Management of polycystic ovary syndrome: the role of primary care

The 2023 international guideline for the assessment and management of polycystic ovary syndrome introduces several key changes. The updated guideline emphasises holistic patient care that addresses the associated cardiometabolic risks and complications and psychosocial challenges.
- Polycystic ovary syndrome (PCOS) is a common endocrine disorder associated with psychological challenges (e.g. anxiety, depression, negative body image) and cardiometabolic complications (e.g. overweight and obesity, insulin resistance, tolerance, diabetes, cardiovascular disease).
- The 2023 international guideline for PCOS assessment and management introduces several changes, including the addition of anti-Müllerian hormone levels to assess polycystic ovary morphology and differences in the diagnostic criteria between adults and adolescents.
- Greater awareness of psychological features associated with PCOS, including eating disorders, and their impact on body image and quality of life is needed.
- Women with PCOS are at increased risk of cardiovascular disease and, potentially, of cardiovascular mortality.
- Women with PCOS have higher-risk pregnancies (e.g. gestational diabetes, preeclampsia, small-for-gestational-age birthweight), but assisted reproductive technology does not significantly increase the risks.
- Hirsutism can be managed with cosmetic therapies, the combined oral contraceptive pill and anti-androgens, with consideration of the associated risks.
- GPs are well placed to use a whole-person approach to management, which can include lifestyle interventions, weight and fertility management, optimising preconception health and addressing psychosocial aspects.
Polycystic ovary syndrome (PCOS) is a common endocrine disorder affecting women of reproductive age, including gender-diverse individuals with female reproductive organs. About 8 to 13% of women are diagnosed with PCOS worldwide according to the Rotterdam criteria, which include hyperandrogenism, oligo- or anovulation and polycystic ovary morphology (PCOM).1
The new international evidence-based guideline for the assessment and management of PCOS published in 2023 emphasises the importance for healthcare professionals to address PCOS holistically, including emotional wellbeing. PCOS is associated with a high prevalence of moderate to severe anxiety and depressive symptoms in adults and depressive symptoms in adolescents; low health-related quality of life; negative body image; eating disorders or disordered eating; and potential increased risk of psychosexual dysfunction.2 The experiences of infertility, overweight or obesity, hirsutism, the uncertainty of prolonged amenorrhoea and loss of feminine identity can significantly impact mental health.3,4 Additionally, the use of hormonal medications may affect mood, and psychological symptoms can impact engagement in lifestyle interventions and self management. The 2023 guideline also highlights cardiometabolic risk factors as key complications of PCOS, including underlying insulin resistance (independent of body mass index [BMI]). This results in increased risks of impaired glucose tolerance, type 2 diabetes and composite cardiovascular disease including ischaemic heart disease, myocardial infarction and stroke.
The management of PCOS is informed by the woman’s current priorities, needs, values and preferences, and shared decision- making is required. This comprises a whole-person approach, with continuity and co-ordination of care that is well aligned with general practice. GPs are the most commonly attended healthcare professionals in Australia, with 83.6% of people in Australia consulting a GP compared with 38.9% consulting a medical specialist in 2021 to 2022.5 GPs provide a person-centred, comprehensive approach to health care, and are the experts in managing the uncertainty of a low-risk environment, and complexities of comorbidity and polypharmacy.6 The generalist approach is beneficial in the management of PCOS as GPs are skilled at integrating scientific evidence and guideline recommendations with a profound understanding of the psychosocial and cultural context of the individual. Additionally, well-supported primary healthcare systems improve patient outcomes, reduce overall healthcare costs and mitigate negative health outcomes in marginalised populations.7-11 In this article, the new PCOS guideline is outlined, highlighting what is new and describing its relevance to general practice.
