Premature ovarian insufficiency. Not ‘too young for menopause’

Premature ovarian insufficiency, which is defined as loss of ovarian activity before the age of 40 years, has negative health impacts. Prompt diagnosis, evaluation of cause and sequelae, psychological support and institution of hormone replacement therapy are essential components of management.
Premature ovarian insufficiency (POI), also known as premature menopause or premature ovarian failure, is defined as the loss of ovarian activity before the age of 40 years.1 It may occur spontaneously or secondary to medical treatments. Management should incorporate consideration of symptoms experienced, psychological health and desire for fertility, as well as the long-term sequelae related to bone and cardiovascular health. Hormone replacement therapy is recommended until the age of natural menopause.
Resources are available to assist with the care of women affected by POI developed by Monash Centre for Health Research and Implementation, Melbourne (MCHRI), and available online (https://mchri.org.au/guidelines-resources/
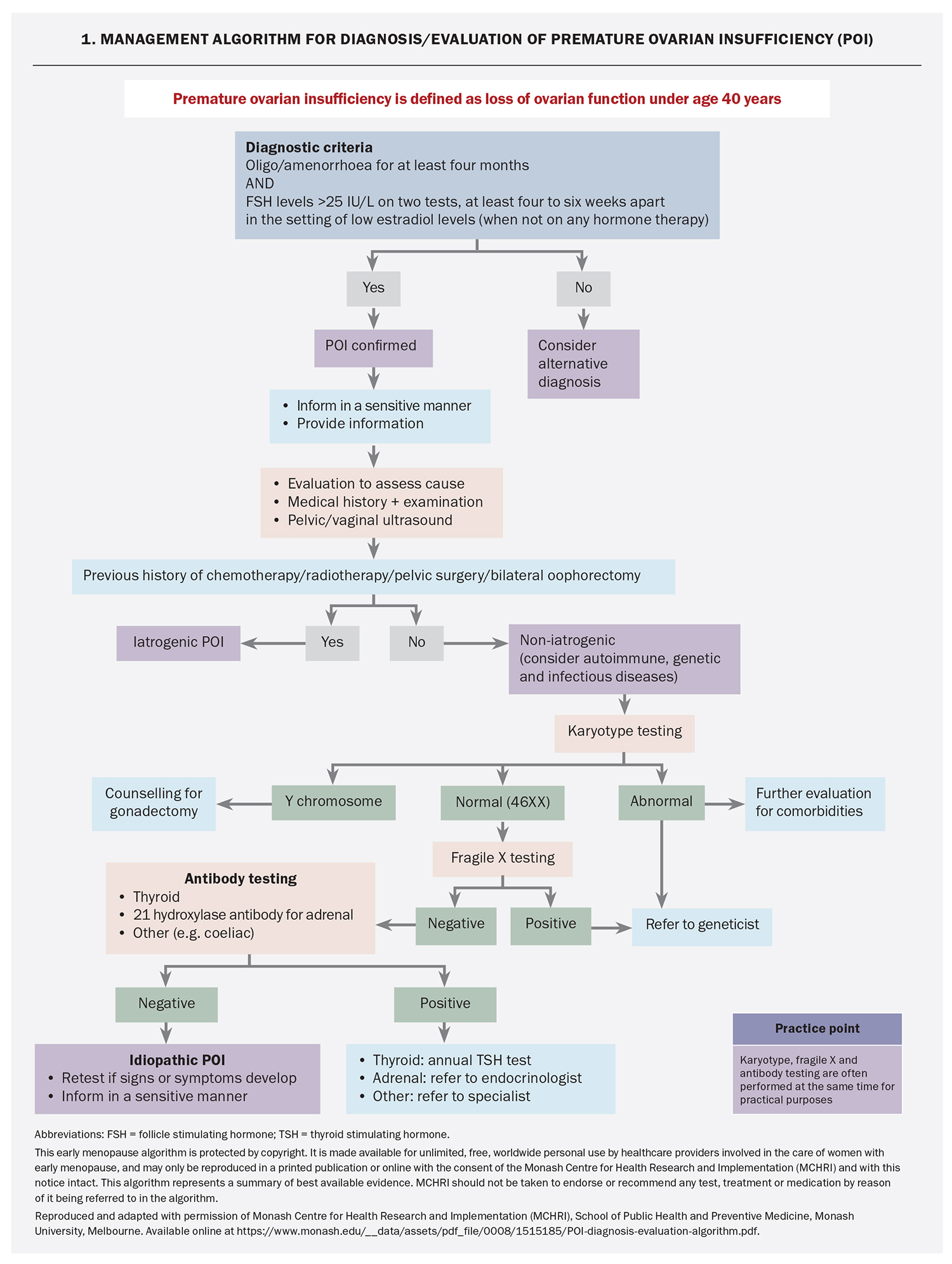
