ATTR cardiac amyloidosis: update on recent disease-modifying therapies
Transthyretin amyloidosis (ATTR) is a systemic illness that results in significant morbidity involving multiple organ systems, with cardiac involvement being the leading cause of mortality. Recent advances in targeted medical therapy provide therapeutic options for patients with previously limited treatment options. The advent of disease-modifying therapies for ATTR provides the opportunity for clinicians to initiate these agents early in a patient’s clinical course and improve outcomes.
- Systemic amyloidosis is a group of disorders caused by the extracellular deposition of insoluble, misfolded fibrillar proteins, resulting in organ dysfunction and eventually death; the most common types are immunoglobulin light chain (AL) amyloidosis and transthyretin (TTR) amyloidosis (ATTR).
- Cardiac involvement with heart failure and arrhythmias as well as neurological involvement are common in patients with systemic amyloidosis.
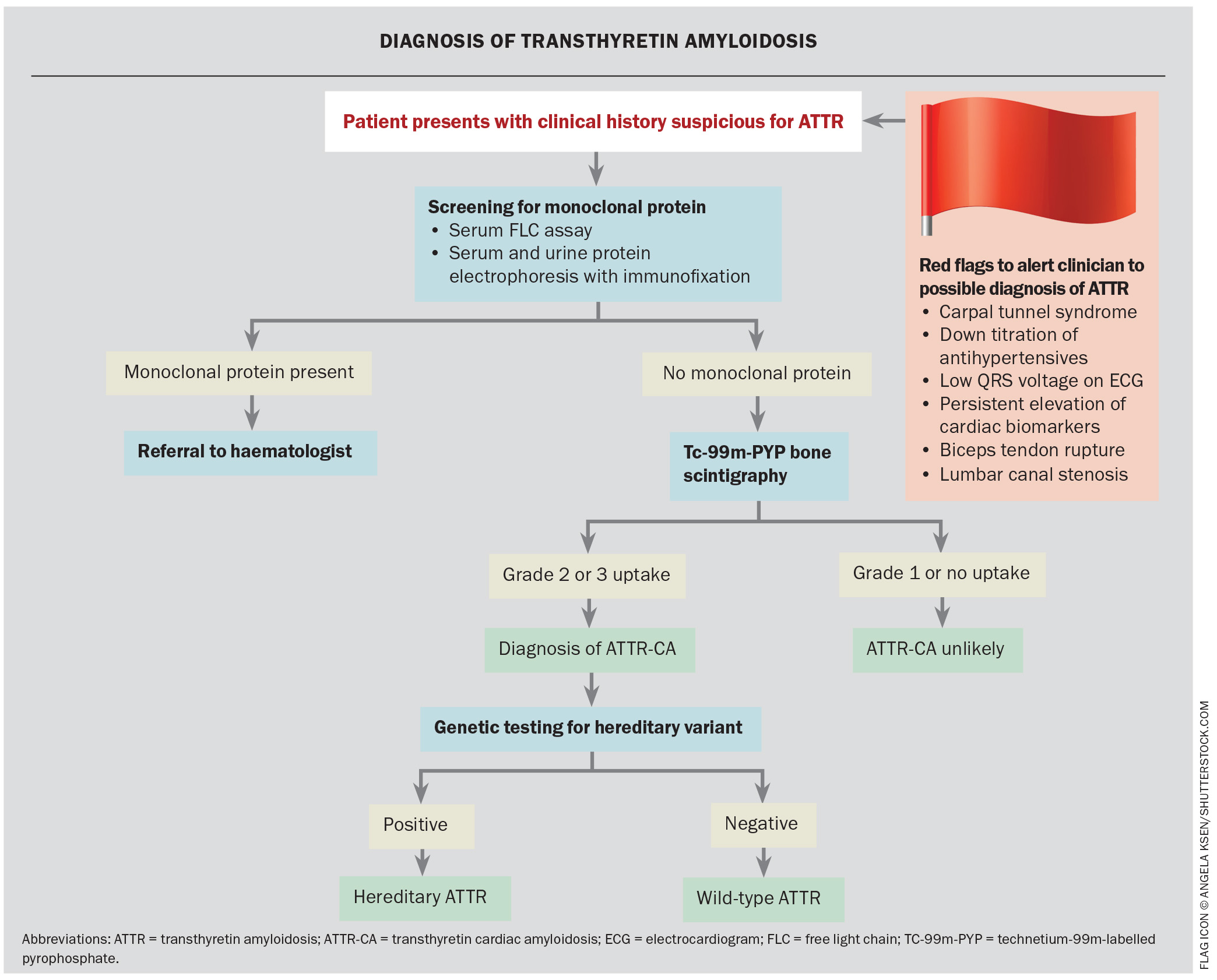
- Early diagnosis of ATTR cardiac amyloidosis (ATTR-CA) is crucial to allow initiation of treatment before significant morbidity has developed; however, diagnosis is often delayed with patients reviewed by multiple specialists before a diagnosis is confirmed.