Diagnosis of polycystic ovary syndrome
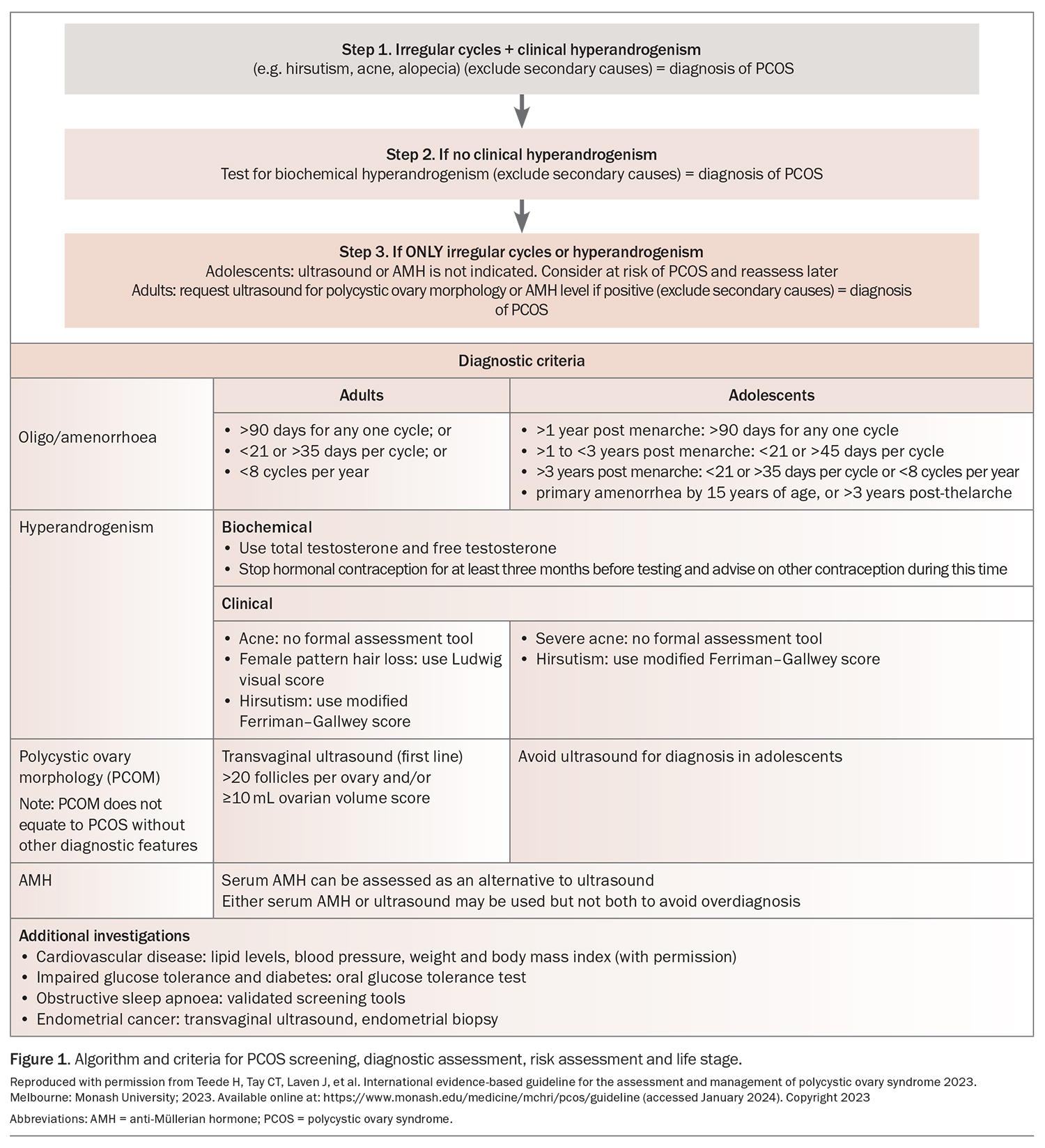
PCOS was previously defined using the Rotterdam criteria. The changes from the 2018 guideline are listed in Box 1. The 2023 international guideline introduces new criteria, with the addition of anti-Müllerian hormone (AMH) levels as an additional test to help determine PCOM in adults. Figure 1 lists the specific features for each diagnostic criterion outlined below. A practice tool for screening, diagnostic assessment, risk assessment and life stage can be found online (Algorithm 1: https://www.mchri.org.au/guidelines-resources/health-professionals/pcos-practice-tools/).
Oligo/amenorrhoea
In adults and adolescents who are more than three years postmenarche to perimenopause, irregular menstrual cycles are defined as those lasting less than 21 days or more than 35 days, or fewer than eight cycles per year. In adolescents less than three years postmenarche, irregular cycles in the first year postmenarche are a normal part of the pubertal transition. Irregular cycles after the first year are defined as any one cycle lasting more than 90 days or, for adolescents one to three years postmenarche, cycles lasting less than 21 days or more than 45 days. This criterion includes primary amenorrhea by the age of 15 years or more than three years post-thelarche.
Hyperandrogenism
Biochemical hyperandrogenism
Total and free testosterone levels, which can be estimated using the free androgen index (FAI), should be used for assessment. Androgen levels cannot be reliably assessed while the woman is taking the combined oral contraceptive pill (COCP). Consider withdrawal for more than three months with alternative contraception. If testosterone levels or the FAI are not elevated, consider assessing androstenedione and dehydroepiandrosterone sulphate levels. However, they both have poorer specificity than testosterone, with the latter being associated with a greater age-related decline. Note that adolescents usually have adult levels of testosterone by 12 to 15 years of age.
