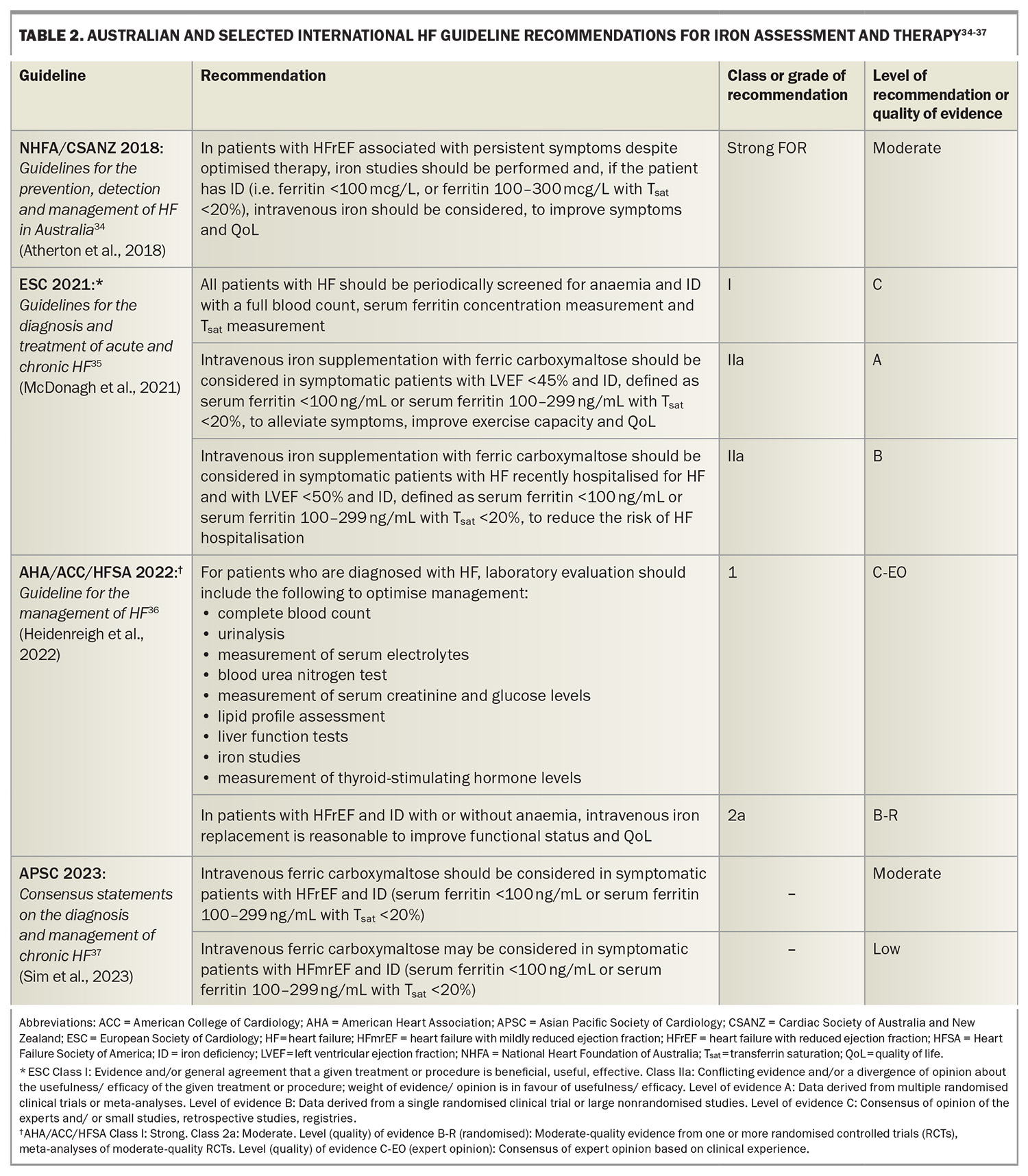
Iron deficiency in heart failure – more than just anaemia
Iron deficiency is common in patients with heart failure and is associated with poorer outcomes, irrespective of the presence or absence of anaemia. Intravenous iron replenishment in these patients improves exercise capacity, symptoms and quality of life, and likely reduces heart failure hospitalisation.
- Iron is an essential micronutrient for many cellular functions, including both haematopoietic and cellular metabolic processes.
- Iron deficiency is common in patients with heart failure and often occurs independently of anaemia, and is associated with poor clinical outcomes, including increased mortality.
- Patients with heart failure are considered iron deficient with a serum ferritin level less than 100 mcg/L, or serum ferritin level 100 to 300 mcg/L combined with transferrin saturation less than 20%.
- Intravenous iron replenishment in patients with heart failure and iron deficiency improves exercise capacity, symptoms and quality of life. Recent evidence suggests that intravenous iron replenishment likely also reduces heart failure hospitalisation and is a cost-effective treatment. Oral iron supplementation does not appear to be effective.
In 2017, more than half a million (about 511,000) people in Australia had heart failure (HF), representing 2.1% of the population, with a projected estimate of 657,000 patients by 2025.1 However, Australian studies suggest that HF may be under-recognised in the primary care setting, contributing to lower rates of guideline-directed therapies for this vulnerable patient cohort.2,3 Patients with HF represent a high proportion of primary care presentations, on average visiting their GPs 12.4 times a year and representing 3.6% of all patients presenting to primary practice.4,5 Therefore, primary care practitioners have a unique opportunity and are well positioned to help optimise their patients’ HF management.
Iron deficiency (ID) is estimated to affect 2% of men and 9% of women in developed countries.6,7 Patients with HF are at greater risk of ID compared with the general population, and although exact estimates vary, the prevalence of ID in the HF population ranges from 37% to 61%, depending on differences in the cohorts studied.8-12 Patients with HF have altered iron uptake and turnover, contributing to higher rates of ID. Patients with HF and ID have a poor prognosis, associated with increased hospitalisation and mortality.9,11 ID is, therefore, an important therapeutic target, with intravenous iron replenishment shown to improve exercise capacity, symptoms and quality of life (QoL) and likely reduce HF hospitalisations.13,14
This article discusses the mechanisms of ID in patients with HF, the role of iron in cardiac function and the evidence and guidelines supporting intravenous iron replenishment in patients with HF and ID.
