What’s on the horizon for heart failure management?
Despite major advances in the treatment of heart failure over the past three decades, the prognosis for most patients remains guarded, particularly for those with acute decompensated heart failure. Telemedicine and remote monitoring are likely to play an increasingly important role in supporting GPs to manage patients, especially those in rural and remote communities. Several emerging drugs and devices show considerable promise in further improving the outlook for these patients.
- Advances in remote monitoring of patients with chronic heart failure (HF), including implantable pulmonary arterial pressure monitors, allow GPs to detect and intervene to prevent clinical worsening, reducing the need for rehospitalisation.
- Sodium–glucose co-transporter-2 (SGLT2) inhibitors improve survival and reduce hospitalisation in patients with HF with reduced ejection fraction, with or without diabetes.
- HF with preserved ejection fraction (HFpEF) remains an area of unmet need, with no drug yet shown to improve survival; trials of novel agents, including SGLT2 inhibitors, are underway.
- Several promising drugs are under investigation for treating transthyretin cardiac amyloidosis, which likely accounts for 10 to 15% of patients with HFpEF.
- There are various devices under investigation that can be implanted using minimally invasive techniques to treat certain subgroups of patients, such as the mitral clip for patients with severe functional mitral regurgitation and interatrial septal devices for those with HFpEF.
- Mobile extracorporeal membrane oxygenation retrieval teams allow critically ill patients with HF to be retrieved from rural and remote sites.
Despite major advances in the treatment of patients with chronic heart failure (HF) over the past three decades, many challenges remain. Most, if not all, advances have been in the management of patients with chronic HF with reduced ejection fraction (HFrEF), which has recently been defined in the Australian HF guidelines as a left ventricular ejection fraction (LVEF) of less than 50%.1 As yet, no therapies have been found to improve the survival of patients with HF with preserved ejection fraction (HFpEF). Acute decompensated heart failure (ADHF) is another area of unmet need, with many novel agents failing to provide meaningful clinical benefits when evaluated in large-scale (Phase III) clinical trials. Nonetheless, there has been a steady pipeline of novel monitoring devices and treatments that have been investigated across these three broad categories (Table), some of which show considerable promise and are likely to be incorporated into clinical practice in the next few years.
Multidisciplinary care, telemedicine and monitoring
Multidisciplinary care involves a team of health professionals, which usually includes the GP, medical specialist, HF nurse specialist and pharmacist. Ideally, a physiotherapist, occupational therapist and social worker should also form part of the team. Formal engagement in multidisciplinary HF programs has been shown to reduce HF hospitalisation and mortality by 25%.2
High-speed internet access has made it possible to transfer large volumes of data between patient and clinician. Telemedicine is a broad term encompassing all health care delivered remotely; in Australia, it has come to be most often applied to the concept of videoconference-delivered clinical consultations. In HF care, telemedicine can provide for clinical consultations between the specialist and the patient’s GP, with the patient present, to optimise team collaboration. It can also allow for limited clinical follow up directly in the patient’s home. When patients are unable to attend in-centre HF rehabilitation, telemedicine allows for this to be delivered directly into their homes. Initial pilot studies have shown promise, and it is likely that further larger scale trials will be undertaken.
A more studied aspect of remotely delivered HF care is better termed telemonitoring, which allows the use of internet-connected devices, such as scales, sphygmomanometers, oxygen saturation probes and blood glucose monitors, to monitor aspects of patients’ health status in their own homes.
Urinary sodium monitoring
In a recent study, a low spot urinary sodium concentration and no increase in the urinary sodium level in response to intravenous diuretics were associated with poor diuretic response, renal tubular injury and high risk of one-year mortality.3 The use of this simple measure may help guide immediate dose adjustments of loop diuretic therapy. In addition, for postdischarge planning, this measure may help identify those patients with ADHF who are at increased risk of early readmission and mortality and hence require early consideration for advanced HF therapies. Although there are limited data on the use of urinary sodium monitoring of patients with HF in the community, the test is simple to perform and could easily be incorporated into a multidisciplinary care program.
