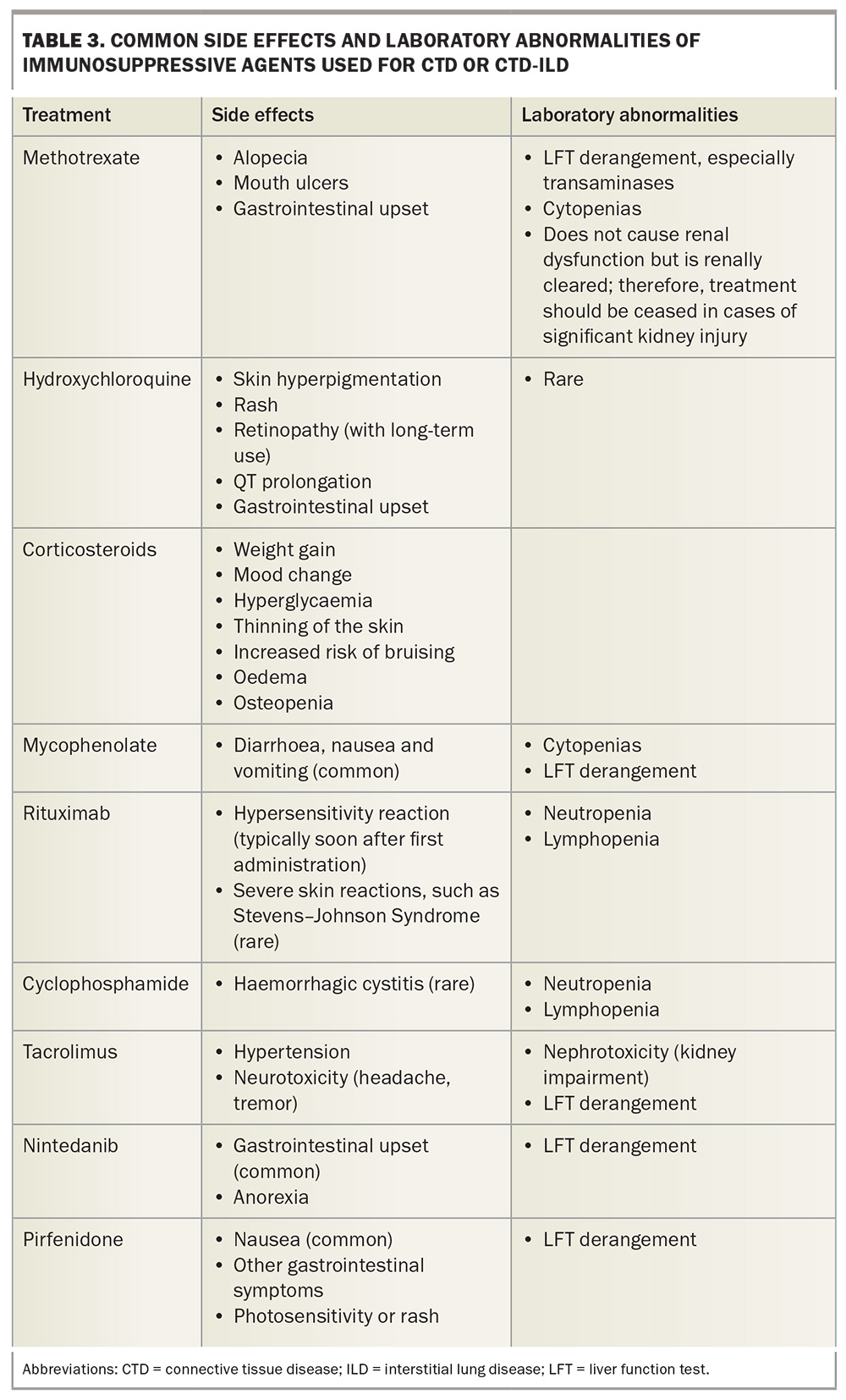
Ongoing CTD-ILD management requires a multidisciplinary team including rheumatologists or immunologists, respiratory physicians, radiologists, allied health professionals, histopathologists, GPs and the patient. The landscape of pharmacological management in CTD-ILD is changing with the use of both corticosteroids and other immunosuppressive agents, in addition to newer treatments such as antifibrotic agents (e.g. nintedanib), depending on clinical features. Although pharmacological treatment decisions are typically driven by specialists, GPs play an important role in monitoring these patients in the intervals between specialist visits and supporting patients on what is often a stressful journey. Additionally, GPs must be aware of common adverse effects and laboratory abnormalities of immunosuppressive treatment as they may be the first to encounter these effects (Table 3). GPs are in a unique position to disseminate their experience of managing these cases among their colleagues, trainees and allied medical professionals.