- Technetium-99m (Tc-99m) bone scintigraphy is essential in the diagnosis of ATTR-CA and is often performed when there is clinical suspicion based on a transthoracic echocardiogram.
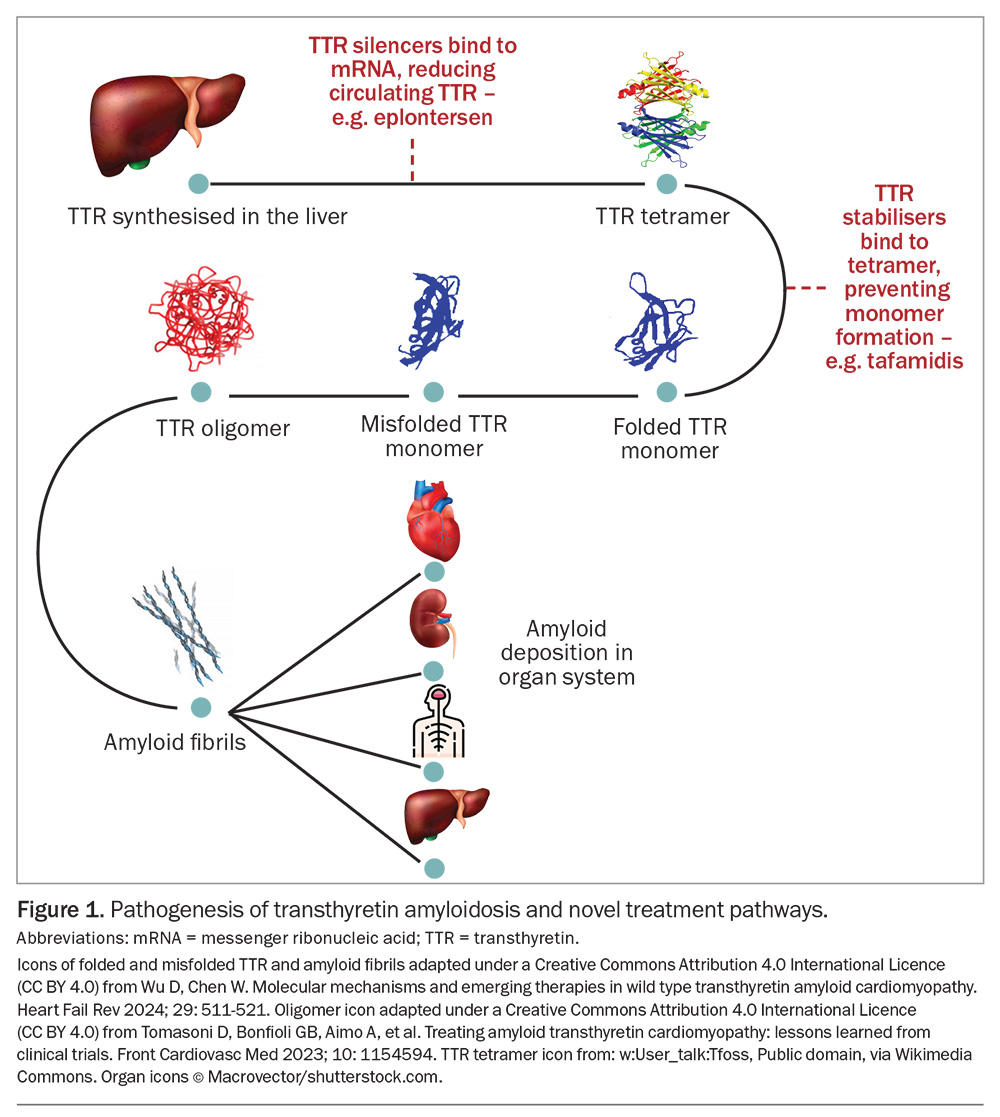
- Novel therapeutics in the form of TTR stabilisers and silencers represent a revolutionary development inthe management of ATTR-CA. TTR stabilisers bind toTTR, stabilise this protein and prevent fibril formation. TTR silencers aim to reduce the amount of circulating TTR.