Why do patients with heart failure become iron deficient?
Dietary iron is reduced to ferrous iron in the duodenal and jejunal lumen, which then enters the enterocytes, and is exported into the circulation by ferroportin, situated on the basolateral membrane of the enterocyte.15 Ferrous iron (Fe2+), once it enters the circulation, is then oxidised to ferric iron (Fe3+) and transported in the circulation, bound to plasma transferrin. This complex is then taken up by the liver, spleen and bone marrow, where it is stored as ferritin.
Hepcidin is an important small peptide that acts as a regulatory hormone to maintain iron homeostasis. Hepcidin is formed and released by the liver. The level of circulating hepcidin is increased in states of inflammation and iron overload.16 Hepcidin inhibits gastrointestinal iron absorption by binding and degrading ferroportin on the enterocyte basolateral membrane, thereby preventing the export of ferrous iron from the enterocyte into the circulation. Iron is not actively secreted; however, the action of hepcidin results in iron accumulation within the enterocyte itself, and eventual excretion via shedding of the intestinal cells. Ferroportin is also present in the macrophages of the reticuloendothelial system, where the action of hepcidin results in iron sequestration in the reticuloendothelial system and a reduction in the availability of iron for utilisation, a process known as reticuloendothelial block. This explains the reduced efficacy of oral iron supplementation in improving clinical status in patients with HF and ID.
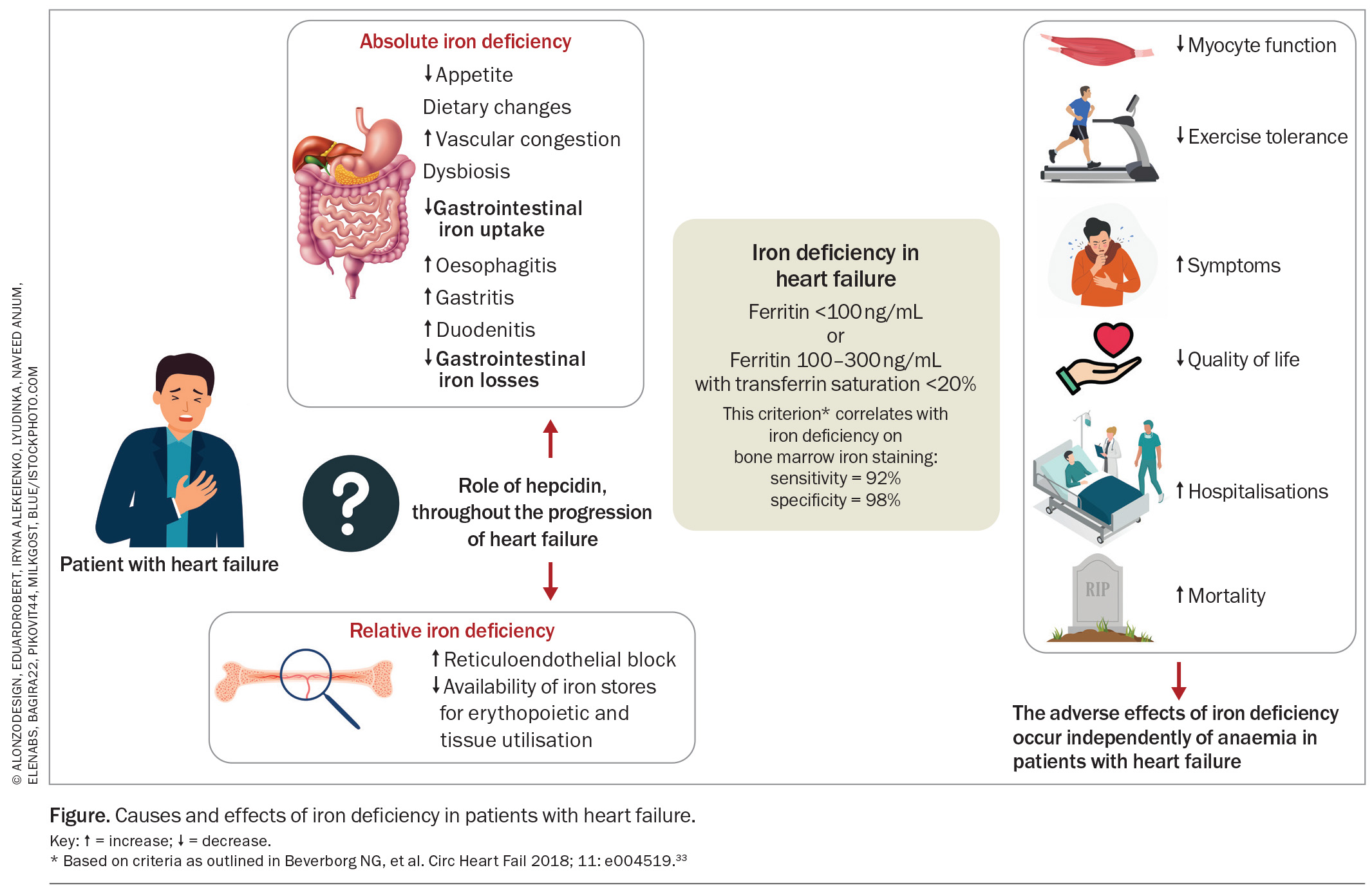
ID may be absolute or functional. Absolute ID reflects depleted body stores and is reflected by a low serum ferritin level. Functional ID reflects the impaired availability and utilisation of iron owing to reticuloendothelial block, which is reflected by low transferrin saturation (Tsat).17,18 Patients with HF are at increased risk of both absolute and functional ID.18
HF increases the risk of ID through a number of multifactorial and complex mechanisms.8,19,20 First, patients with HF have reduced gastrointestinal iron uptake, mediated via poor appetite, dietary changes, gastrointestinal ischaemia or venous congestion, dysbiosis of the gut microbiome and concurrent medications, including proton pump inhibitors and histamine H2 receptor antagonists.21 Second, patients with HF are at risk of increased gastrointestinal losses through occult bleeding associated with oesophagitis, gastritis, duodenitis or gastrointestinal ulceration. Importantly, although most patients with HF are on concomitant antiplatelet or anticoagulants for associated cardiovascular indications, meta-analyses have shown that this does not correlate with increased rates of ID in this population.12 Third, and importantly, the HF syndrome is characterised by a chronic inflammatory state.21,22 Chronic inflammation results in high levels of hydroxyl molecules (OH–) in the intestinal wall, which may inhibit the absorption of ferrous iron (Fe2+) and its conversion to ferric iron (Fe3+).