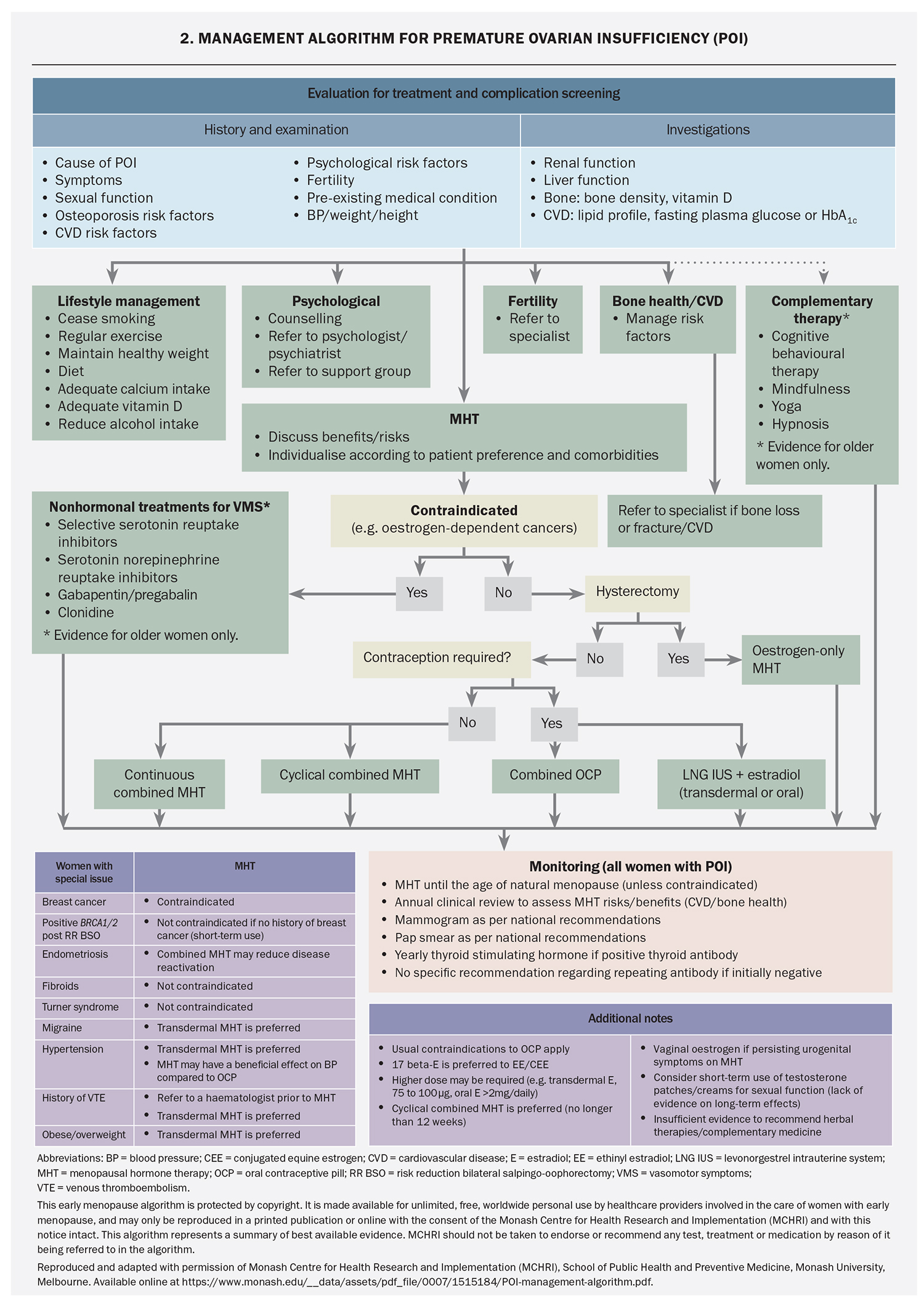
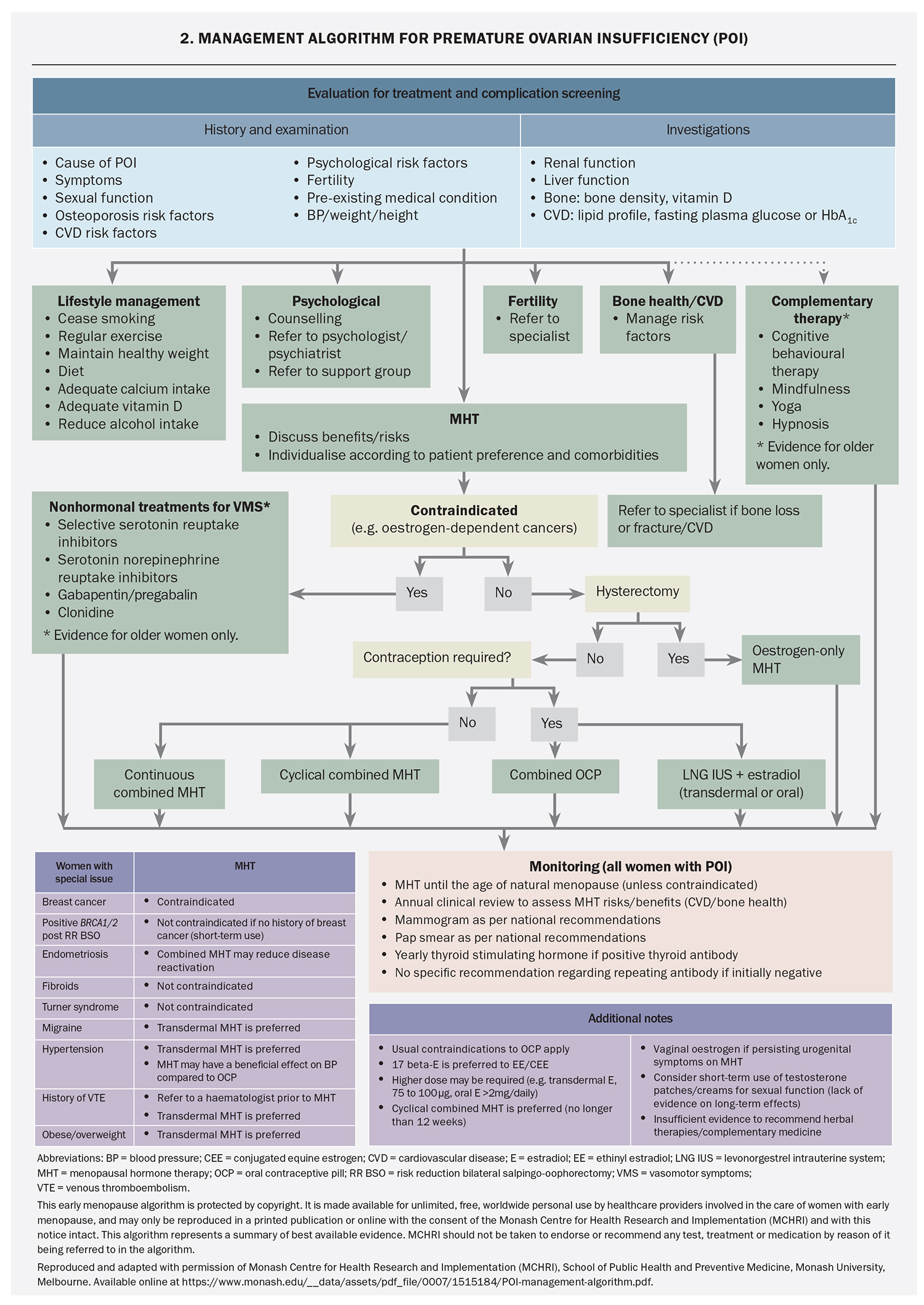
health-professionals/early-menopause-practice-tools/). These include algorithms for the diagnosis and evaluation and for the management of POI, which are based on a systematic search of menopause guidelines, AGREE (Appraisal of Guidelines for Research and Evaluation) evaluation and extraction of recommendations related to POI. The algorithms are shown below. The evidence-based Ask Early Menopause app is freely available that provides quality information for women with early menopause or POI (Figure).