Systemic amyloidosis is a group of disorders caused by the deposition of insoluble, misfolded fibrillar proteins, resulting in organ dysfunction and eventually death.1 Several identified proteins can lead to systemic amyloidosis, with clinical manifestations dependent on the precursor protein and organ involved. Cardiac involvement with heart failure and arrhythmias as well as neurological involvement are common.2
The most frequently encountered types of systemic amyloidosis are immunoglobulin light chain (AL) amyloidosis and transthyretin (TTR) amyloidosis (ATTR).3 ATTR is further characterised into hereditary ATTR (ATTRv), caused by a mutation with amino acid substitution in the TTR gene sequence on chromosome 18 (autosomal dominant inheritance), and wild-type ATTR (ATTRwt).4,5
There have been recent advances in the management of amyloidosis with the development of specific transthyretin (TTR) stabilisers, TTR silencers and TTR depleters.6 This review focuses on the recent novel therapeutic agents for the management of ATTR. The authors have previously published a detailed review on the presentation and diagnosis of ATTR cardiac amyloidosis (ATTR-CA) in Cardiology Today in May 2024.7
Clinical presentation
Early diagnosis of ATTR-CA is crucial to allow initiation of treatment before significant morbidity has developed. Diagnosis is often delayed, with patients reviewed by multiple specialists before the diagnosis is confirmed.8,9 ATTR-CA is predominately diagnosed in patients over 70 years of age and is more common in men than women.
Cardiac involvement may present with features of decompensated heart failure (with preserved ejection fraction [HFpEF]), which includes peripheral oedema, paroxysmal nocturnal dyspnoea and orthopnoea. Conduction abnormalities, including complete heart block warranting a pacemaker and arrhythmias such as atrial fibrillation (AF), are common. Patients on established antihypertensive therapies who are now intolerant to these medications due to hypotension should also prompt consideration of ATTR-CA.
Noncardiac red flag features suggestive of ATTR include a history of bilateral carpal tunnel syndrome, lumbar canal stenosis and biceps tendon rupture; symptoms from carpal tunnel syndrome may predate cardiac symptoms by about 10 years. Although these features may be indicative of ATTR-CA, they are not used to grade disease severity. Red flag features that should prompt suspicion for ATTR-CA and a diagnostic algorithm are summarised in the Flowchart.
Diagnosis of ATTR-CA
Patients suspected of having ATTR-CA require testing for monoclonal gammopathy to screen for AL amyloidosis.10 This includes serum free light chain (kappa and lambda) testing as well as serum and urine protein electrophoresis with immunofixation. A positive screen is determined by an abnormal kappa to lambda ratio in free light chain testing.11
Technetium-99m (Tc-99m) bone scintigraphy is essential in the diagnosis of ATTR-CA and is often performed when there is clinical suspicion based on a transthoracic echocardiogram (TTE). The bisphosphonate tracers accumulate in myocardium where TTR has deposited and, therefore, myocardial uptake indicates cardiac involvement. A negative monoclonal protein screen with a positive Tc-99m bone scintigraphy has high sensitivity and specificity for ATTR-CA, allowing noninvasive diagnosis without cardiac biopsy. Genetic testing is performed to further delineate ATTRv and ATTRwt.