Clinical hyperandrogenism
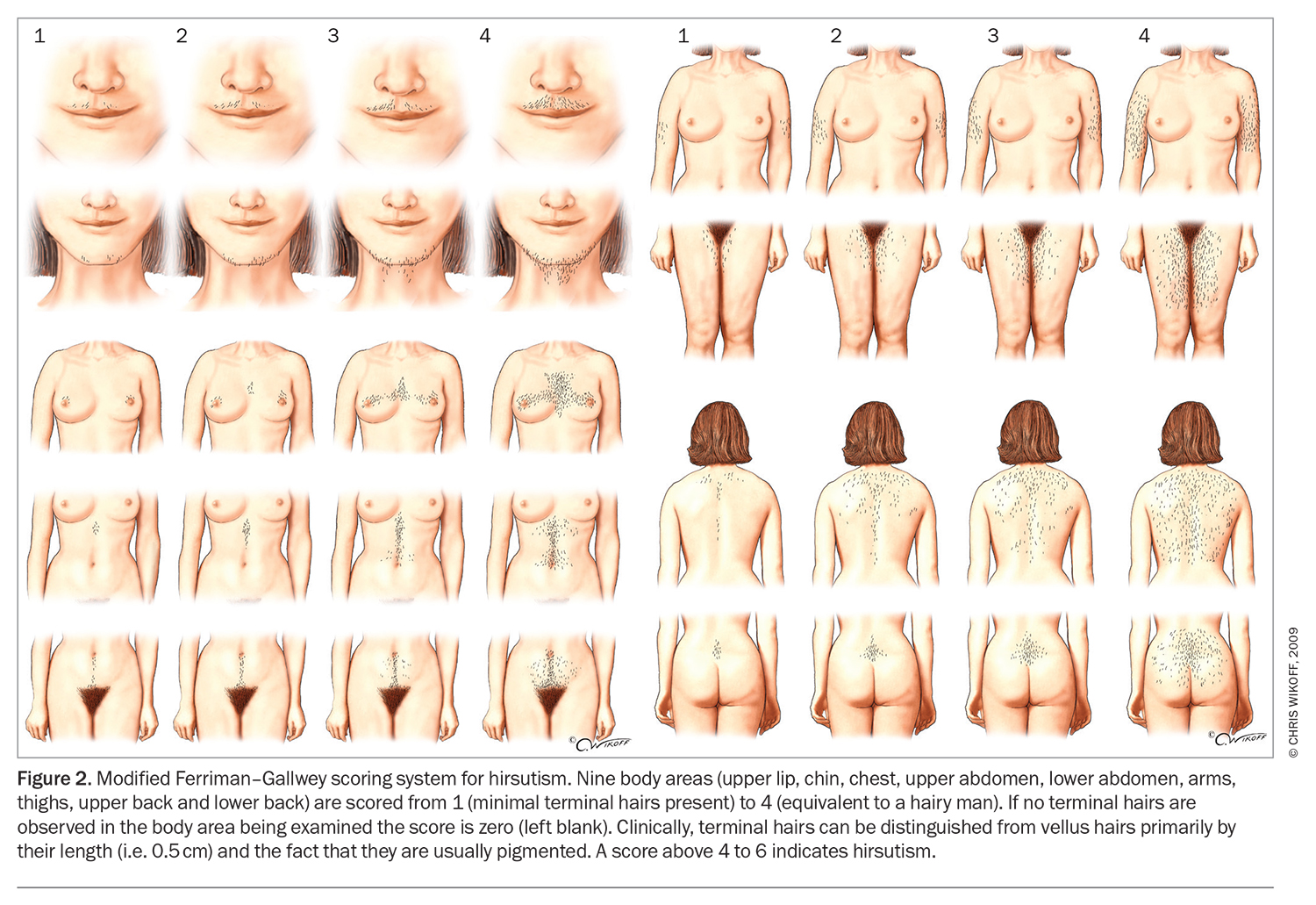
The presence of hirsutism alone should be considered predictive of biochemical hyperandrogenism and PCOS in adults. A modified Ferriman–Gallwey score of 4 to 6 should be used to detect hirsutism (Figure 2), recognising that previous or ongoing treatment can limit clinical assessment and that the score may be less useful in women of Asian or Middle Eastern ethnicity, as it was developed on the basis of a predominantly Caucasian population.12 Female pattern hair loss and acne in isolation (without hirsutism) are relatively weak predictors of biochemical hyperandrogenism.
Polycystic ovary morphology
In adolescents, PCOM cannot be accurately determined because of the high incidence of multifollicular ovaries at this life stage. Recommendations for determining PCOM as a criterion for the diagnosis of PCOS are only relevant for individuals more than eight years postmenarche.
In adults, pelvic ultrasound to assess the follicle number per ovary (FNPO), follicle number per cross-section (FNPS) and ovarian volume (OV) is an accurate assessment of PCOM. The threshold for PCOM in adults can be either of the following:
- an FNPO of 20 or higher (using a high-frequency ultrasound probe) in at least one ovary
- an OV of 10 mL or greater or FNPS of 10 or higher in at least one ovary if using older technology or if the image quality is insufficient to allow for an accurate assessment of follicle counts throughout the entire ovary.
Anti-Müllerian hormone
A new criterion is that reported laboratory AMH levels can be assessed in adults to determine PCOM, noting that levels vary throughout the menstrual cycle, may be suppressed by current or recent COCP use and decrease with age and increasing BMI. There are strong correlations between circulating AMH levels and the antral follicle count on ultrasound in PCOS, with a meta-analysis reporting a sensitivity and specificity of 0.79 and 0.87, respectively, for AMH levels as a diagnostic indicator for PCOS in adults. The sensitivity and specificity are lower in adolescents and, therefore, measuring AMH levels is not recommended as a diagnostic test in this group.
Management of cardiometabolic and psychological effects
A diagnosis of PCOS is accompanied by an increased risk of metabolic complications (including diabetes), cardiovascular disease and sleep disorders. GPs play an essential role in diagnostic management, including the assessment and management of cardiovascular risk, glycaemic status, obstructive sleep apnoea risk and emotional wellbeing.