Notably, whereas hepcidin plays a key mediator role in both acute and chronic inflammation, the precise role of hepcidin in HF and ID is less clear. Studies have demonstrated that asymptomatic patients with HF have markedly increased levels of circulating hepcidin; however, with increasing HF severity, this progresses to decreased levels of circulating hepcidin, despite the presence of increased chronic inflammation.18 The reason underlying the drop in hepcidin levels in more advanced stages of HF remains unclear, but a reduced hepatic capacity to produce hepcidin may be contributory. Hepcidin may, therefore, contribute to reduced gastrointestinal iron absorption and enhanced reticuloendothelial block at certain stages of HF, but its exact role at different timepoints through the progression of HF and, particularly, its role in the more advanced stages of HF require further evaluation. Lower circulating hepcidin levels, in both acute and chronic settings, are independently associated with higher mortality in patients with HF.18,23
Role of iron in cardiac function
Iron has a well-recognised role in erythropoiesis, and ID remains the leading cause of anaemia in both the developed and developing world.24 Beyond its role in erythropoiesis, however, iron is also an essential micronutrient for a wide variety of cellular processes, including oxygen transport and storage, electron transfer reactions, mitochondrial respiration, gene regulation, cellular immunity and cellular reproduction.25,26 The importance of iron in these extrahaematopoietic, metabolic cellular processes may explain the fatigue that occurs in non-anaemic patients with ID.27 Iron has a crucial role in metabolic functioning; therefore, maintaining adequate iron availability is particularly important for cells with a high metabolic demand, such as cardiac and skeletal myocytes.9
Therefore, even though ID has traditionally been linked with anaemia, it is important to distinguish the two conditions and emphasise that they do not necessarily coexist. ID is substantially more prevalent than anaemia in patients with HF.28 ID is also present in about 32% of patients with HF who are non-anaemic.9 Studies have also shown that in HF, ID is poorly linked with red cell indices, indicating that ID in these patients should be viewed independently of their erythropoietic status.29,30