Management of ATTR-CA
The management of patients with ATTR-CA involves symptomatic therapy and disease-modifying therapies.
Symptomatic therapy in ATTR-CA
It is recommended that patients with ATTR-CA monitor their salt and fluid intake. Loop diuretic therapy remains the first-line diuretic in managing heart failure; however, patients with ATTR-CA are at higher risk of over-diuresis than other heart failure populations. Aldosterone antagonists and thiazide diuretics can be considered as additive agents to loop diuretics but patients taking these medications require close monitoring for hypotension and acute kidney injury.
Primary care physicians may at times be the first point of medical contact in the community for patients with ATTR-CA during periods of fluid overload, and caution should be taken to avoid profound increases in diuretic therapy without close monitoring of blood pressure. Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are increasingly being used to help with diuresis in patients with ATTR-CA.
Autonomic dysfunction manifesting as postural hypotension occurs often. Traditionally used therapies in orthostatic hypotension, such as increased salt and fluid intake, may be poorly tolerated in patients with concomitant heart failure. Compression stockings can be used as a nonpharmacological option and midodrine as a pharmacological option.
Importantly, traditionally used neurohormonal agents (ACE inhibitors, angiotensin receptor blockers, etc) are poorly tolerated in patients with ATTR-CA.
AF is common in patients with ATTR-CA but can often be difficult to manage. Beta blockers can be used judiciously, but their use can be limited by symptomatic hypotension and heart block. Calcium channel blockers have been associated with increased risk of local myocardial tissue toxicity and heart block. Amiodarone may be considered in patients with AF in whom beta-blocker therapy is not tolerated or cannot be uptitrated. Patients with ATTR-CA and AF are at increased stroke risk regardless of their CHA2DS2-VASc score and, therefore, should be on anticoagulation therapy with either direct oral anticoagulants or warfarin.12
Conduction disease is common, with patients often requiring pacemaker therapy.
Emerging therapeutic options for ATTR
Disease-modifying therapies for ATTR-CA comprise TTR stabilisers and silencers and fibril disrupters. Previously, liver transplantation and fibril disrupters were the only available disease-modifying therapy for ATTR-CA. Fibril disrupters included doxycycline, tauroursodeoxycholic acid and epigallocatechin-3-gallate (a green tea extract), with limited clinical evidence of their efficacy.13 Diflunisal, an NSAID, was a nonspecific fibril stabiliser, again with limited evidence.14 Novel therapeutics in the form of TTR stabilisers and silencers represent a revolutionary development in the management of ATTR-CA (Figure 1).
TTR stabilisers
TTR stabilisers bind to TTR, stabilise this protein and prevent fibril formation. Tafamidis, the only available specific TTR stabiliser in Australia, binds to the thyroxine binding site on TTR to prevent ATTR tetramers from dissociating into monomers, thus slowing monomer fibril formation and deposition.15 Early diagnosis of ATTR-CA is essential for patients on tafamidis therapy as it slows disease progression.
ATTR-ACT was a multicentre, international, double-blinded randomised trial that compared tafamidis with placebo in patients with ATTR-CA. Following 30 months of treatment, tafamidis reduced all-cause mortality (29.5% vs 42.9%; hazard ratio [HR], 0.70; 95% confidence interval [CI], 0.51 to 0.96) and cardiovascular hospitalisations (relative risk ratio 0.68; 95% CI, 0.56 to 0.81) in patients with ATTR-CA with New York Heart Association (NYHA) class I-II symptoms.16 This was demonstrated in patients with ATTRv and ATTRwt. A higher rate of hospitalisations was noted in patients with NYHA class III symptoms, which was thought to be due to longer survival of patients with more advanced disease once started on tafamidis. The improved mortality was apparent after 18 months in ATTR-ACT and has been demonstrated to continue out to 58 months of treatment.17 Tafamidis has also demonstrated improved quality-of-life scores in patients with ATTR-CA.18 Patients with NYHA class IV symptoms, AL amyloidosis, a history of liver or heart transplantation and an estimated glomerular filtration rate (eGFR) less than 25mL/min/1.73 m2 were excluded from ATTR-ACT.