All patients with chronic health conditions have an increased risk of psychological comorbidities, such as anxiety and depression, so it is important to consider this when assessing patients with CTD-ILD. This is often supplemented by functional decline and reduced exercise tolerance associated with ILD. GPs are highly skilled at assessing and managing these comorbidities if they arise.
Preventive health is a particularly important issue for patients with CTD-ILD, who typically are at an increased risk of infection, cardiovascular disease and osteoporosis, among other conditions. In particular, GPs play an important role in ensuring patients with CTD-ILD are up to date with their vaccinations (e.g. influenza, shingles, pneumococcal, COVID-19). The Australian Rheumatology Association has an up-to-date online resource for patients and GPs on their website.37 In addition to the Australian Immunisation Handbook, there are recent major international guidelines regarding vaccination specifically for patients of rheumatology.38 With respect to infection, it is generally recommended that all patients on immunosuppressive treatment withhold their treatment temporarily (except corticosteroids) for a short period of time while unwell. GPs can contact the treating specialists regarding immunosuppressive treatment in the context of infection if there are any concerns, especially given the increased risk of rarer opportunistic infections.
Conclusion
CTD and ILD are two heterogenous groups of conditions that have a bidirectional relationship; both are a cause of the other. It is important to appreciate that a patient with idiopathic ILD may subsequently develop a CTD over time; thus, clinicians must maintain suspicion for the early and often subtle signs of an evolving CTD and determine how to appropriately investigate these. Optimal management of CTD-ILD typically involves a large multidisciplinary team. GPs play a key role in this team in recognising new symptoms, identifying adverse effects of treatment and ensuring that preventive health and comorbidities are addressed. Early patient referral and discussion with specialists are recommended given the complex nature of CTD-ILD. MT
COMPETING INTERESTS: Dr Neuen: None. Dr Parker was a speaker in a conference session sponsored by Boehringer Ingelheim Pty Ltd.
References
1. American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2012; 165: 277-304.
2. Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733-748.
3. Kaul B, Cottin V, Collard HR, Valenzuela C. Variability in global prevalence of interstitial lung disease. Front Med 2021; 8: 751181.
4. Watanabe K, Handa T, Tanizawa K, et al. Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias. Respir Med 2011; 105: 1238-1247.
5. Fischer A, Swigris JJ, Bois RM du, et al. Anti-synthetase syndrome in ANA and anti-Jo-1 negative patients presenting with idiopathic interstitial pneumonia. Respir Med 2009; 103: 1719-1724.
6. Demoruelle MK, Mittoo S, Solomon JJ. Connective tissue disease-related interstitial lung disease. Best Pr Res Clin Rheumatol 2016; 30: 39-52.
7. Spagnolo P, Bonniaud P, Rossi G, Sverzellati N, Cottin V. Drug-induced interstitial lung disease. Eur Respir J 2022; 60: 2102776.
8. Mehta P, Redhead G, Nair A, Sparks JA, Porter JC. Can we finally exonerate methotrexate as a factor in causing or exacerbating fibrotic interstitial lung disease in patients with rheumatoid arthritis? Clin Rheumatol 2022; 41: 2925-2928.
9. Chen Z, Wu Z, Ning W. Advances in molecular mechanisms and treatment of radiation-induced pulmonary fibrosis. Transl Oncol 2019; 12: 162-169.
10. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline. Thorax 2008; 63(Suppl 5): v1.
11. Jee AS, Adelstein S, Bleasel J, et al. Role of autoantibodies in the diagnosis of connective-tissue disease ILD (CTD-ILD) and interstitial pneumonia with autoimmune features (IPAF). J Clin Med 2017; 6: 51.
12. Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69: 1809.
13. Nihtyanova SI, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long‐term survival in systemic sclerosis. Arthritis Rheumatol 2014; 66: 1625-1635.
14. Qiu M, Nian X, Pang L, Yu P, Zou S. Prevalence and risk factors of systemic sclerosis‐associated interstitial lung disease in East Asia: a systematic review and meta‐analysis. Int J Rheum Dis 2021; 24: 1449-1459.
15. Hallowell RW, Paik JJ. Myositis-associated interstitial lung disease: a comprehensive approach to diagnosis and management. Clin Exp Rheumatol 2022; 40: 373-383.