Terminology
The term ‘menopausal hormone therapy’ (MHT) is now preferred when referring to oestrogen and progestin preparations for managing menopause. However, the term ‘hormone replacement therapy’ (HRT) is used in this article for women with premature ovarian insufficiency (and is also used in the European Society of Human Reproduction and Embryology [ESHRE] guideline),1 as this reflects the fact that in this setting women are using preparations to replace hormones that would normally be produced by the ovaries. In addition, some young women find the term MHT less appealing than HRT.
Case scenario
Karima is a 35-year-old woman who presents to her GP with an eight-month history of amenorrhoea, hot flushing, hair loss and loss of libido that is affecting her relationship with her husband. She and her husband have two daughters but have been hoping to have more children, particularly as in their culture a son is very much desired. Karima is a nonsmoker and does not drink alcohol. She does not take any medications and has no previous medical or surgical history.
What investigations should be performed to determine the cause of Karima’s secondary amenorrhoea?
Although the most common causes of secondary amenorrhoea are pregnancy, polycystic ovary syndrome and hypothalamic or pituitary disease, POI should also be considered in a young woman with oligo- or amenorrhoea. Spontaneous POI affects up to 4% of women.2
Diagnostic criteria for POI are oligo- or amenorrhoea for at least four months and FSH levels in the postmenopausal range (>25 IU/L) on two tests performed at least four to six weeks apart (Flowchart 1).1,3,4 Women should not be taking hormonal medication at the time of testing. Anti-Müllerian hormone (AMH) testing is not recommended for the diagnosis of POI, although it is used by specialists for fertility assessment.1,3
Karima’s initial investigations include a negative pregnancy test and show normal TSH and prolactin levels. Her FSH is elevated (68 IU/L and 101 IU/L) and her estradiol low (86 pmol/L and 76 pmol/L) on two occasions, six weeks apart, confirming the diagnosis of spontaneous POI.
What could be the cause of Karima’s POI and what investigations should be performed?
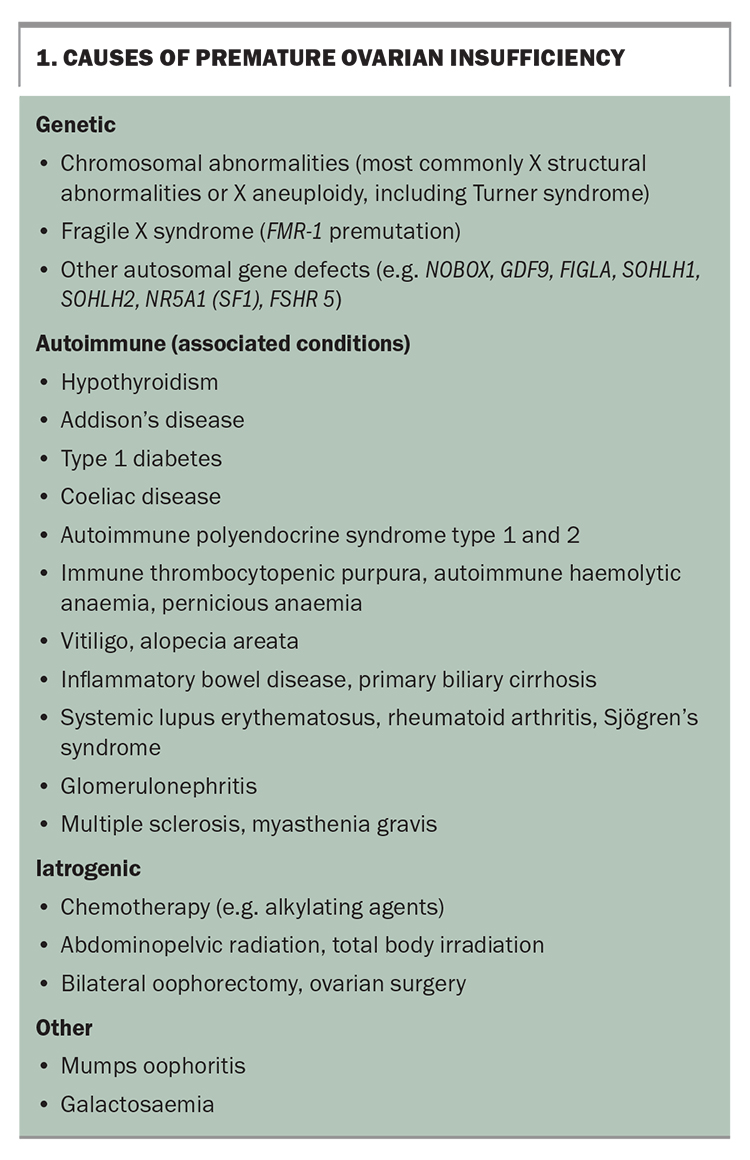
Known causes of POI include genetic, autoimmune and infectious diseases and iatrogenic factors (Box 1). Iatrogenic POI is becoming more common with advances in cancer treatment and risk-reducing bilateral oophorectomy. The risk of iatrogenic POI depends on a patient’s age, type and dose of chemotherapy, and dose and field of radiotherapy.4 Most cases of POI are unexplained or idiopathic and may reflect gene mutations that currently are not readily detected.6