The data supporting the use of tafamidis in the management of ATTR peripheral neuropathy have been mixed and it is not TGA approved for this indication.19
Tafamidis administered orally at a dose of 61 mg daily currently has PBS listing for the treatment of patients with ATTRwt or ATTRv with confirmed ATTR-CA with NYHA class I-II symptoms, TTE demonstrating interventricular wall thickness greater than 12 mm, prior episodes of heart failure requiring hospitalisation or current diuretic therapy with eGFR greater than 25 mL/min/1.73 m2. Tissue biopsy is required in the presence of a coexistent monoclonal gammopathy. A cardiologist or physician experienced in managing amyloidosis is required to initiate therapy and to provide further repeat prescriptions. It is recommended that patients on tafamidis have their renal and hepatic function monitored on a six-monthly basis. In the ATTR-ACT trial the overall incidence and type of adverse events were similar in the tafamidis and placebo arms.16
TTR silencers
TTR silencers aim to reduce the amount of circulating TTR; there are currently two classes of TTR silencers: antisense oligonucleotides (ASO) and small interfering RNA (siRNA).20
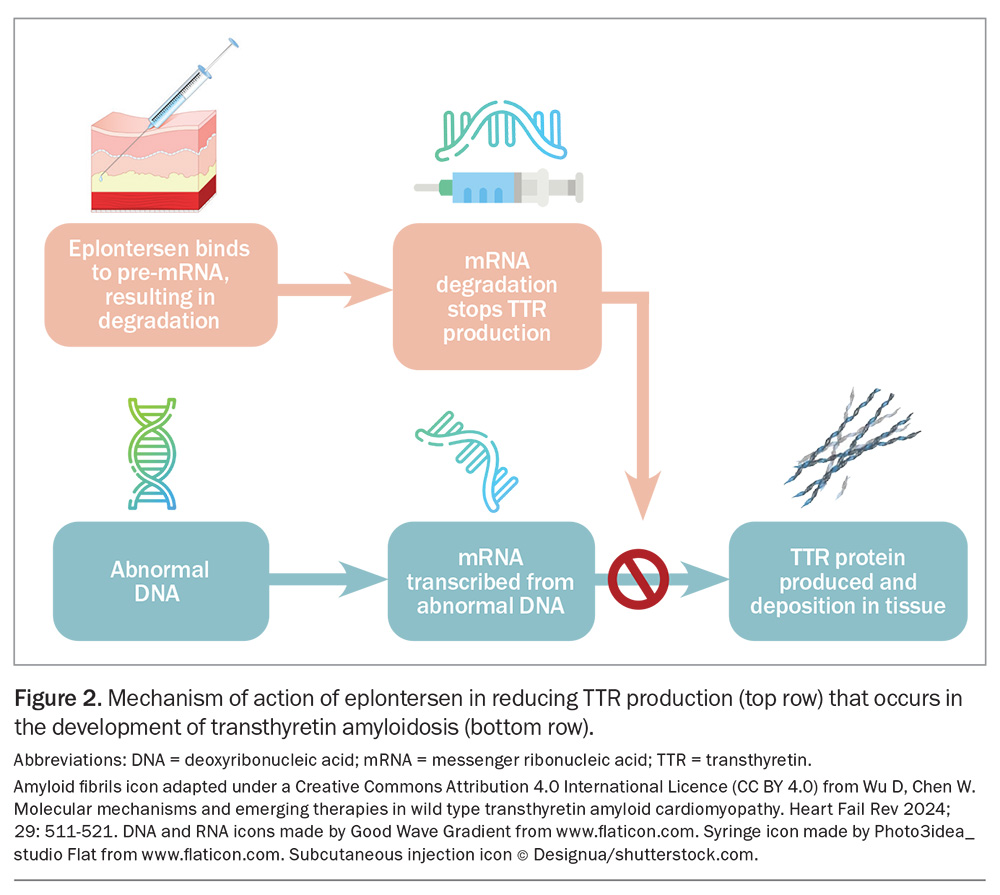
ASO bind directly to TTR mRNA in an untranslated region, resulting in activation of RNase H1 that cleaves the TTR mRNA. This results in reduced expression of TTR mRNA and, therefore, reduced TTR synthesis.21 Eplontersen is a ligand-conjugated ASO (conjugated with N-acetylgalactosamine moieties), to support preferential uptake by the liver, which is the predominant source for TTR production (Figure 2).22 Conjugation with N-acetylgalactosamine differentiates eplontersen from inotersen, another ASO TTR silencer previously evaluated for the treatment of ATTR. Eplontersen is administered at a dose of 45 mg, as a monthly subcutaneous injection.