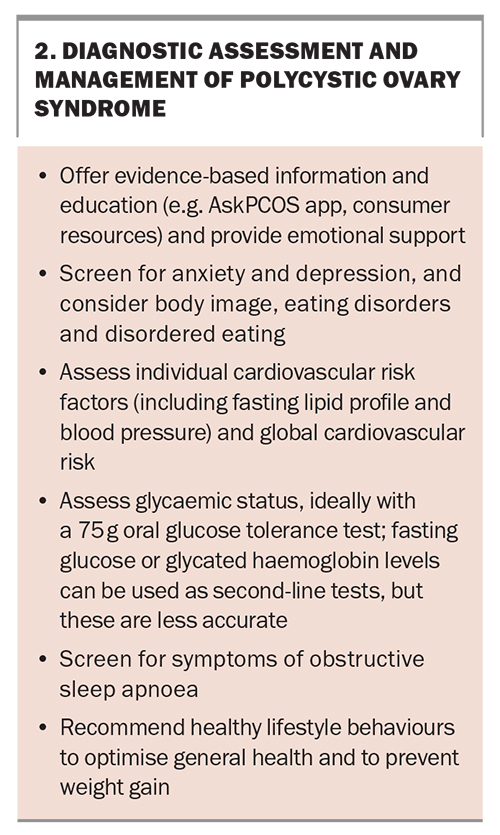
The initial management of PCOS after diagnosis involves identifying women’s key concerns and priorities, screening for cardiometabolic and psychological outcomes and offering education and lifestyle advice in terms of long-term benefits for reproductive and metabolic health (Box 2). Information provision is a key gap noted by patients and, thus, directing patients to reliable sources of information is important.13-15 One evidence-based resource is the AskPCOS mobile application (https://www.askpcos.org/).
Cardiometabolic outcomes
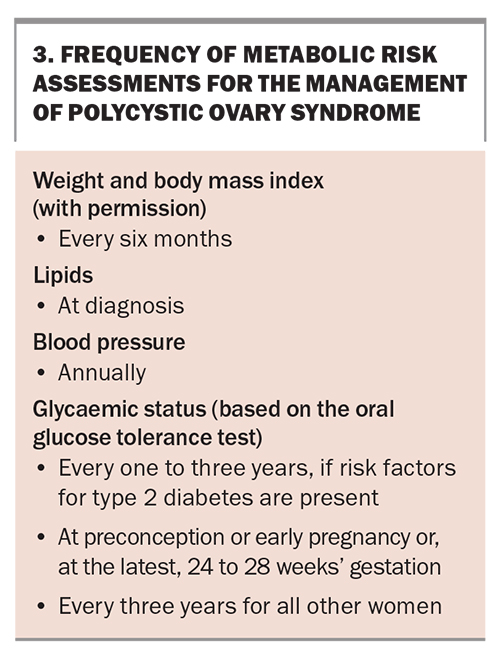
Women with PCOS have an increased risk of impaired fasting glucose levels, impaired glucose tolerance and type 2 diabetes, regardless of age and BMI. GPs can play a significant role in preventing or mitigating the impact of metabolic complications in PCOS by counselling patients about the increased risk, assessing glycaemic status using a 75 g oral glucose tolerance test (OGTT) at diagnosis and at regular intervals after diagnosis (depending on the individual risk), providing lifestyle advice and other guidance for the prevention of metabolic complications, and referring to a multidisciplinary team as needed. Women with PCOS are also considered to have an increased risk of cardiovascular disease and should be assessed for cardiovascular risk factors (including lipid levels and blood pressure) at diagnosis and thereafter according to the individual risk (Box 3).
Lifestyle and weight management
Weight management is a priority for many women with PCOS and was identified as a priority to address in the guidelines by healthcare professionals and consumers internationally.16 Women with PCOS are more likely to have excess weight compared with age-matched controls, and a BMI greater than 30 kg/m2 increases the risk of insulin resistance, hyperandrogenism, metabolic dysfunction and reproductive complications, such as oligo/amenorrhoea.2 The 2023 guideline highlights the insufficient evidence on individual lifestyle strategies and a growing recognition of environmental and systems-based drivers of excess weight. Evidence on the contribution of lifestyle factors to excess weight in women with PCOS is conflicting. Underlying hormonal dysfunction might predispose an individual to intrinsic factors, such as an altered metabolic rate; however, the evidence for this is limited and inconsistent.
Lifestyle interventions (exercise alone or a combination of exercise, dietary intervention and behavioural strategies) should be recommended for all women with PCOS, with a focus on the overall health benefits and prevention of weight gain. Lifestyle interventions improve central adiposity, hirsutism and metabolic and lipid outcomes, but they are not more effective than usual care or minimal interventions for excess weight and a high BMI. Although there is limited evidence for the effectiveness of behavioural interventions in PCOS, evidence from general population guidelines can be extrapolated to individuals with PCOS. Behavioural interventions may include goal setting (using the Specific, Measurable, Achievable, Relevant and Timebound [SMART] approach), self-monitoring, problem solving and reinforcing changes. There is a lack of evidence supporting one type of diet composition over another for all PCOS outcomes and, therefore, general population guidelines could be applied. An emphasis on tailoring dietary changes to food preferences, avoiding restrictive and nutritionally unbalanced diets and considering the barriers and facilitators of adopting dietary changes (including socioeconomic, cultural and psychological factors), as well as family engagement, are recommended. Awareness of the potential of disordered eating and eating disorders when discussing weight management and lifestyle interventions is important. A practice tool for lifestyle management in PCOS is available online (Algorithm 3: https://mchri.org.au/guidelines-resources/health-professionals/pcos-practice-tools/).