Chromosomal abnormalities are found in 10 to 12% of women with POI, and may cause primary or secondary amenorrhoea.7,8 If Y chromosomal material is detected then there is an elevated risk of gonadoblastoma, and gonadectomy is usually advised.9 Sporadic and familial fragile X (FMR-1) premutation has been reported in up to 7.5% and 13% of Caucasian women with POI, respectively, but it is less prevalent in Indian women and Chinese women with POI.1,10,11 The presence of this mutation has implications for a woman’s offspring and other family members. If any genetic abnormalities are detected then the woman should be referred to genetic services.
On exclusion of genetic causes, an autoimmune screen should be performed, including 21-hydroxylase antibody (21OH-Ab) or adrenocortical antibodies for adrenal autoimmunity and anti-thyroid peroxidase antibodies (TPO-Ab). If 21OH-Ab is positive, the woman should be referred to an endocrinologist for further assessment of adrenal function. If the woman is TPO-Ab positive, TSH should be measured yearly because of the increased risk of hypothyroidism secondary to Hashimoto thyroiditis.
In practice, karyotype, fragile X testing and autoantibody testing may be requested at the same time. Ovarian antibody testing is nonspecific and is not recommended. Testing for other autoimmune disorders may be indicated depending on initial history and examination.
What are the initial steps in the management of Karima’s symptoms and concerns?
POI can be a devastating diagnosis for many women, especially when it is unexpected and no underlying cause has been found. Early referral to psychology services and support groups (e.g. Daisy Network) may be beneficial (Flowchart 2). In addition, careful assessment for depression or anxiety is important. Lifestyle recommendations for women with POI are directed at menopausal symptom control, cardiovascular disease risk reduction and bone health. Women should be given appropriate information or referred to good quality resources – examples of useful websites are given in Box 2.
Baseline investigations are directed at determining choice of hormone replacement therapy, assessing fertility, and evaluating cardiovascular and osteoporosis risk. They include renal and liver function, pelvic ultrasound, AMH level, lipid profile, fasting plasma glucose, calcium, phosphate and vitamin D measurements and bone mineral density (BMD) scanning. Cervical screening should be up to date.