Diagnosis of iron deficiency in heart failure
Iron status is ordinarily assessed by measuring serum ferritin levels, with a standard threshold of less than 30 mcg/L being diagnostic for ID in the general population. This cutoff in an unselected (non-HF) population gives a sensitivity of 92% and specificity of 98% for ID, and a positive predictive value of 83%, when assessed against a gold standard of bone marrow aspirate or documented response to iron therapy.31
However, the interpretation of serum ferritin in inflammatory states is problematic, as ferritin is an acute-phase reactant that rises in acute inflammation. The standard cutoff of serum ferritin, therefore, becomes insensitive for ID in proinflammatory conditions such as HF. This was demonstrated in a study, wherein out of 37 patients with HF, 73% had ID as confirmed on bone marrow aspiration, despite a mean serum ferritin of 75 ± 59 mcg/L in the ID group.32 Inflammation also impairs iron availability and utilisation for red cell production, leading to reticuloendothelial block, an iron sequestration syndrome and iron-restricted erythropoiesis, a state known as functional ID.27 This is not reflected by serum ferritin levels, but rather reflected in a low Tsat. Currently, in patients with HF, a diagnosis of ID can be made if:
- serum ferritin level is less than 100 mcg/L (absolute ID), OR
- serum ferritin level is 100 to 300 mcg/L, combined with Tsat less than 20% (functional ID).
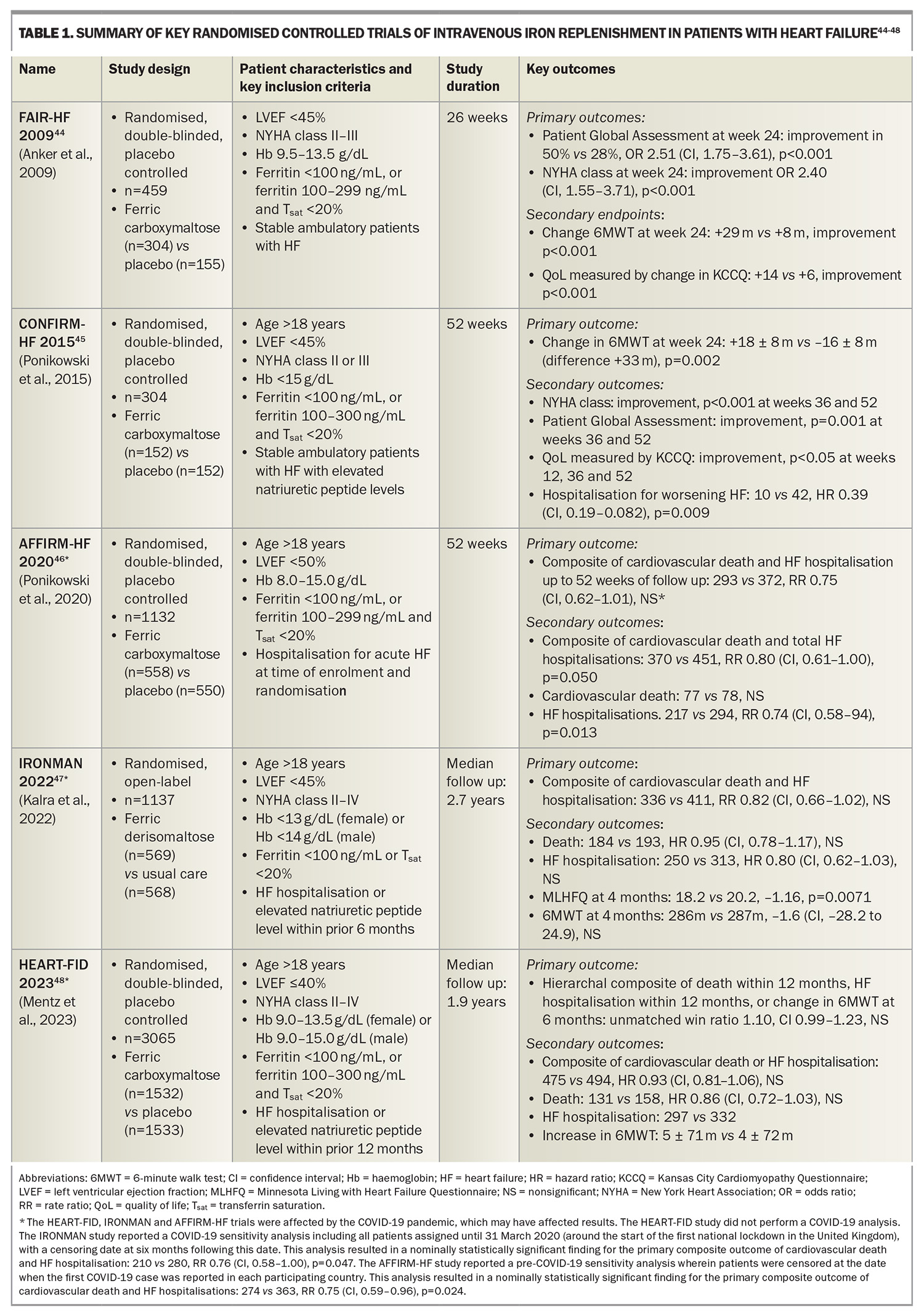
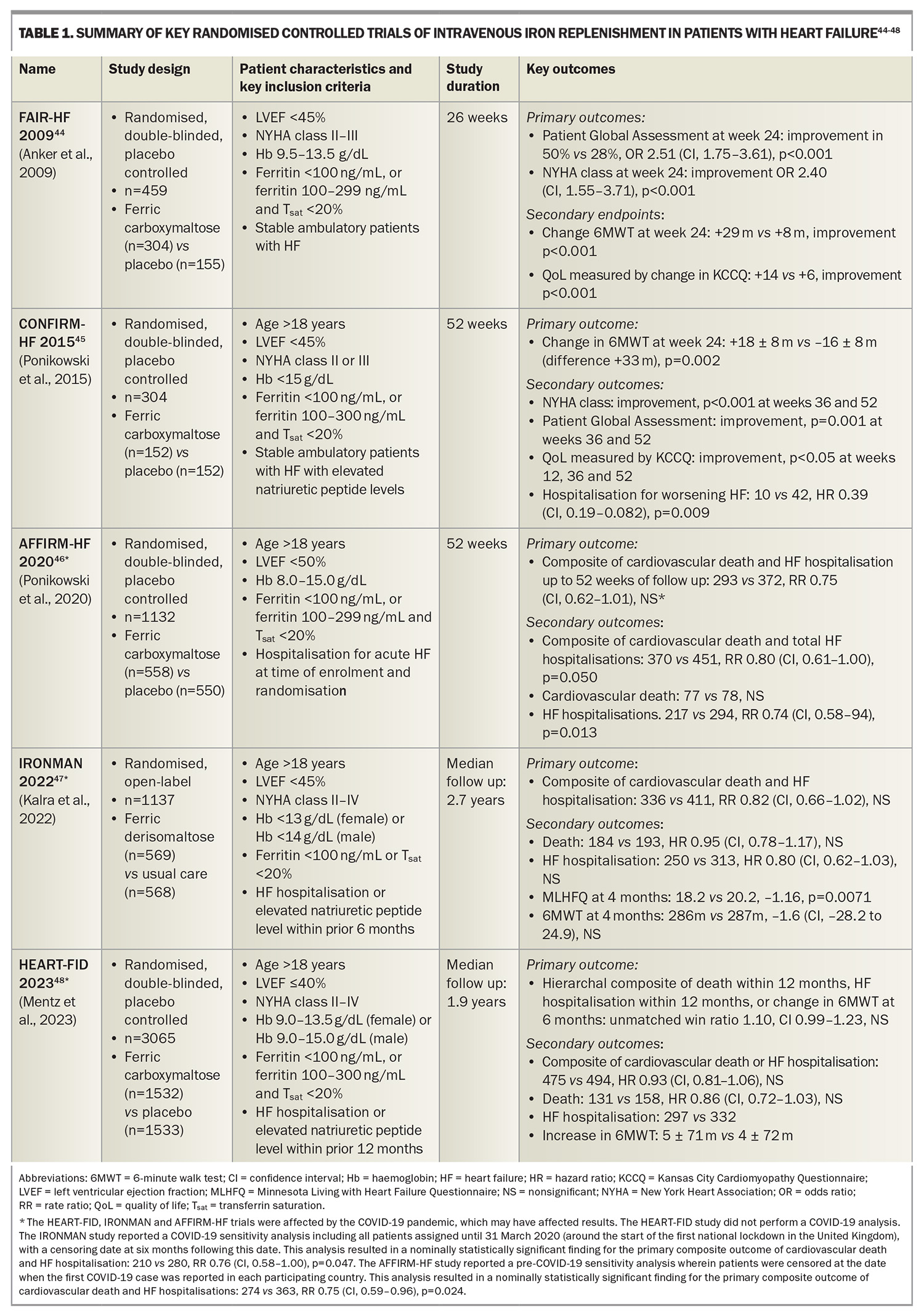
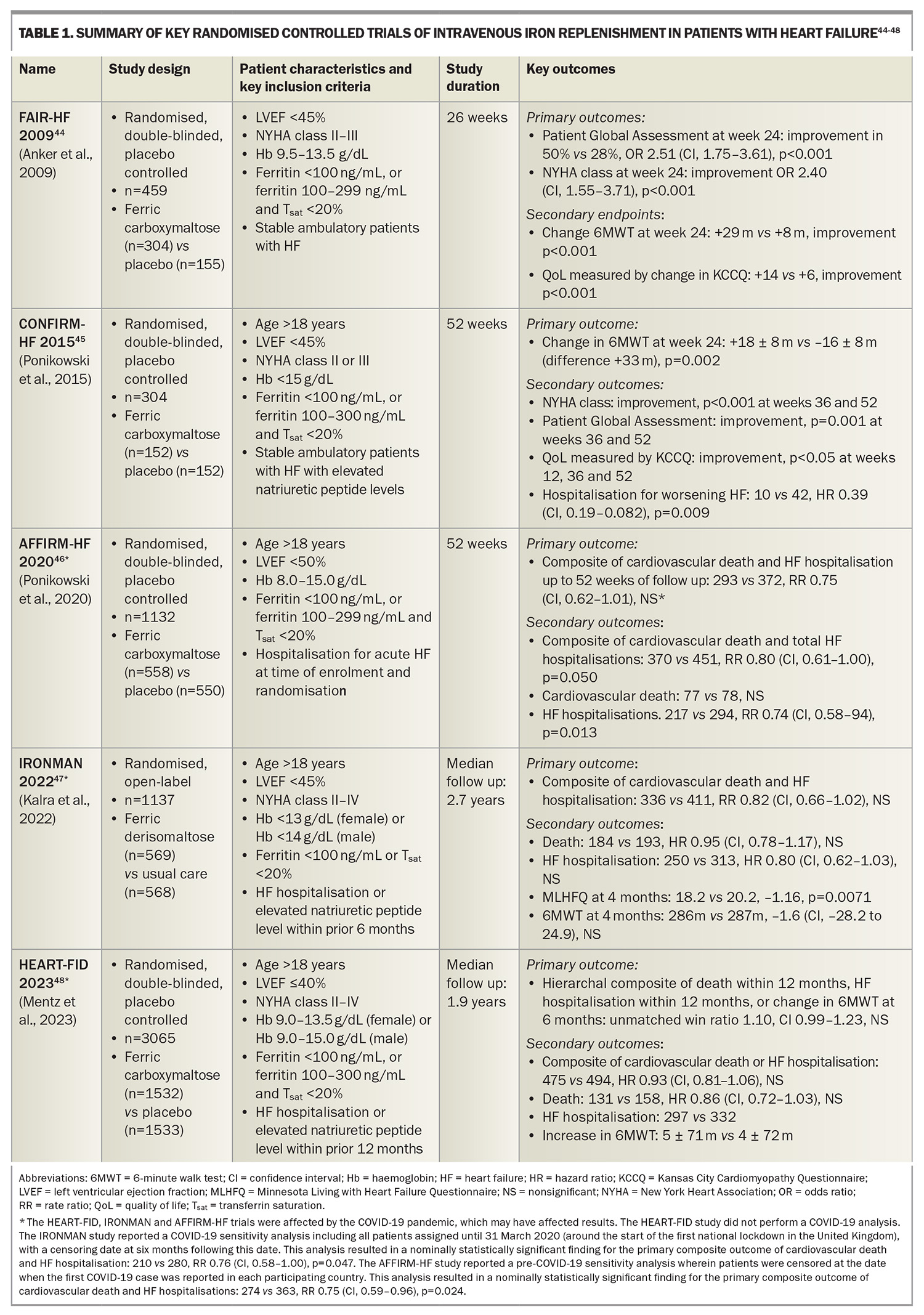
This criterion is based on cutoffs used in landmark clinical trials assessing iron replacement in patients with HF and ID (Table 1). This criterion was shown to yield a sensitivity of 82% and specificity of 72% when assessed against a gold standard of bone marrow iron staining in a cohort of patients with HF undergoing coronary artery bypass grafting surgery.33 This criterion has since been incorporated into Australian, European, American and Asia-Pacific HF guidelines.34-37 This is also consistent with diagnostic thresholds for ID in other chronic inflammatory conditions including chronic kidney disease and inflammatory bowel disease.27