Implantable pulmonary artery pressure monitoring
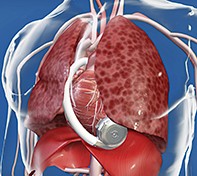
The most successful monitoring device for patients with HF, an implantable pulmonary artery pressure monitoring device (CardioMEMS), is already in widespread use in the United States and increasingly in Europe. It is a leadless and battery-free pressure sensor implanted in a branch of the left pulmonary artery through a femoral venous approach (Figure 1). Patients are provided with a pillow containing a radio antenna, which they use on a daily basis to interrogate the device and transmit a pressure tracing of their pulmonary artery to the treating HF team (see video at www.cardiovascular.abbott/us/en/hcp/products/heart-failure/cardiomems-hf-system.html). Rises in pulmonary artery pressure have been shown to precede HF hospitalisation by up to 21 days, which allows ample opportunity for the treating clinicians to modify HF therapies (usually diuretics) to reduce the risk of hospitalisation.
In the pivotal randomised single-blind CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial, overall heart failure hospitalisation was reduced by 33%.4,5 The trial included patients with New York Heart Association (NYHA) Class III breathlessness and at least one hospitalisation in the preceding 12 months, irrespective of their LVEF. The device appeared even more effective in patients with HFpEF, with an incidence rate ratio of 0.3 compared with the control group in the annualised rate of hospitalisation for HF at 18-month follow up (p<0.0001).4
Lack of a funding mechanism means this device is not readily accessible in Australia, although three sites in the country have now implanted some devices. Even if funding were available, a strategy for implementing this monitoring in Australia would need to be developed, perhaps through a system of certified implantation and monitoring programs, similar to transplant and pulmonary hypertension programs.
Device sensor algorithms
Modern pacemakers and implantable cardiac defibrillators have an array of sensors that can provide evidence of worsening HF. One of the earliest measures developed and tested was thoracic electrical impedance, based on the premise that when lungs filled with water, their electrical impedance fell. Unfortunately, those trials failed to reduce hospitalisation rates, possibly because of suboptimal specificity (leading to unnecessary hospitalisation) or because the trials did not mandate any action on the part of the treating physician.6
More recently, manufacturers have amalgamated an array of parameters – including thoracic impedance, heart rate variability, resting heart rate, intensity of heart sounds, respiratory rate, sleep posture, patient activity levels and percentage of time paced – into proprietary algorithms. These algorithms produce a single number that triggers an alert to the patient’s treating clinician when it crosses a certain threshold. The clinician can then adjust treatment to prevent symptomatic decompensation and subsequent hospitalisation.7 To date, these algorithms have only been applied retrospectively to show that they predicted hospitalisation, but trials are underway to prospectively provide the alerts to treating clinicians to demonstrate that they actually improve HF outcomes.
Drugs
Several classes of drugs are under evaluation as possible adjuncts to existing evidence-based drug therapies in patients with HFrEF or as potentially novel drug therapies in patients with HFpEF (Table).
SGLT-2 inhibitors
Arguably, the most promising drugs are the sodium-glucose co-transporter-2 (SGLT-2) inhibitors, which were developed as a treatment for type 2 diabetes. These drugs block glucose reabsorption in the proximal convoluted tubule, resulting in glycosuria. Although the SGLT-2 inhibitors have induced only modest lowering of glycated haemoglobin (HbA1c) levels, they have several favourable haemodynamic and metabolic actions, including osmotic diuresis, lowering of blood pressure and weight reduction.
Large clinical trials designed to establish the cardiovascular safety of these drugs in patients with type 2 diabetes have shown a surprising benefit in several major clinical endpoints, including a significant reduction in mortality.8 The most dramatic and consistent benefit across several trials has been a reduction in incident HF and hospitalisations for ADHF.8 Most patients included in these studies did not have HF at baseline, suggesting that these drugs may help prevent development of symptomatic HF in patients with type 2 diabetes. Although a reduction in development of symptomatic HF could be explained by the diuretic action of these drugs, the reduction in mortality is harder to explain, given the lack of evidence for mortality reduction with loop or thiazide diuretics.