When and how should hormone replacement therapy be prescribed?
Recommendations for HRT in women with POI (Flowchart 2)are derived from observational studies and small clinical trials of women with POI due to Turner syndrome, bilateral oophorectomy or chemotherapy, or idiopathic in origin, and from evidence in women experiencing menopause at the usual age. As well as being the most effective treatment for vasomotor symptoms, HRT has been shown to improve genitourinary symptoms, sleep and emotional wellbeing. HRT is also important for long-term health by reducing the risk of cardiovascular disease, cognitive dysfunction and osteoporosis.12-14
HRT ameliorates the impact of POI on bone health, and has beneficial effects on cardiovascular and metabolic health, including lowering blood pressure and improving endothelial function and lipid profile.15-23 In women with Turner syndrome, HRT has been shown to improve liver function and increase lean body mass.21,24,25 HRT is recommended for women with POI until the average age of natural menopause (51 years); however, the optimal HRT preparation is unknown. Delay in initiation of HRT is associated with lower BMD, smaller uterine size and decreased quality of life. The choice of HRT depends on the cause of POI, need for pubertal induction, need for contraception, comorbidities and patient preference. Some women may prefer a regular withdrawal bleed and thus cyclical therapy (12 to 14 days of added progestogen per month) is appropriate, whereas others may prefer no withdrawal bleeds with a continuous progestogen regimen. A cyclical regimen stimulating active functioning of the endometrium with regular proliferation and withdrawal bleeding may be preferable in women aiming for pregnancy by oocyte donation, with amenorrhoea potentially indicating pregnancy.1
There is evidence that physiological estradiol may confer a greater benefit for BMD compared with ethinylestradiol or conjugated estrogens.16,19,20 Recommended doses for bone protection are at least 2 mg oral estradiol daily or 100 mcg transdermal estradiol weekly or twice weekly.25,26 Studies in postmenopausal women of typical age indicate that micronised progesterone has advantages in regard to breast cancer and venous thromboembolism risk compared to other progestogens.27,28 Transdermal estradiol with cyclical micronised progesterone most closely approximates ‘body-identical’ HRT. Tibolone and the oral combined preparation of bazedoxifene acetate and conjugated estrogens have not been studied in women with POI.
HRT is not contraceptive. Estradiol-containing combined oral contraceptives (COCs) have not been studied in POI, although may be considered in women desiring contraception. Continuous or long cycle use of COCs is preferred for bone health, avoiding the non-oestrogen inactive tablets.26 The levonorgestrel-releasing intrauterine system used in combination with transdermal estradiol is a good option for women requiring contraception.
Principles for HRT prescription for women affected by POI are similar to those for women experiencing menopause at the usual age. A list of HRT (MHT) preparations available in Australia can be found on the Australasian Menopause Society website (www.menopause.org.au/hp/information-sheets/
426-ams-guide-to-equivalent-mht-hrt-doses). The usual contraindications apply, and include current or suspected oestrogen-dependent cancers and active liver, cardiovascular or venous thromboembolic disease. Although the evidence is limited, HRT does not appear to increase breast cancer risk in women with POI, and the risk appears to be less with oestrogen alone than with combined oestrogen and progestin in studies of postmenopausal women of typical age. BRCA gene mutation carriers can use short-term HRT following prophylactic bilateral salpingo-oophorectomy without an apparent increase in breast cancer risk.29 After the age of 51 years (average age of natural menopause), consideration of whether to use HRT/MHT is the same as for any postmenopausal woman.
There are limited data for the use of testosterone therapy in POI. One small trial in women with Turner syndrome showed that testosterone therapy improved lipid profile, BMD, body composition, cognitive function, quality of life and sexual desire.30 However, studies of women with normal karyotype idiopathic POI indicated that testosterone was no different to placebo for BMD or quality of life.17,31
Karima was advised to use HRT rather than the COC for management of her POI due to her desire for fertility. A cyclical HRT regimen was chosen because the development of amenorrhoea may indicate pregnancy.