Iron deficiency in patients with heart failure is associated with poor outcomes
Findings of animal studies have suggested that ID has deleterious effects on cardiac myocyte function. A study showed that rats induced with ID anaemia developed cardiac hypertrophy by 12 weeks and left ventricular dilatation with pulmonary congestion by 20 weeks.38 In rats with ID anaemia, intraperitoneal iron infusion improved exercise endurance when compared with saline placebo, independent of changes in haemoglobin levels.39
Studies in human cohorts have demonstrated that ID in patients with HF is associated with a range of deleterious clinical outcomes, including poorer exercise capacity, symptoms and QoL. A study showed that patients with HF and ID had poorer exercise capacity with lower mean oxygen consumption on cardiopulmonary exercise testing, a relationship seen independent of haemoglobin levels.40 ID has also been shown to predict exercise intolerance, irrespective of the patient’s underlying haemoglobin level or severity of HF.41 Two studies showed that ID was associated with worse QoL measures, and multivariate analysis in both these studies confirmed that ID, not anaemia, is independently associated with poorer QoL.42,43
ID is associated with increased mortality in patients with HF.9,40 International pooled analysis showed that patients with HF and ID had significantly higher mortality at six months (8.7% vs 3.6%) and persisting out to a median follow up of 1.9 years.12 Further, ID was shown to be an independent predictor for mortality (hazard ratio 1.42, confidence interval [CI] 1.14–1.77, p=0.002), whereas anaemia was not. The effects of ID in patients with HF are illustrated in the Figure.33
Clinical trial evidence for iron replacement in patients with heart failure
Numerous randomised controlled clinical trials have been conducted to assess the role and clinical benefits of intravenous iron replacement in patients with heart failure with reduced ejection fraction (HFrEF) and ID. The key randomised control trials are summarised in Table 1.