The positive HF outcomes from SGLT-2 inhibitors in patients with diabetes led to the hypotheses that, firstly, SGLT-2 inhibitors will reduce mortality and HF hospitalisations in patients with type 2 diabetes and established symptomatic HF; and, secondly and perhaps more provocatively, SGLT-2 inhibitors will reduce mortality and HF hospitalisations in patients with HF but without diabetes, including those with HFpEF. These hypotheses are being tested in ongoing Phase III clinical trials, with answers expected over the next 12 to 24 months. The topline results of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial, comparing the SGLT-2 inhibitor dapagliflozin with placebo in patients with HFrEF, were presented in September 2019 at the annual congress of the European Society of Cardiology and have now been published.9 The trial reported that dapagliflozin significantly reduced HF hospitalisation and mortality in patients with HFrEF, both with and without diabetes.
Transthyretin cardiac amyloidosis drugs
Another promising development with implications for the treatment of a substantial proportion of patients with HFpEF is the emergence of effective therapies for treating cardiac amyloidosis resulting from accumulation of amyloid fibrils formed from misfolding of transthyretin protein. This form of cardiac amyloidosis, which can be detected noninvasively with conventional nuclear medicine bone scans, has been estimated to account for 10 to 15% of cases of HFpEF with echocardiographic left ventricular hypertrophy.10
Several drug treatments have been developed with the aim of preventing or reversing cardiac amyloid deposition. These include (orally active) transthyretin stabilisers – tafamidis, AG10 and diflunisal – and (injectable) inhibitors of transthyretin synthesis – patisiran and inotersen.11 Although patisiran and inotersen have mainly been investigated in patients with polyneuropathy caused by transthyretin amyloid, these drugs also show favourable effects on cardiac structure and function. Tafamidis has been shown to delay symptomatic progression and improve survival in patients with cardiac amyloidosis.12
Angiotensin receptor–neprilysin inhibitors
Sacubitril-valsartan, an angiotensin receptor-neprilysin inhibitor that demonstrated superior efficacy to enalapril in patients with chronic HFrEF, has recently been shown to be superior to enalapril in patients hospitalised with acute decompensated HFrEF. Sacubitril-valsartan resulted in a greater reduction in N-terminal B-type natriuretic peptide levels over the subsequent eight weeks; more importantly, fewer patients in the sacubitril-valsartan group required rehospitalisation during follow up.13
Sacubitril-valsartan has also been compared with valsartan in the large Phase III Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients with Preserved Ejection Fraction (PARAGON-HF) trial. The primary results of the PARAGON-HF trial were presented at the European Society of Cardiology congress and simultaneously published.14 Although the trial showed a trend favouring sacubitril-valsartan across a range of endpoints and in some subgroups, overall the trial failed to meet its primary endpoint of reduced HF hospitalisation and mortality.
Direct myosin activators
Omecamtiv mecarbil, a direct myosin activator, is being investigated in a Phase III clinical trial of patients with HFrEF. This trial is now fully recruited, with results expected in 2020. The drug is an orally active positive inotropic agent that has shown favourable effects on surrogate endpoints in Phase II clinical trials.15,16
Devices
Mitral clip
Functional mitral regurgitation complicating chronic HFrEF is common and associated with poorer survival. Two major trials of a percutaneously delivered mitral clip device (Figure 2) to treat functional mitral regurgitation have recently been published, with one (Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation [MITRA-FR]) showing no benefit and the other (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation [COAPT]) showing improved survival and reduced HF hospitalisation.17 Although these trials appear to have produced conflicting findings, a detailed analysis of the entry criteria for each shows that patients entered into COAPT had less severe ventricular enlargement and more severe mitral regurgitation than those enrolled in MITRA-FR. This suggests that, within the population of patients with HFrEF, there is a subgroup with disproportionate mitral regurgitation who will benefit from this intervention.17
Interatrial septal devices
In patients with HF, most breathlessness is driven by pulmonary congestion. The mechanism of pulmonary congestion is high pulmonary capillary wedge pressure, which is in turn caused by the inability of blood to drain from the left atrium into the left ventricle and onwards to the systemic circulation. Patients with Lutembacher syndrome, who have severe mitral stenosis and an atrial septal defect (ASD), have been shown to have fewer symptoms and better outcomes than those with mitral stenosis and no ASD.18 It was therefore hypothesised that creating an ASD in patients with elevated left atrial pressures would reduce their symptoms.