What other options can be offered if Karima prefers not to have HRT?
HRT is the best treatment in POI and should be strongly recommended unless there are contraindications. If HRT is contraindicated, nonhormonal options may be considered for vasomotor symptoms. Serotonin noradrenaline reuptake inhibitors, selective serotonin reuptake inhibitors, clonidine, and gabapentin have all been shown to reduce vasomotor symptoms in women with a history of breast cancer (off-label use except for clonidine).32 Cognitive behavioural therapy has been shown to reduce vasomotor symptoms and improve mood, sleep, sexual function and quality of life. Nonhormonal vaginal moisturisers or lubricants can be used for urogenital symptoms.1 Cardiovascular health should be monitored carefully, and referral to an endocrinologist for management of bone health should be considered.
What fertility advice should be given to Karima?
Spontaneous resumption of ovarian activity has been reported in 25% of women with idiopathic POI,33 usually within the first year after diagnosis, with a spontaneous pregnancy rate of 5%.34 Therefore, contraception needs to be considered in the choice of HRT if a woman does not want to become pregnant. However, in the case of desired fertility, women with POI should be referred to a fertility specialist for assessment. Currently, oocyte donation is the best method to achieve a pregnancy.
Karima and her husband were referred to a fertility specialist.
Conclusion
POI can have profound effects on a woman’s physical and psychosocial wellbeing that require multimodal assessment and management. Prompt diagnosis, institution of individualised HRT, psychological support, provision of information and monitoring for long-term sequelae are essential components of management. Referral to psychological, genetic, endocrinology and fertility services may also be required. MT
COMPETING INTERESTS: Associate Professor Xu: None. Amanda Vincent has received honoraria from Besins.
References
1. European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, Anderson R, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016; 31: 926-937.
2. Golezar S, Ramezani Tehrani F, Khazaei S, Ebadi A, Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric 2019; 22: 403-411.
3. National Institute for Health and Care Excellence. Menopause: diagnosis and management. NICE guideline [NG23]. NICE; 2015. Available online at: www.nice.org.uk/guidance/ng23/chapter/Recommendations (accessed September 2019).
4. Baber RJ, Panay N, Fenton A; IMS Writing Group. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016; 19: 109-150.
5. Chemaitilly W, Li Z, Krasin MJ, et al. Premature ovarian insufficiency in childhood cancer survivors: a report from the St. Jude lifetime cohort. J Clin Endocrinol Metab 2017; 102: 2242-2250.
6. Jiao X, Zhang H, Ke H, et al. Premature ovarian insufficiency: phenotypic characterization within different etiologies. J Clin Endocrinol Metab 2017; 102: 2281-2290.
7. Jiao X, Qin C, Li J, et al. Cytogenetic analysis of 531 Chinese women with premature ovarian failure. Hum Reprod 2012; 27: 2201-2207.
8. Kalantari H, Madani T, Zari Moradi S, et al. Cytogenetic analysis of 179 Iranian women with premature ovarian failure. Gynecol Endocrinol 2013;
29: 588-591.
9. Michala L, Goswami D, Creighton SM, Conway GS. Swyer syndrome: presentation and outcomes. BJOG 2008; 115: 737-741.
10. Tosh D, Rao KL, Rani HS, Deenadayal DA, Murty US, Grover P. Association between fragile X premutation and premature ovarian failure: a case-control study and meta-analysis. Arch Gynecol Obstet 2014; 289: 1255-1262.
11. Guo T, Qin Y, Jiao X, Li G, Simpson JL, Chen ZJ. FMR1 premutation is an uncommon explanation for premature ovarian failure in Han Chinese. PLoS One 2014; 9: e103316.
12. Cintron D, Lipford M, Larrea-Mantilla L, et al. Efficacy of menopausal hormone therapy on sleep quality: systematic review and meta-analysis. Endocrine 2017; 55: 702-711.
13. Piccioni P, Scirpa P, D’Emilio I, et al. Hormonal replacement therapy after stem cell transplantation. Maturitas 2004; 49: 327-333.