The landmark FAIR-HF trial randomised stable, ambulatory patients with HFrEF and ID to ferric carboxymaltose or placebo.44 The trial revealed that iron replenishment with intravenous ferric carboxymaltose improved exercise capacity (as measured by the 6-Minute Walk Test [6MWT]), symptoms (as measured based on the Patient Global Assessment [PGA] and New York Heart Association [NYHA] classification) and QoL (as measured using the Kansas City Cardiomyopathy Questionnaire [KCCQ]) at 26 weeks of follow up. These findings were confirmed in the CONFIRM-HF trial, which similarly found that iron replenishment with intravenous ferric carboxymaltose resulted in improved exercise capacity (as measured by the 6MWT), symptoms (as measured based on the PGA and NYHA classification) and QoL (as measured using the KCCQ), as well as a reduction in HF hospitalisations at 52 weeks of follow up.45
Subsequently, more recent cardiovascular outcome trials (CVOTs) have assessed the role of intravenous iron replenishment and their effects on ‘hard’ endpoints of cardiovascular death and HF hospitalisations. These include the AFFIRM-HF, IRONMAN and HEART-FID trials.46-48
The AFFIRM-HF trial randomised patients hospitalised for acute HF with ID to intravenous ferric carboxymaltose or placebo, with a primary composite endpoint of cardiovascular death and HF hospitalisation. Although the findings supported a trend of improved outcomes in the iron treatment group, this trial narrowly failed to meet statistical significance (rate ratio [RR] 0.75, CI 0.62–1.91, not significant). The study was partially interrupted by the COVID-19 pandemic.46
The IRONMAN trial randomised patients with a prior HF hospitalisation or elevated levels of natriuretic peptides within the preceding six months, and ID, to intravenous ferric derisomaltose or usual care (unblinded study), assessed against a primary composite endpoint of cardiovascular death and HF hospitalisation. Similarly, this trial supported a trend of improved outcomes in the iron treatment group, but narrowly failed to meet statistical significance (RR 0.82, CI 0.66–1.02, not significant), and was also partially interrupted by the COVID-19 pandemic.47
It is prudent to acknowledge that the COVID-19 pandemic impacted the results of these two trials. Sensitivity analyses reported by both of these trials (performed by censoring patients following the COVID-19 outbreak) would have yielded nominally statistically significant primary outcomes for both trials (see Table 1, footnote).
The HEART-FID trial randomised patients with a prior HF hospitalisation or elevated levels of natriuretic peptides within the preceding 12 months, and ID, to intravenous ferric carboxymaltose or placebo, assessed against a primary hierarchal composite outcome of death, HF hospitalisation or change in 6MWT results. Although the findings supported a trend towards improved outcomes in the iron treatment group, this trial narrowly failed to meet statistical significance (unmatched win ratio 1.10, CI 0.99–1.23, not significant).48
An individual patient data meta-analysis of the CONFIRM-HF, AFFIRM-HF and HEART-FID studies demonstrated that intravenous ferric carboxymaltose reduced one of the co-primary endpoints comprising total cardiovascular hospitalisations and cardiovascular deaths, to 52 weeks (RR 0.86, CI 0.75–0.98, p=0.029).13 This was predominantly driven by reductions in HF hospitalisation (RR 0.84, CI 0.71–0.98, p=0.025) with no apparent difference in cardiovascular death. There was no significant heterogeneity in treatment effects across prespecified subgroups of age, sex and baseline serum ferritin. Of note, the IRONMAN study was excluded from this meta-analysis as the study used a different criterion for ID, the treatment included ferric derisomaltose and the study was unblinded; however, sensitivity analysis incorporating the IRONMAN study censored at 12 months also confirmed this co-primary endpoint.
No established role for oral iron replenishment
The role of high-dose, oral iron polysaccharide repletion was examined in the IRONOUT HF trial, which showed no difference in the clinical outcomes of exercise capacity (based on the peak oxygen uptake and 6MWT) or QoL (as measured using the KCCQ).49 Furthermore, oral iron replenishment produced only minimal improvements in iron stores. Therefore, there is currently insufficient evidence to recommend oral iron replenishment in patients with HF and ID, but this is currently the subject of further study (IVOFER-HF trial 2017-005053-37).
Practical recommendations
Australian and international HF guideline recommendations for iron replacement are summarised in Table 2.
Iron studies should be conducted at the time of diagnosis of HF, and at regular intervals, including at each HF hospitalisation. Intravenous iron replenishment should be considered in patients with HFrEF, persistent symptoms and ID to improve symptoms and QoL. In the context of newer evidence, intravenous iron replenishment may reduce HF hospitalisation.13
Importantly, it should be noted that these recommendations for ferric carboxymaltose were first introduced to HF guidelines following the results of the FAIR-HF study, which showed an improvement in functional outcomes. They first appeared in the Australia and New Zealand Guidelines in 2011 and ESC Guidelines in 2012.50,51 Subsequent to this, the more recent three moderate-sized CVOTs (AFFIRM-HF, IRONMAN and HEART-FID) suggest that although iron replacement may reduce HF hospitalisation, these studies did not meet their primary endpoints and failed to show any improvement in mortality.46-48 Guideline recommendations for iron replenishment in patients with HF and ID are, therefore, to improve symptoms and QoL, and may likely reduce HF hospitalisation, but has not been shown to improve mortality.
Intravenous administration has the advantage of bypassing the gastrointestinal tract, thereby avoiding issues with gastrointestinal uptake present in patients with HF and the consequent proinflammatory state. Ferric carboxymaltose (in Australia, Ferinject) is the formulation used in most clinical trials with evidence in the HF population.52
Cost effectiveness
Cost-effectiveness analyses have suggested that intravenous ferric carboxymaltose is a cost-effective treatment when modelled in the United Kingdom, the USA, Switzerland and Italy using outcomes from the AFFIRM-AHF trial and in Sweden, Spain and South Korea when modelled on the FAIR-HF outcomes.53-56 No cost-effectiveness analyses have been conducted in an Australian context and this remains an opportunity for future research; however, the average cost for a HF hospitalisation in Australia has previously been reported to be $6,699 in a HF therapy cost-effectiveness analysis.57
Future directions and unanswered questions
First, it remains controversial how to best diagnose ID in patients with HF. Diagnostic cutoffs that are currently used are based on those used in other chronic inflammatory conditions, including chronic kidney disease and inflammatory bowel disease, and are based on the criteria used to define ID in the FAIR-HF study. However, previous work has shown that both low serum iron levels and a reduced Tsat may have better test characteristics (i.e. sensitivity, specificity) than the currently used definition.33 Investigations are also continuing to examine alternative markers of iron deficiency, including serum transferrin receptor levels and the serum transferrin receptor–ferritin index.
