Update on diabetes technologies: what’s available, what’s coming?

Advances in diabetes treatment technologies that can help people with type 1 diabetes manage their condition include continuous subcutaneous insulin infusion via insulin pumps and continuous glucose monitoring. GPs and practice nurses have an important role in helping their patients navigate the practical challenges of using these technologies.
- Technologies available in Australia to help people with type 1 diabetes manage their glucose levels include insulin pumps and continuous glucose monitoring (CGM) systems.
- Insulin pumps are battery-powered devices that infuse short-, rapid- or ultrarapid-acting insulin analogues into the subcutaneous tissue via a self-inserted cannula.
- CGM systems comprise a self-inserted glucose sensor attached to a transmitter that wirelessly transmits data to a receiver, such as a smart phone or insulin pump.
- GPs can help their patients who use diabetes technology with practical considerations, including day-to-day device attachment and skin problems, travelling with diabetes technology, and more complex events, such as technology failure.
The importance of tightly maintaining blood glucose levels in the optimum range has been known for decades. Accordingly, current guidelines suggest that the glycated haemoglobin (HbA1c) level should be less than 53 mmol/mol (7%) for both children and adults living with type 1 diabetes.1 Achieving this glucose target is complicated by individual variability in insulin requirements and limitations in the pharmacokinetics and pharmacodynamics of available insulin products. Hypoglycaemia and the fear of hypoglycaemia have also been significant barriers to improving glycaemic profile.
Over the past decade, the evolution of technology has reshaped the management of diabetes, particularly type 1 diabetes. Advances in diabetes treatment technologies offer the potential to improve the blood glucose levels of people with type 1 diabetes and to reduce the burden of living with this condition.
This article summarises the most common, currently used diabetes treatment technologies in Australia today. These include insulin pumps for continuous subcutaneous insulin infusion, continuous and flash glucose monitoring devices, and glucose-responsive automated insulin delivery systems, also known as hybrid closed-loop systems. We focus on practical day-to-day management advice for people with diabetes using these systems and those managing them.
Continuous subcutaneous insulin infusion
Continuous subcutaneous infusion of insulin via an insulin pump has been possible for almost four decades. However, only in the past two decades have these pumps become broadly available.
What are insulin pumps?
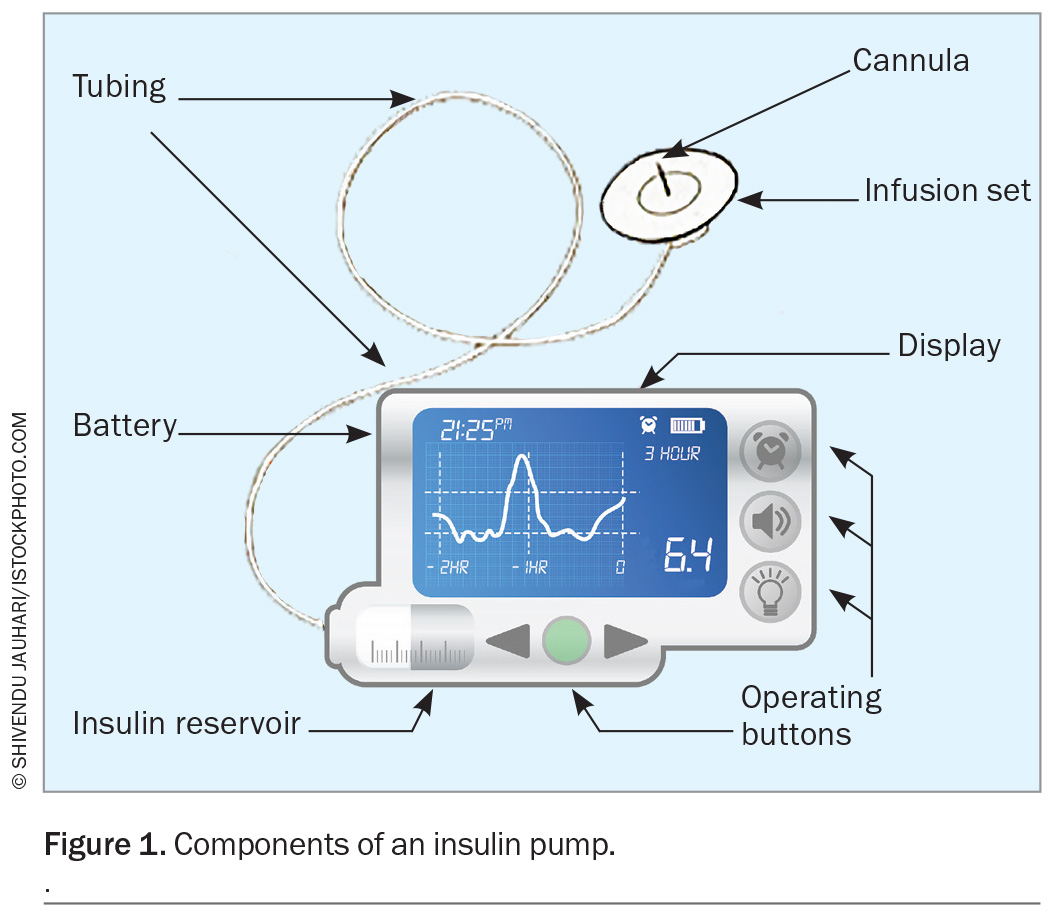
Insulin pumps are small battery-powered devices that infuse short-acting, rapid-acting or ultrarapid-acting insulin analogues into the subcutaneous tissue via self-inserted teflon or steel cannulas (Figure 1). These pumps are programmed with multiple settings, based on the time of day, and are tailored to each individual. Multiple basal profiles can be programmed for different circumstances, such as exercise, work or sick days. Alternatively, temporary basal rates can be used to adjust insulin delivery for a set period of time in the event of increased or decreased insulin requirements.