Weight stigma
Weight stigma refers to the social devaluation of individuals because of excess body weight and includes negative attitudes, stereotypes and prejudice about excess body weight. This is a key issue highlighted in the 2023 guideline. The experience of weight stigma is associated with negative health and behavioural outcomes, including poorer health behaviours (e.g. exercise avoidance and greater consumption of food), avoidance of preventative health screening and impaired mental health, including depression, anxiety, disordered eating and body image disturbance.2 Weight stigma by healthcare professionals has been reported by women with and without PCOS seeking health advice.17-19
GPs should aim to practise weight inclusiveness ,with acceptance of and respect for body size diversity and a focus on health at every size (Box 4). These approaches include asking permission to discuss and measure weight, using destigmatising terms, such as ‘higher weight’ instead of ‘overweight’ or ‘obesity’, and ensuring appropriate equipment is available for patients of all sizes.
Medical therapy for weight management
For anthropometric and metabolic outcomes, metformin can be considered in adults with a BMI greater than 25 kg/m2. Antiobesity medications (e.g. orlistat or GLP-1 agonists semaglutide, liraglutide and exenatide) can be considered in adults with a higher BMI or other risk factors; however, there is limited evidence on the efficacy of antiobesity medications, especially semaglutide, for women with PCOS. Inositol may also be considered. There are nine stereo-isomers of inositol, of which myo-inositol is the most abundant in the human body. Insulin stimulates the endogenous conversion of myo-inositol to D-chiro-inositol. Myo-inositol and D-chiro-inositol are available in supplement form, including as monotherapy and in combination with other ingredients. However, there is very limited evidence that inositol, taken as a supplement, helps with weight management, and metformin is preferred over inositol for central adiposity.20
In Australia, TGA-listed products are self-assessed for safety and quality, whereas products obtained overseas will have variable regulatory standards. Myo-inositol and D-chiro-inositol are not currently recommended for weight management, regardless of the type and doses, because of the lack of quality evidence. GPs should engage in shared decision-making with patients. This includes making them aware of the differences in quality control processes between supplement products and pharmacological products and helping patients to balance financial risk against perceived or potential benefits. GPs can support informed decision-making while acknowledging individual values and preferences.
Bariatric and metabolic surgery for weight management
Recommendations for bariatric surgery are based on studies conducted in the general population. When bariatric surgery is performed in women with PCOS, they should be counselled about the potential rapid return of fertility. Effective contraception should commence prior to surgery and continue until a stable weight is achieved, usually after one year. This aims to avoid increased risks of growth restriction, premature birth, small-for-gestational-age birthweight and prolonged hospital admission for the neonate if pregnancy occurs soon after surgery.
Emotional wellbeing
Psychological issues have been generally under-recognised in the context of PCOS.21 GPs play an important role in the detection and assessment of psychological features associated with PCOS. All adults and adolescents with PCOS should be screened for anxiety and depression, in addition to being offered appropriate referrals and management (depending on the clinical expertise) if moderate or severe anxiety or depressive symptoms are detected. Permission to discuss psychosexual function should be sought, noting that a diagnosis requires the combination of low psychosexual function and related distress. GPs are encouraged to be aware of the increased risk of the development of a negative body image and eating disorders or disordered eating (e.g. bingeing or excessive dietary restriction). More information can be found on the online practice tool for screening, diagnostic assessment and treatment of emotional wellbeing (Algorithm 2: https://mchri.org.au/guidelines-resources/health-professionals/pcos-practice-tools/).
Fatigue and sleep
Women with PCOS have a nearly 10-fold higher risk of developing obstructive sleep apnoea compared with women without PCOS.2 Women presenting with suggestive symptoms (e.g. snoring in combination with waking unrefreshed from sleep, daytime somnolence or fatigue) should be screened using validated tools, such as the Berlin questionnaire, or referred for assessment and a formal sleep study.
Management of reproductive health
Irregular periods
After lifestyle interventions and education, the COCP is recommended for managing irregular menstrual cycles in women with PCOS. Metformin can also be considered for irregular cycles if the COCP is contraindicated, not accepted or not tolerated.2 Inositol may be considered for managing irregular cycles if it is the woman’s preference, noting the lack of clear benefit and low risk of side effects.20 Owing to the lack of high-quality evidence for inositol use to improve the symptoms of PCOS, no recommendations on specific types, doses or combinations can be made. Other forms of contraception should be considered and discussed with women using metformin or inositol.