Second, there remains ongoing debate as to the precise role of intravenous iron replacement in patients with HFrEF and concomitant ID, particularly in light of the three recent CVOTs failing to meet the primary endpoints and failing to show benefits in terms of cardiovascular mortality. Of note, although individual patient meta-analyses of these studies suggest an improvement in heart failure hospitalisation, this is hypothesis-generating only and does not constitute a randomised clinical trial finding.
Third, the current guideline recommendations for iron replacement pre-date the results of these three CVOTs and should be interpreted in this context.
Fourth, intravenous iron supplementation is not without risk, and the potential hazards need to be considered prior to prescribing, including risks of increased allergy and infusion reactions, inflammation, infection, hypophosphataemia and concerns of oncogenesis seen in animal models.
Fifth, the cost effectiveness of intravenous iron has not been assessed specifically in the Australian context.
Sixth, the role of oral iron replenishment is not yet established. Oral iron repletion has a theoretical appeal as it is cheaper than intravenous iron, readily available, and does not bypass the hepcidin system, which physiologically maintains iron homeostasis. Therefore, serious toxicity from oral iron supplementation is exceedingly rare. However, the IRONOUT HF trial failed to show functional benefits of oral iron replenishment and showed only a minimal improvement in iron stores; thus, the role of oral iron replenishment is not yet established and warrants further investigation.49
Finally, the role of iron replenishment in heart failure with preserved ejection fraction (HFpEF) is less clear and warrants further study. The prevalence of ID in HFpEF reaches 59%; however, studies evaluating iron replenishment in this area are lacking, with the recently published FAIR-HFpEF trial being discontinued because of slow recruitment after only 39 patients.58,59
Conclusion
Patients with HF are prone to developing ID, which may occur concurrently with, or independently of, anaemia. ID in patients with HF is associated with poor outcomes including a reduced exercise capacity, increased symptoms, reduced QoL and increased mortality. Randomised controlled trials have established that intravenous iron replacement improves exercise capacity, symptoms and QoL in patients with HF and concomitant ID. Meta-analyses have suggested that intravenous iron replenishment likely reduces HF hospitalisation. However, three recent moderate- sized CVOTs of intravenous iron replenishment failed to meet primary endpoints and did not show any improvements in mortality.
Australian and international HF guidelines currently recommend that intravenous iron replenishment be considered in patients with HF and ID. There are ongoing studies in this area to further define the role and benefits of intravenous iron replenishment. Primary care physicians have a unique opportunity and an important role in assessing and optimising their patients’ HF therapies, including iron replenishment. MT
COMPETING INTERESTS: Dr Chan: None. Professor Sindone has received honoraria, speaker fees, consultancy fees, travel support, is a member of advisory boards or has appeared on expert panels for: Abbott, Alphapharm, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL, Edwards, Eli Lilly, Glaxo Smith Kline, HealthEd, Inside Practice, Menarini, Merck Sharp and Dohme, Moderna, Mylan, Novartis, Novo Nordisk, Orion, Otsuka, Pfizer, Pharmacosmos, Roche, Sanofi, Servier and Vifor. He has a leadership role at the Cardiac Society of Australia and New Zealand, National Heart Foundation of Australia, Asian Pacific Society of Cardiology, NSW Agency for Clinical Innovation and National Cardiac Australia. He holds stock or stock options in National Cardiac Australia.
References
1. Chen L, Booley S, Keates AK, Stewart S. Snapshot of heart failure in Australia. Melbourne: Mary MacKillop Institute for Health Research, Australian Catholic University; 2017. Available online at: https://www.acu.edu.au/about-acu/news/2017/june/snapshot-of-heart-failure-in-australia (accessed February 2025).
2. Sindone AP, Haikerwal D, Audehm RG, et al. Clinical characteristics of people with heart failure in Australian general practice: results from a retrospective cohort study. ESC Heart Failure 2021; 8: 4497-4505.
3. Liew D, Audehm RG, Haikerwal D, et al. Epidemiology of heart fail: Study of Heart failure in the Australian Primary carE setting (SHAPE). ESC Heart Fail 2020; 7: 3871-3880.
4. Britt H, Miller GC, Henderson J, et al. General practice activity in Australia 2014-15. Sydney: Sydney University Press; 2015.
5. Taylor CJ, Valenti L, Britt H, et al. Management of chronic heart failure in general practice in Australia. Aust Fam Phys 2016; 45: 823-827.
6. Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet 2007; 370: 511-520.
7. Hegde N, Rich MW, Gayomali C. The cardiomyopathy of iron deficiency. Tex Heart Inst J 2006; 33: 340-344.
8. Wong CC, Ng AC, Kritharides L, Sindone AP. Iron deficiency in heart failure: looking beyond anaemia. Heart Lung Circ 2016; 25: 209-216.
9. Jankowska E, Rozentryt P, Witkowska A, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010; 31: 1872-1880.
10. Parikh A, Natarajan S, Lipsitz S, Katz S. Iron deficiency in community- dwelling U.S. adults with self-reported heart failure in NHANES III: prevalence and associations with anemia and inflammation. Circ Heart Fail 2011; 4: 599-606.
11. Kasner M, Aleksandrov A, Westermann D, et al. Functional iron deficiency and diastolic function in heart failure with preserved ejection fraction. Int J Cardiol 2013; 168: 4652-4657.
12. Klip I, Comin-Colet J, Voors A, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575-582.
13. Ponikowski P, Mentz RJ, Hernandez AF, et al. Efficacy of ferric carboxymaltose in heart failure with iron deficiency: an individual patient data meta-analysis. Eur Heart J 2023; 44: 5077-5091.
14. Sindone AP, Diener W, Comin-Colet J. Systematic review and meta-analysis of intravenous iron carbohydrate complexes in HFrEF patients with iron deficiency. ESC Heart Fail 2023; 10: 44-56.
15. Ebner N, von Haehling S. Iron deficiency in heart failure: a practical guide. Nutrients 2013; 5: 3730-3739.
16. Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol 2009; 122: 78-86.
17. Deicher R, Hörl WH. New insights into the regulation of iron homeostasis. Eur J Clin Invest 2006; 36: 301-309.
18. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34: 816-826.