The clinical benefit of eplontersen in ATTRv was demonstrated in the NEURO-TTRansform trial.23 Patients treated with eplontersen had a mean reduction in serum TTR concentration of 81.7% after 65 weeks. There was clinical benefit with better outcomes in modified Neuropathy Impairment Score +7 and Norfolk Quality of Life Questionnaire–Diabetic Neuropathy scores after 65 weeks compared with placebo. The most common adverse events with the eplontersen group were vitamin A deficiency (about 9%) and vomiting (9%).24
Eplontersen has not been studied in patients who have received a liver transplant or have moderate or severe liver or renal dysfunction. It has received approval from the US Food and Drug Administration but has not yet been approved by the TGA at the time of writing this article.
SiRNAs bind to RNA-induced silencing complex resulting in the cleavage of target messenger RNA, facilitating degradation.25 These medications require adequate cellular uptake and targeted organ activity. Patisiran, a SiRNA formulation with lipid nanoparticles, allows targeted delivery to hepatocytes. It has PBS approval (Section 100) for adult patients with ATTRv suffering from stage 1 or 2 polyneuropathy without evidence of heart failure. It is administered as 300 mcg/kg intravenously every three weeks.
In the APOLLO trial, patisiran resulted in a median reduction in serum TTR of 81.0% at 18 months.26 This trial also demonstrated improved outcomes in patients with ATTRv when assessed by neuropathy questionnaires and quality-of-life scores, with benefit in both autonomic and sensorimotor components of polyneuropathy.26 The utility of patisiran in the APOLLO-B trial was demonstrated by reduced decline in the 6-minute walk test (6MWT) after 12 months (median change from baseline in the patisiran group −8.15 metres [95% CI, −16.4 to 1.5] vs −21.35 metres [95% CI, −34.05 to −7.52] in the placebo group).27 There was no difference in the composite secondary end-point of death from any cause, cardiovascular events and change from baseline in 6MWT distance at 12 months.27 Adverse events observed with patisiran include peripheral oedema (30% in the patisiran group vs 22% in the placebo group) and infusion reactions (19% in the patisiran group vs 9% in the placebo group), which may manifest as back pain, flushing, muscle spasms, abdominal pain and diarrhoea.26
The natural function of TTR is as a transporter of vitamin A; therefore, it is recommended that patients on TTR silencers receive vitamin A supplementation at the recommended daily dose.28 Ingestion of a higher dose of vitamin A is not recommended as plasma only contains 1% of total body vitamin A, with the liver being its main reservoir.29 A decrease in vitamin A level did not always correspond with ocular events in the NEURO-TTRansform trial.23 Ophthalmology review is recommended if patients are suspected of having ocular symptoms suggesting vitamin A deficiency-related ocular symptoms, such as dry eyes or night blindness.
Conclusion
Disease-modifying therapies provide new treatment options for patients with ATTR-related cardiomyopathy and neuropathy. Tafamidis is a PBS-listed TTR stabiliser that improves all-cause mortality and cardiovascular outcomes in patients with ATTR-CA and NYHA class I-II symptoms. TTR silencers have shown benefit, especially in neuropathic symptoms in ATTRv. The development and ongoing research into the use of disease-modifying therapies in ATTR provides promise for a progressive disease that previously had very limited treatment options. MT
COMPETING INTERESTS: None.
References
1. Muchtar E, Dispenzieri A, Magen H, et al. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med 2021; 289: 268-292.
2. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet 2016; 387: 2641-2654.