16. Opinc AH, Makowska JS. Antisynthetase syndrome – much more than just a myopathy. Semin Arthritis Rheum 2021; 51: 72-83.
17. Gerami P, Schope JM, McDonald L, Walling HW, Sontheimer RD. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol 2006; 54: 597-613.
18. Dai Y, Wang W, Yu Y, Hu S. Rheumatoid arthritis–associated interstitial lung disease: an overview of epidemiology, pathogenesis and management. Clin Rheumatol 2021; 40: 1211-1220.
19. Habib HM, Eisa AA, Arafat WR, Marie MA. Pulmonary involvement in early rheumatoid arthritis patients. Clin Rheumatol 2011; 30: 217-221.
20. Zhuo J, Lama S, Knapp K, et al. Epidemiology and clinical characteristics of interstitial lung disease in patients with rheumatoid arthritis from the JointMan database. Sci Rep 2023; 13: 11678.
21. Mittoo S, Fell C. Pulmonary manifestations of systemic lupus erythematosus. Semin Respir Crit Care Med 2014; 35: 249-254.
22. Eisenberg H, Dubois EL, Sherwin RP, Balchum OJ. Diffuse interstitial lung disease in systemic lupus erythematosus. Ann Intern Med 1973; 79: 37.
23. Toyoda Y, Koyama K, Kawano H, et al. Clinical features of interstitial pneumonia associated with systemic lupus erythematosus. Respir Investig 2019; 57: 435-443.
24. Borg EJ ter, Groen H, Horst G, Limburg PC, Wouda AA, Kallenberg CGM. Clinical associations of antiribonucleoprotein antibodies in patients with systemic lupus erythematosus. Semin Arthritis Rheum 1990; 20: 164-173.
25. Gunnarsson R, Aaløkken TM, Molberg Ø, et al. Prevalence and severity of interstitial lung disease in mixed connective tissue disease: a nationwide, cross-sectional study. Ann Rheum Dis 2012; 71: 1966.
26. Flament T, Bigot A, Chaigne B, Henique H, Diot E, Marchand-Adam S. Pulmonary manifestations of Sjögren’s syndrome. Eur Respir Rev 2016; 25: 110-123.
27. Alba MA, Flores-Suárez LF, Henderson AG, et al. Interstital lung disease in ANCA vasculitis. Autoimmun Rev 2017; 16: 722-729.
28. Goh NSL, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis. Am J Respir Crit Care Med 2012; 177: 1248-1254.
29. Goh NS, Hoyles RK, Denton CP, et al. Short‐term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol 2017; 69: 1670-1678.
30. Kondoh Y, Makino S, Ogura T, et al. 2020 guide for the diagnosis and treatment of interstitial lung disease associated with connective tissue disease. Respir Investig 2021; 59: 709-740.
31. Hughes M, Allanore Y, Chung L, Pauling JD, Denton CP, Matucci-Cerinic M. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat Rev Rheumatol 2020; 16: 208-221.
32. Gunnarsson R, Hetlevik SO, Lilleby V, Molberg Ø. Mixed connective tissue disease. Best Pr Res Clin Rheumatol 2016; 30: 95-111.
33. Wells M, Alawi S, Thin KYM, et al. A multidisciplinary approach to the diagnosis of antisynthetase syndrome. Front Med 2022; 9: 959653.
34. Heimovski FE, Simioni JA, Skare TL. Systemic lupus erythematosus and Raynaud’s phenomenon*. A Bras Dermatol 2015; 90: 837-840.
35. Dugan EM, Huber AM, Miller FW, Rider LG, International Myositis Assessment and Clinical Studies Group. Photoessay of the cutaneous manifestations of the idiopathic inflammatory myopathies. Dermatol Online J 2009; 15: 1.
36. Elahmar H, Feldman BM, Johnson SR. Management of calcinosis cutis in rheumatic diseases. J Rheumatol 2022; 49: jrheum.211393.
37. Australian Rheumatology Association (ARA). Vaccinations in Rheumatology. New Farm: ARA; 2024. Available online at: https://rheumatology.org.au/For-Patients/Vaccinations-in-Rheumatology (accessed January 2025).
38. Bass AR, Chakravarty E, Akl EA, et al. 2022 American College of Rheumatology Guideline for vaccinations in patients with rheumatic and musculoskeletal diseases. Arthritis Care Res 2023; 75: 449-464.