19. Hughes CM, Woodside JV, McGartland C, et al. Nutritional intake and oxidative stress in chronic heart failure. Nutr Metab Cardiovasc Dis 2012; 22: 376-382.
20. Sindone A. Iron deficiency in heart failure: more than just fatigue. Cardiology Today 2018; 8(2): 75-78.
21. Murphy SP, Kakkar R, McCarthy CP, Januzzi Jr JL. Inflammation in heart failure: JACC state-of-the-art review. JACC 2020; 75: 1324-1340.
22. Reina-Couto M, Pereira-Terra P, Quelhas-Santos J, Silva-Pereira C, Albino-Teixeira A, Sousa T. Inflammation in human heart failure: major mediators and therapeutic targets. Front Physiol 2021; 12: 746494.
23. Jankowska EA, Kasztura M, Sokolski M, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014; 35: 2468-2476.
24. Jankowska E, Ponikowski P. Molecular changes in myocardium in the course of anemia or iron deficiency. Heart Fail Clin 2010; 6: 295-304.
25. Beard J. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr 2001; 131: S568-S579.
26. Cairo G, Bernuzzi F, Recalcati S. A precious metal: iron, an essential nutrient for all cells. Genes Nutr 2006; 1: 25-39.
27. Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis 2018; 2018: 9394060.
28. Cleland JG, Zhang J, Pellicori P, et al. Prevalence and outcomes of anemia and hematinic deficiencies in patients with chronic heart failure. JAMA Cardiol 2016; 1: 539-547.
29. Tkaczyszyn M, Comín‐Colet J, Voors AA, et al. Iron deficiency and red cell indices in patients with heart failure. Eur J Heart Fail 2018; 20: 114-122.
30. Alnuwaysir RI, Beverborg NG, van Der Meer P. Fluctuating iron levels in heart failure: when and where to look at? Eur J Heart Fail 2022; 24: 818-820.
31. Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem 1998; 44: 45-51.
32. Nanas J, Matsouka C, Karageogopoulos D. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol 2006; 48: 2485-2489.
33. Grote Beverborg N, Klip IT, Meijers WC, et al. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail 2018; 11: e004519.
34. Atherton JJ, Sindone A, De Pasquale CG, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ 2018; 27: 1123-1208.
35. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2021; 42: 3599-3726.
36. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022; 79: e263-e421.
37. Sim D, Lin W, Sindone A, et al. Asian Pacific Society of Cardiology consensus statements on the diagnosis and management of chronic heart failure. J Asia Pac Soc Cardiol 2023; 2: e10.
38. Naito Y, Tsujino T, Matsumoto M, Sakoda T, Ohyanagi M, Masuyama T. Adaptive response of the heart to long-term anemia induced by iron deficiency. Am J Physiol Heart Circ Physiol 2009; 296: 585-593.
39. Willis W, Gohil K, Brooks G, Dallman P. Iron deficiency: improved exercise performance within 15 hours of iron treatment in rats. J Nutr 1990; 120: 909-916.
40. Okonko D, Mandal A, Missouris C, Poole-Wilson P. Disordered iron homeostasis in chronic heart failure. JACC 2011; 58: 1241-1251.
41. Jankowska E, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Cardiac Fail 2011; 17: 899-906.
42. Comin-Colet J, Enjuanes C, Gonzalez G, et al. Iron deficiency is a key determinant of health related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail 2013; 15: 1164-1172.
43. Enjuanes C, Klip I, Bruguera J, et al. Iron deficiency and health-related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol 2014; 174: 268-275.
44. Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. New Engl J Med 2009; 361: 2436-2448.
45. Ponikowski P, Van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36: 657-668.
46. Ponikowski P, Kirwan BA, Anker SD, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. The Lancet 2020; 396: 1895-1904.
47. Kalra PR, Cleland JG, Petrie MC, et al. Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): an investigator-initiated, prospective, randomised, open-label, blinded-endpoint trial. Lancet 2022; 400: 2199-2209.
48. Mentz RJ, Garg J, Rockhold FW, et al. Ferric carboxymaltose in heart failure with iron deficiency. New Engl J Med 2023; 389: 975-986.
49. Lewis GD, Malhotra R, Hernandez AF, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA 2017; 317: 1958-1966.
50. Krum H, Jelinek MV, Stewart S, Sindone A, Atherton JJ. 2011 update to National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand Guidelines for the prevention, detection and management of chronic heart failure in Australia, 2006. Med J Aust 2011; 194: 405-409.
51. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787-1847.
52. Sindone A, Doehner W, Manito N, et al. Practical guidance for diagnosing and treating iron deficiency in patients with heart failure: why, who and how? J Clin Med 2022; 11: 2976.
53. McEwan P, Ponikowski P, Davis JA, et al. Ferric carboxymaltose for the treatment of iron deficiency in heart failure: a multinational cost‐effectiveness analysis utilising AFFIRM‐AHF. Eur J Heart Fail 2021; 23: 1687-1697.
54. Hofmarcher T, Borg S. Cost-effectiveness analysis of ferric carboxymaltose in iron-deficient patients with chronic heart failure in Sweden. J Med Econ 2015; 18: 492-501.
55. Comin-Colet J, Rubio-Rodriguez D, Rubio-Terres C, et al. A cost-effectiveness analysis of ferric carboxymaltose in patients with iron deficiency and chronic heart failure in Spain. Rev Esp Cardiol (Engl Ed) 2015; 68: 846-851.
56. Lim E, Sohn H, Lee H, Choi S. Cost-utility of ferric carboxymaltose (Ferinject) for iron-deficiency anemia patients with chronic heart failure in South Korea. Cost Eff Resour Alloc 2014; 12: 19.
57. Adena MA, Hamann G, Sindone AP. Cost-effectiveness of ivabradine in the treatment of chronic heart failure. Heart Lung Circ 2019; 28: 414-422.
58. Beale AL, Warren JL, Roberts N, Meyer P, Townsend NP, Kaye D. Iron deficiency in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Open Heart 2019; 6: e001012.
59. von Haehling S, Doehner W, Evertz R, et al. Ferric carboxymaltose and exercise capacity in heart failure with preserved ejection fraction and iron deficiency: the FAIR-HFpEF trial. Eur Heart J 2024; 45: 3789-3800.