14. Webber L, Anderson RA, Davies M, Janse F, Vermeulen N. HRT for women with premature ovarian insufficiency: a comprehensive review. Hum Reprod Open 2017; 2017: 1-11.
15. Prior JC, Vigna YM, Wark JD, et al. Premenopausal ovariectomy-related bone loss: a randomized, double-blind, one-year trial of conjugated estrogen or medroxyprogesterone acetate. J Bone Miner Res 1997; 12: 1851-1863.
16. Crofton PM, Evans N, Bath LE, et al. Physiological versus standard sex steroid replacement in young women with premature ovarian failure: effects on bone mass acquisition and turnover. Clin Endocrinol (Oxf) 2010; 73: 707-714.
17. Popat VB, Calis KA, Kalantaridou SN, et al. Bone mineral density in young women with primary ovarian insufficiency: results of a three-year randomized controlled trial of physiological transdermal estradiol and testosterone replacement. J Clin Endocrinol Metab 2014; 99: 3418-3426.
18. Kodama M, Komura H, Kodama T, Nishio Y, Kimura T. Estrogen therapy initiated at an early age increases bone mineral density in Turner syndrome patients. Endocr J 2012; 59: 153-159.
19. Cartwright B, Robinson J, Seed PT, Fogelman I, Rymer J. Hormone replacement therapy versus the combined oral contraceptive pill in premature ovarian failure: a randomized controlled trial of the effects on bone mineral density. J Clin Endocrinol Metab 2016; 101: 3497-3505.
20. Cintron D, Rodriguez-Gutierrez R, Serrano V, Latortue-Albino P, Erwin PJ, Murad MH. Effect of estrogen replacement therapy on bone and cardiovascular outcomes in women with turner syndrome: a systematic review and meta-analysis. Endocrine 2017; 55: 366-375.
21. Gravholt CH, Naeraa RW, Nyholm B, et al. Glucose metabolism, lipid metabolism, and cardiovascular risk factors in adult Turner’s syndrome. The impact of sex hormone replacement. Diabetes Care 1998; 21: 1062-1070.
22. Langrish JP, Mills NL, Bath LE, et al. Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension 2009; 53: 805-811.
23. Kalantaridou SN, Naka KK, Papanikolaou E, et al. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab 2004; 89: 3907-3913.
24. Elsheikh M, Hodgson HJ, Wass JA, Conway GS. Hormone replacement therapy may improve hepatic function in women with Turner’s syndrome. Clin Endocrinol (Oxf) 2001; 55: 227-231.
25. Cleemann L, Holm K, Kobbernagel H, et al. Dosage of estradiol, bone and body composition in Turner syndrome: a 5-year randomized controlled clinical trial. Eur J Endocrinol 2017; 176: 233-242.
26. Gazarra LBC, Bonacordi CL, Yela DA, Benetti-Pinto CL. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause 2020; 27: 1110-1116.
27. Stanczyk FZ, Hapgood JP, Winer S, Mishell DR Jr. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev 2013; 34: 171-208.
28. Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ 2019; 364: k4810.
29. Gordhandas S, Norquist BM, Pennington KP, Yung RL, Laya MB, Swisher EM. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol 2019; 153: 192-200.
30. Zuckerman-Levin N, Frovola-Bishara T, Militianu D, Levin M, Aharon-Peretz J, Hochberg Z. Androgen replacement therapy in Turner syndrome: a pilot study. J Clin Endocrinol Metab 2009; 94: 4820-4827.
31. Guerrieri GM, Martinez PE, Klug SP, et al. Effects of physiologic testosterone therapy on quality of life, self-esteem, and mood in women with primary ovarian insufficiency. Menopause 2014; 21: 952-961.
32. Santen RJ, Stuenkel CA, Davis SR, Pinkerton JV, Gompel A, Lumsden MA. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab 2017; 102: 3647-3661.
33. Bachelot A, Nicolas C, Bidet M, et al. Long-term outcome of ovarian function in women with intermittent premature ovarian insufficiency. Clin Endocrinol (Oxf) 2017; 86: 223-228.
34. van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update 1999; 5: 483-492.