This hypothesis has been tested with two research devices: a simple shunt device without a valve (Corvia’s InterAtrial Shunt Device [IASD]; Figure 3) and a shunt device with a valve to ensure unidirectional flow (V-Wave). The valve device was assessed in a pilot study in patients with HFpEF and HFrEF and showed acceptable safety and symptomatic improvements.19 V-Wave has announced a pivotal randomised controlled double-blind study of 500 patients for its device. The device without a valve has undergone more extensive research, largely confined to patients with HFpEF.20,21 Both these devices showed significant reductions in pulmonary capillary wedge pressures and improved patient exercise tolerance in initial unblinded studies. A pilot randomised double-blind sham-controlled study of the IASD also confirmed significant improvements in exercise tolerance and quality of life and a signal for reduced hospitalisation (but was not powered for this endpoint).22 A large randomised double-blind, sham-controlled study of the IASD is recruiting and is powered to detect reductions in hospitalisation and mortality for patients with HFpEF.
Cardiac contractility modulation
Cardiac contractility modulation (CCM) is not a new technology, but it has only recently gained regulatory approval outside of Europe. The CCM device looks superficially like a pacemaker, with a pulse generator and battery (Figure 4). It is implanted, like a pacemaker, in a subcutaneous pocket (although usually in the right subclavian region, unlike a pacemaker), with two leads in the heart: a sensing lead in the right atrium and a pacing lead in the right ventricle. Earlier versions of the device required two leads in the right ventricle. It works by delivering a biphasic high-voltage bipolar signal to the right ventricular septum during the absolute refractory period.
The proposed mechanism of benefit defies simple explanation. It is proposed that the electrical signal delivered elicits an acute increase in global contractility by improving cardiomyocyte calcium handling and, with time, reverses the fetal myocyte gene programming associated with HF, subsequently producing reverse remodelling. The benefit is seen when the electrical current is delivered for five to 12 hours per day, with longer durations not producing greater benefit.23
CCM is indicated in patients with a narrow QRS complex and persistent HF symptoms despite optimal medical therapy. The greatest benefits seem to be in patients with less severe reductions in ejection fraction. CCM has shown reductions in HF hospitalisation and improvements in exercise tolerance in small randomised controlled trials. The individual trials of CCM to date have been too small and underpowered to show mortality benefits.24 Between concerns about the number of leads in the patient’s heart (potentially two leads for CCM plus another two for an implantable cardioverter defibrillator), the high cost of the device and the lack of proven mortality benefit, the technology has not gained much acceptance in Australia.
His bundle pacing
Although right ventricular pacing has been the mainstay of bradyarrhythmia management for decades, it has been associated with a heightened risk of HF proportional to the degree of right ventricular apical pacing. In patients with HF, biventricular pacing (pacing the left and right ventricles simultaneously) has been shown to unequivocally improve HF outcomes in patients with a broad QRS complex. Pacing the His bundle and proximal bundle branches is intuitively attractive, as it should provide a more physiological stimulus to ventricular depolarisation and subsequent ventricular contraction.25
His bundle pacing (HBP) has not been a feasible option until recently, owing to incomplete understanding of the anatomical and physiological properties of the His bundle, the difficulties of placing the pacing lead on the His bundle and the higher voltages (and thus reduced battery life) required to do so. With more knowledge, dedicated steerable catheters and larger capacity batteries, HBP has now become feasible and is gaining considerable interest.
HBP results in a narrow, physiological QRS complex with synchronous right and left ventricular pacing, unlike traditional right ventricular pacing, which can cause significant ventricular dyssynchrony. In small studies, HBP has been shown to improve the LVEF of patients with HF who have needed atrioventricular nodal ablation for uncontrollable symptomatic atrial fibrillation.26 Compared with right ventricular pacing, HBP has been shown to preserve ejection fraction and significantly reduce HF hospitalisation in patients requiring more than 20% ventricular pacing.27
Not all patients meeting criteria for biventricular pacing have cardiac anatomy suitable for it, and one-third of those who do have suitable anatomy do not respond to conventional biventricular pacing. Thus, HBP is an attractive alternative. In the His Bundle Pacing versus Coronary Sinus Pacing for Cardiac Resynchronization Therapy (His-SYNC) pilot trial, patients with HFrEF and a broad QRS complex were randomly assigned to receive either HBP or biventricular pacing. The trial results were confounded by a large number of crossovers between the groups but, when analysed according to the treatment received, those assigned to HBP showed greater QRS narrowing and a trend towards greater echocardiographic improvement.28 Clinical outcomes between the groups were similar. Larger randomised controlled clinical trials directly comparing biventricular pacing and HBP are in progress.