The GP plays an important role in shared decision-making on the use of the COCP and other potential therapies for irregular periods. General population guidelines for COCP prescribing should be consulted. GPs have significant experience in prescribing the COCP and are well placed to provide information on its efficacy and safety and consider individual relative and absolute contraindications and the impact of the COCP on PCOS-specific features, such as excess weight, mental health and cardiovascular risk factors. Progestin-only oral and long-acting reversible contraceptives may also be considered for endometrial protection, based on general population guidelines, acknowledging that evidence on other hormonal and metabolic effects in women with PCOS is limited.2,22 A practice tool for pharmacological treatment for nonfertility indications is available online (Algorithm 4: https://mchri.org.au/guidelines-resources/health-professionals/pcos-practice-tools/).
Fertility
Fertility is a major concern for women with PCOS. The role of the GP includes co-creation of a lifelong reproductive health plan with adults and adolescents. One of the most important measures is the provision of accurate and age-appropriate information, including reassurance that pregnancy can often be successfully achieved either naturally or with infertility treatment. Other components of a reproductive health plan include:
- discussions of contraception if pregnancy is not currently desired
- counselling (using a weight-inclusive approach) on the adverse impact of excess weight on reproductive outcomes such as clinical pregnancy rates, miscarriage and live birth rates
- provision of routine preconception care
- lifelong provision of optimal management of comorbidities such as diabetes, hypertension and psychological conditions.
A practice tool is available online for the management of infertility in women with PCOS (Algorithm 5: https://mchri.org.au/guidelines-resources/health-professionals/pcos-practice-tools/).
First-line management for all women intending to conceive comprises optimising preconception health and lifestyle. Modifiable risk factors that impact fertility, such as a higher BMI, smoking status, alcohol consumption, prescribed and recreational drug use, untreated sexually transmitted infections, nutritional status, coeliac disease and poor dental health, should be addressed. Women with PCOS have a higher risk of hypertension, preeclampsia and gestational diabetes in pregnancy, and blood pressure and glycaemic status (using an OGTT) should be checked in the preconception period and managed appropriately. Other usual preconception measures should be undertaken as for all women. Of note, folic acid is recommended to be commenced at least one month prior to pregnancy at a dose of 0.4 mg. For women with an increased risk of neural tube defects, including those with a BMI greater than 30 kg/m2, a higher dose of 5 mg is recommended.23
GPs are likely to diagnose female infertility associated with PCOS and play an essential role in conducting the initial workup for couples experiencing infertility, facilitating timely referrals to fertility specialists for more complex workup and discussing potential treatments. Although PCOS is the most common cause of anovulatory infertility, there may be other coexisting male and female infertility factors (e.g. endometriosis, tubal factors), and a comprehensive assessment of both individuals is required. Women with PCOS who have regular menstrual cycles may still experience anovulation, and luteal phase progesterone levels can be checked for evidence of ovulation.24 Women with PCOS and infertility due to anovulation alone may be considered for tubal patency testing if their male partner returns a normal semen analysis. The test should be offered on an individual basis prior to ovulation induction, taking into account the cost, complexity, inconvenience, discomfort and availability of treatment, as well as the individual risk for tubal pathology (e.g. history of pelvic inflammatory disease).
The first-line pharmacological treatment for anovulatory infertility in PCOS is letrozole, an aromatase inhibitor prescribed off-label by fertility specialists that acts as an ovulation-inducing agent. Other options include clomiphene citrate plus metformin, clomiphene alone, metformin alone and gonadotrophins. In vitro fertilisation is a third-line medical treatment, which is offered if first- or second-line ovulation induction therapies have failed. If women do not wish to be referred to fertility services or use letrozole or clomiphene, it is reasonable for GPs to offer metformin alone, noting that metformin is not as efficacious as letrozole and clomiphene for ovulation induction.
Pregnancy
GPs also play an important role in caring for pregnant women with PCOS. The 2023 guideline highlights that women with PCOS have higher-risk pregnancies, including higher risks of gestational weight gain, miscarriage, gestational diabetes, gestational hypertension, preeclampsia, intrauterine growth restriction, small-for-gestational-age and low-birth-weight neonates, preterm delivery and need for Caesarean section. However, women with PCOS should be reassured that assisted reproductive technology does not confer additional risks of miscarriage, preterm birth, impaired fetal growth and need for Caesarean section compared with the risks observed in women without PCOS. Additionally, despite the increased risk of gestational diabetes, women with PCOS are not considered to have increased risks of large-for-gestational-age neonates, macrosomia and instrumental delivery.25-29 GPs can facilitate early lifestyle intervention for pregnant women with PCOS to reduce the risk of excess gestational weight gain and other complications. If not already checked in the preconception period, an OGTT should be offered at the first antenatal visit and repeated at 24 to 28 weeks’ gestation.
Hirsutism
Clinical hyperandrogenism has a significant impact on emotional wellbeing and quality of life and is one of the areas of major concern in women with PCOS. A key change in the 2023 guideline is the recommendation for the use of cosmetic therapies, which may include mechanical laser and light therapies. Both cosmetic therapies and the COCP are first-line recommendations for the management of hirsutism, often caused by hyperandrogenism.