3. Ravichandran S, Lachmann HJ, Wechalekar AD. Epidemiologic and survival trends in amyloidosis, 1987-2019. N Engl J Med 2020; 382: 1567-1568.
4. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2019; 73: 2872-28791.
5. Adams D, Koike H, Slama M, Coelho T. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol 2019; 15: 387-404.
6. Rosenblum H, Castano A, Alvarez J, Goldsmith J, Helmke S, Maurer MS. TTR (transthyretin) stabilizers are associated with improved survival in patients with TTR cardiac amyloidosis. Circ Heart Fail 2018; 11: e004769.
7. Geenty P, Kodsi M, Thomas L. Transthyretin cardiac amyloidosis: hiding in plain sight? Cardiology Today 2024; 14(1): 77-82.
8. Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation 2019; 140: 16-26.
9. Lousada I, Comenzo RL, Landau H, Guthrie S, Merlini G. Patient experience with hereditary and senile systemic amyloidoses: a survey from the Amyloidosis Research Consortium. Orphanet J Rare Dis 2015; 10: P22.
10. Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133: 2404-2412.
11. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem 2002; 48: 1437-1444.
12. Donnellan E, Elshazly MB, Vakamudi S, et al. No association between CHADS-VASc score and left atrial appendage thrombus in patients with transthyretin amyloidosis. JACC: Clin Electrophysiol 2019; 5: 1473-1474.
13. Marques N, Azevedo O, Almeida AR, et al. Specific therapy for transthyretin cardiac amyloidosis: a systematic literature review and evidence‐based recommendations. J Am Heart Assoc 2020; 9: e016614.
14. Lohrmann G, Pipilas A, Mussinelli R, et al. Stabilization of cardiac function with diflunisal in transthyretin (ATTR) cardiac amyloidosis. J Card Fail 2020; 26: 753-759.
15. Castaño A, Drachman BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev 2015; 20: 163-178.
16. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379: 1007-1106.
17. Elliott P, Drachman BM, Gottlieb SS, et al. Long-term survival with tafamidis in patients with transthyretin amyloid cardiomyopathy. Circ Heart Fail 2022; 15: e008193.
18. Hanna M, Damy T, Grogan M, et al. Impact of tafamidis on health-related quality of life in patients with transthyretin amyloid cardiomyopathy (from the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial). Am J Cardiol 2021; 141: 98-105.
19. Coelho T, Maia LF, Martins da Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology 2012; 79: 785-792.
20. Aimo A, Castiglione V, Rapezzi C, et al. RNA-targeting and gene editing therapies for transthyretin amyloidosis. Nat Rev Cardiol 2022; 19: 655-667.
21. Viney NJ, Guo S, Tai LJ, et al. Ligand conjugated antisense oligonucleotide for the treatment of transthyretin amyloidosis: preclinical and phase 1 data. ESC Heart Fail 2021; 8 :652-661.
22. Brannagan TH 3rd, Berk JL, Gillmore JD, et al. Liver-directed drugs for transthyretin-mediated amyloidosis. J Periph Nerv Syst 2022; 27: 228-237.
23. Coelho T, Marques W, Jr, Dasgupta NR, et al. Eplontersen for hereditary transthyretin amyloidosis with polyneuropathy. JAMA 2023; 330: 1448-1458.
24. AstraZeneca. Wainua [eplontersen] prescribing information. US Food and Drug Administration. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217388s000lbl.pdf (accessed November 2024).
25. Coelho T, Adams D, Silva A, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 2013; 369: 819-829.
26. Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 2018; 379: 11-21.
27. Maurer MS, Kale P, Fontana M, et al. Patisiran treatment in patients with transthyretin cardiac amyloidosis. N Engl J Med 2023; 389: 1553-1565.
28. Liz MA, Coelho T, Bellotti V, Fernandez-Arias MI, Mallaina P, Obici L. A narrative review of the role of transthyretin in health and disease. Neurol Ther 2020; 9: 395-402.
29. Olson JA. Serum levels of vitamin A and carotenoids as reflectors of nutritional status. J Natl Cancer Inst 1984; 73: 1439-1444.