Baroreceptor activation therapy
HF is characterised by autonomic imbalance with upregulation of sympathetic activity and downregulation of vagal activity. Baroreceptor activation therapy involves the implantation of a device that activates the carotid sinus, mimicking the effect of elevated blood pressure. Stimulation of the carotid sinus leads to reflex central inhibition of sympathetic activity and upregulation of parasympathetic activity. Initially developed as a treatment for resistant hypertension, baroreceptor activation therapy has been shown in a randomised trial to improve quality of life and functional performance in patients with HFrEF, compared with optimal guideline-directed medical therapy.29 Trials to assess the efficacy of baroreceptor activation therapy on clinical outcomes in both HFrEF and HFpEF are in progress.
Surgery
Surgical options for patients with advanced HF are also evolving, including mechanical circulatory assist devices for both acute and chronic circulatory support.
Heart transplantation
Heart transplantation is limited by donor availability, although recent improvements in Australia’s deceased organ donation rate and donor heart preservation have seen an increase in heart transplant numbers. It is noteworthy that the oldest patient to undergo heart transplantation was 73 years of age at the time of transplantation.
For patients with advanced transthyretin cardiac amyloidosis, combined heart and liver transplantation or heart transplantation in combination with one of the novel drugs mentioned above is possible. For patients with light chain (AL) cardiac amyloidosis (a malignant condition with features that overlap those of multiple myeloma), heart transplantation followed by autologous bone marrow transplantation has been successfully performed.30
Acute circulatory support
Patients with acute cardiogenic shock face an extremely high mortality rate. Intra-aortic balloon pumping has been found not to improve the prognosis for these patients. Advances in the design of pumps and oxygenators have led to the successful use of extracorporeal membrane oxygenation (ECMO) for these critically ill patients; however, this support is generally limited to less than two weeks. This may be sufficient for a patient with fulminant myocarditis to fully recover. The availability of mobile ECMO retrieval teams to travel anywhere in the country has allowed for retrieval of these critically ill patients from rural and remote sites. Although ECMO can provide total cardiopulmonary support, it has several limitations, including that most patients remain intubated and sedated while on ECMO support.
An acute circulatory support device, the Impella pump (Figure 5), has been developed as an alternative to ECMO. This device is a miniature rotary pump that can be placed, either by catheter or surgically, retrogradely across the aortic valve into the left ventricle. The pump draws blood from the left ventricle and ejects it into the ascending aorta. This has the dual benefit of providing circulatory assistance and unloading of the left ventricle. Patients can generally be managed in an awake state, facilitating their assessment and recovery. The device is being used in several Australian centres to treat patients with acute cardiogenic shock or to support patients undergoing high-risk catheter-based or surgical cardiac procedures.
Chronic circulatory support
There are now two widely used continuous-flow ventricular assist devices (VADs): the HeartWare HVAD pump and the HeartMate 3 device (Figure 6).31,32 Both are electrically driven centrifugal flow pumps that are implanted within the pericardium, usually as a left ventricular assist device, although occasionally two pumps are implanted to provide biventricular support. Both VADs are designed for long-term support, and there are now case reports documenting more than 10 years of use. The requirement for a driveline that traverses the skin produces a potential portal for infection, which remains a major long-term complication. Bleeding and thromboembolism, including stroke, are the other major long-term complications of these devices.
In Australia, VADs are only approved for mechanical support of people who are being considered for, or are awaiting, heart transplantation (the so-called bridge indication). Globally, however, most patients undergoing VAD implantation are not being considered for heart transplantation (the so-called destination indication).
Conclusion
HF continues to cause significant morbidity and mortality. A range of new therapies and approaches to therapy are on the horizon, with therapies for HFpEF increasingly gaining research interest. MT
Dr McKenzie has received consulting fees and/or speakers honoraria from Abbott Vascular, Boehringer Ingelheim, AstraZeneca, Medtronic and Novartis and nonfinancial support from Boston Scientific, and has been a principal investigator on projects supported by Corvia Medical, Amgen and Novartis and a clinical investigator on a project supported by CSIRO.