In recommending cosmetic therapies, women can be advised that suitable qualified providers should be sought. GPs can also advise women of the following:
- a greater number of laser treatment sessions may be required in women with PCOS compared with women without PCOS with hirsutism
- the addition of COCP to laser treatment may be more effective than laser treatment alone
- low- and high-fluence lasers have similar efficacies, but low-fluence lasers are associated with less pain
- intense pulsed light therapy appears to be less effective than laser treatment, with no evidence to suggest that home-based kits are effective for hirsutism.
When recommending the COCP, GPs should be aware that there is no clinical advantage of using high-dose (greater than 30 mcg) versus low-dose (less than 30 mcg) ethinylestradiol, and that 35 mcg ethinylestradiol plus cyproterone acetate preparations should be considered second-line therapy over other COCPs because of the higher risk of adverse events including venous thromboembolism. Anti-androgens may be considered for a suboptimal response with six months of COCP or cosmetic therapy, or when the COCP is contraindicated. If a woman becomes pregnant while taking anti-androgens, there is a risk of undervirilisation of the male fetus, and any woman commencing anti-androgens should be counselled about this risk and advised to use effective contraception. When engaging in shared decision-making about other management options, clinicians could consider recommending bariatric or metabolic surgery, noting the limited evidence in the context of PCOS. Metformin should be preferred over inositol for hirsutism.2
Menopause
Limited evidence suggests that menstrual cycles become more regular with age in women with PCOS, whereas the OV and follicle number decrease longitudinally but to a lesser degree than in women without PCOS.30-33 Women with PCOS appear to have a later menopause compared with women without PCOS.34 Clinical and biochemical hyperandrogenism are likelyto persist, but androgen assays are likely to be unreliable at the postmenopause stage.35-38 A diagnosis of PCOS should be considered enduring or lifelong. New-onset, severe or worsening hyperandrogenism (including clinical) should be investigated to rule out androgen-secreting tumours and ovarian hyperthecosis in postmenopause.
Conclusion
GPs play a vital role in the diagnosis and management of PCOS and bring valuable skills and expertise as generalist experts in the provision of whole-person and comprehensive care. The 2023 international PCOS guideline provides evidence-based recommendations to guide clinical care with the aim of optimising health outcomes for all individuals with PCOS. MT
COMPETING INTERESTS : Associate Professor Ee is Deputy Faculty Chair of the RACGP Specific Interests Faculty; is a Member of the RACGP Expert Committee – Research; was a guideline development group member for the 2023 PCOS guideline; and received support to attend PCOS guideline development meetings. Associate Professor Boyle is a Member of the PCOS Guideline Development Group; has received royalities as Editor for the Fundamentals of Obstetrics and Gynaecology textbook (Elsevier); has received travel funds from the Centre for Research Excellence in Women's Health in Reproductive Life (CRE WHiRL, National Health and Medical Research Council [NHMRC]) to attend a PCOS Guideline Development workshop (Italy, November 2023); has received travel funds from Montreal University (Canadian Institutes of Health Research [CIHR] grant) to attend a workshop for the iCARE International Collaborative (Paris, 2024); is a Member of the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) Aboriginal and Torres Strait Islander Women's Health Committee; is a Member of the Migrant and Refugee Health Partnership Council; and developed the first version of the AskPCOS mobile application, with funding through an NHMRC partnership grant.
References
1. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2016; 31: 2841-2855.
2. Teede H, Tay CT, Laven J, et al. International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2023. Melbourne: Monash University; 2023. Available online at: https://www.monash.edu/medicine/mchri/pcos/guideline (accessed January 2024).
3. Wang G, Liu X, Zhu S, Lei J. Experience of mental health in women with polycystic ovary syndrome: a descriptive phenomenological study. J Psychosom Obstet Gynaecol 2023; 44: 2218987.
4. Ee C, Smith C, Moran L, et al. "The whole package deal": experiences of overweight/obese women living with polycystic ovary syndrome. BMC Womens Health 2020; 20: 221.
5. Australian Bureau of Statistics (ABS). Patient experiences. Canberra: ABS; 2023. Available online at: https://www.abs.gov.au/statistics/health/health-services/patient-experiences/latest-release#cite-window1 (accessed January 2024).
6. Tran M, Lawrence R, Abalo C, et al. Improving general practice research in Australia. Australian Journal for General Practitioners 2023; 52: 734-736.
7. Hone T, Macinko J, Trajman A, et al. Expansion of primary healthcare and emergency hospital admissions among the urban poor in Rio de Janeiro Brazil: a cohort analysis . The Lancet Reg Health Am 2022; 15: 100363.
8. Zhao Y, Thomas SL, Guthridge SL, Wakerman J. Better health outcomes at lower costs: the benefits of primary care utilisation for chronic disease management in remote Indigenous communities in Australia’s Northern Territory. BMC Health Serv Res 2014; 14: 463.
9. Ding H, Chen Y, Yu M, et al. The effects of chronic disease management in primary health care: evidence from rural China. J Health Econ 2021; 80: 102539.
10. Sawicki OA, Mueller A, Klaaßen-Mielke R, et al. Strong and sustainable primary healthcare is associated with a lower risk of hospitalization in high risk patients. Sci Rep 2021; 11: 4349.
11. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005; 83: 457-502.
12. Yildiz BO, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update 2010; 16: 51-64.
13. Chiu WL, Kuczynska-Burggraf M, Gibson-Helm M, Teede HJ, Vincent A, Boyle JA. What can you find about polycystic ovary syndrome (pcos) online? Assessing online information on PCOS: quality, content, and user-friendliness. Semin Reprod Med 2018; 36: 50-58.
14. Htet T, Cassar S, Boyle JA, et al. Informing translation: the accuracy of information on websites for lifestyle management of polycystic ovary syndrome. Semin Reprod Med 2018; 36: 80-85.
15. Boyle JA, Xu R, Gilbert E, et al. Ask PCOS: identifying need to inform evidence-based app development for polycystic ovary syndrome. Semin Reprod Med 2018; 36: 59-65.
16. Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017; 102: 604-612.
17. Ismayilova M, Yaya S. What can be done to improve polycystic ovary syndrome (PCOS) healthcare? Insights from semi-structured interviews with women in Canada. BMC Womens Health 2022; 22: 157.
18. Mousa A, Tay CT; Teede H. Technical report for the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Melbourne: Monash University; 2023. Available online at: https://doi.org/10.26180/23625288.v1 (accessed January 2024).
19. Puhl RM, Lessard LM, Himmelstein MS, Foster GD. The roles of experienced and internalized weight stigma in healthcare experiences: perspectives of adults engaged in weight management across six countries. PLoS One 2021; 16: e0251566.
20. Fitz V, Graca S, Mahalingaiah S, et al. Inositol for polycystic ovary syndrome: a systematic review and meta-analysis to inform the 2023 update of the international evidence-based PCOS guidelines. J Clin Endocrinol Metab 2024 Jan 2; e-pub (https://doi.org/10.1210/clinem/dgad762).
21. Hillman SC, Bryce C, Caleyachetty R, Dale J. Women’s experiences of diagnosis and management of polycystic ovary syndrome: a mixed-methods study in general practice. Br J Gen Pract 2020; 70: e322-e329.
22. Oguz SH, Yildiz BO. An update on contraception in polycystic ovary syndrome. Endocrinol Metab (Seoul) 2021; 36: 296-311.
23. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG). Pre-pregnancy counselling: best practice statement. Melbourne: RANZCOG; 2021. Available online at: https://ranzcog.edu.au/resources/statements-and-guidelines-directory/ (accessed January 2024).
24. Rozen G, Stern K. An update on fertility assistance and assisted reproductive technologies. Aust J Gen Pract 2023; 52: 11-17.
25. Khomami MB, Joham AE, Boyle JA, et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity-A systematic review, meta-analysis, and meta-regression. Obes Rev 2019; 20: 659-674.
26. Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 2006; 12: 673-683.
27. Kjerulff LE, Sanchez-Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol 2011; 204: 558.e1-6.
28. Qin JZ, Pang LH, Li MJ, Fan XJ, Huang RD, Chen HY. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol 2013; 11: 56.
29. Yu HF, Chen HS, Rao DP, Gong J. Association between polycystic ovary syndrome and the risk of pregnancy complications: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016; 95: e4863.
30. Elting MW, Korsen TJ, Rekers-Mombarg LT, Schoemaker J. Women with polycystic ovary syndrome gain regular menstrual cycles when ageing. Hum Reprod 2000; 15: 24-28.
31. Vulpoi C, Lecomte C, Guilloteau D, Lecomte P. Ageing and reproduction: is polycystic ovary syndrome an exception? Ann Endocrinol (Paris) 2007; 68: 45-50.
32. Brown ZA, Louwers YV, Fong SL, et al. The phenotype of polycystic ovary syndrome ameliorates with aging. Fertil Steril 2011; 96: 1259-1265.
33. Elting MW, Kwee J, Korsen TJ, Rekers-Mombarg LT, Schoemaker J. Aging women with polycystic ovary syndrome who achieve regular menstrual cycles have a smaller follicle cohort than those who continue to have irregular cycles. Fertil Steril 2003; 79: 1154-1160.
34. Li J, Eriksson M, Czene K, Hall P, Rodriguez-Wallberg KA. Common diseases as determinants of menopausal age. Hum Reprod 2016; 31: 2856-2864.
35. Markopoulos MC, Rizos D, Valsamakis G, et al. Hyperandrogenism in women with polycystic ovary syndrome persists after menopause. J Clin Endocrinol Metab 2011; 96: 623-631.
36. Pinola P, Piltonen TT, Puurunen J, et al. Androgen profile through life in women with polycystic ovary syndrome: a Nordic multicenter collaboration study. J Clin Endocrinol Metab 2015; 100: 3400-3407.
37. Forslund M, Schmidt J, Brännström M, Landin-Wilhelmsen K, Dahlgren E. Reproductive Hormones and Anthropometry: A Follow-Up of PCOS and controls from perimenopause to older than 80 years. J Clin Endocrinol Metab 2021; 106: 421-430.
38. Legro RS, Schlaff WD, Diamond MP, et al. Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab 2010; 95: 5305-5313.