Insulin pumps are also programmed with information to calculate meal and correction boluses. Typically, this information includes insulin to carbohydrate ratios (the grams of carbohydrate that will be disposed of by 1 unit of insulin), insulin sensitivity or correction factors (the mmol/L change in blood glucose per 1 unit of insulin over two to four hours), and target glucose ranges. Advanced bolus features, such as extended bolus delivery, can be used to meet delayed postprandial insulin requirements following high-fat and high-protein meals, such as pizza or ‘fast’ food.2 Active insulin time can be programmed to reduce the risk of insulin ‘stacking’ in some systems.
Integration with continuous glucose monitoring
Over the past decade, insulin pumps have increasingly been integrated with continuous glucose monitoring (CGM) systems to facilitate varying levels of automation for insulin delivery. In some systems, basal insulin delivery is suspended when a low-glucose threshold is reached, or predicted to be reached in the near future, thereby reducing the risk and frequency of hypoglycaemia.
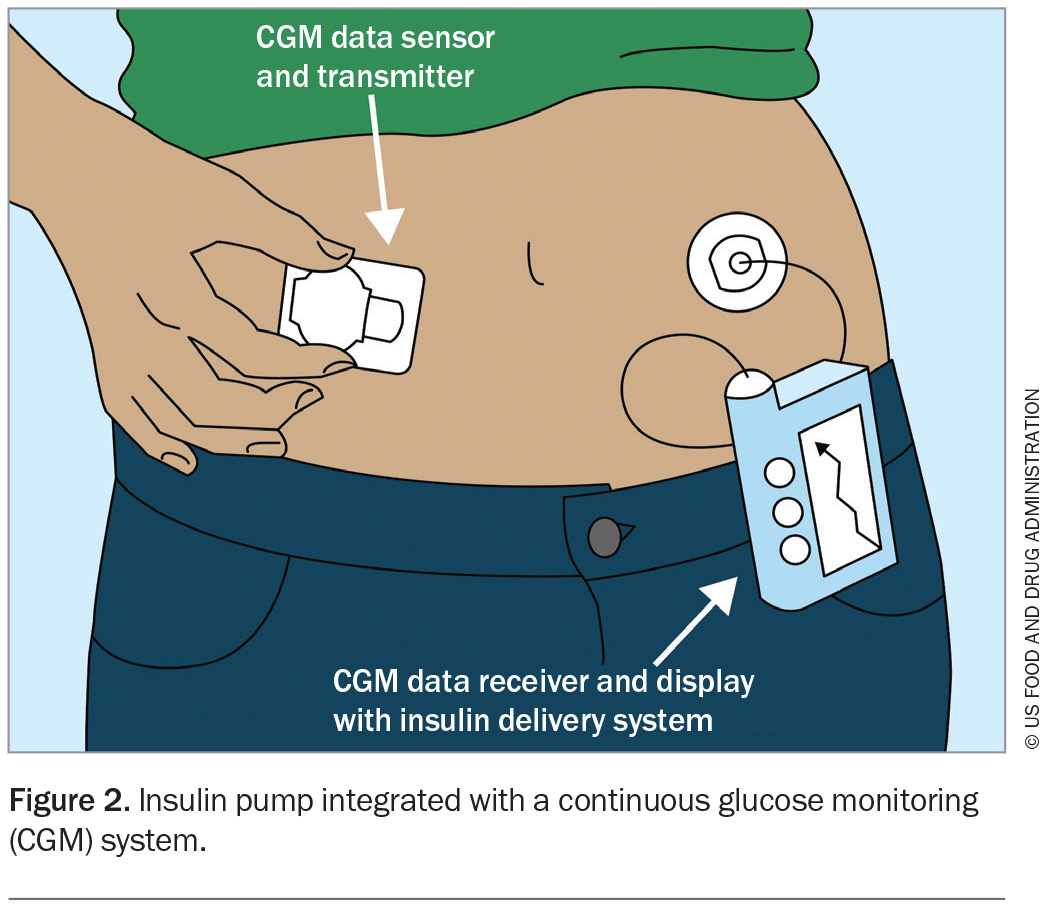
Glucose-responsive automated insulin delivery systems, known as hybrid closed-loop systems, have also been developed and have been available on the Australian market in the past few years (Figure 2). These advanced systems use complex algorithms to increase background insulin delivery in response to sensor glucose readings and to give additional autocorrection bolus doses to minimise hyperglycaemia. They also decrease background insulin delivery to reduce the incidence of hypoglycaemia.
Practical considerations with insulin pumps
Although insulin pumps are associated with improved glycaemic outcomes, they can also be associated with an increased risk of diabetic ketoacidosis. This is because they do not use long-acting insulins and deliver rapid-acting insulin in extremely small amounts. Consequently, if basal insulin delivery is interrupted for more than two hours, circulating insulin levels can fall dangerously low. Resultant insulin deficiency causes the body to metabolise triglycerides and amino acids for energy instead of glucose. Serum levels of glycerol and free fatty acids rise because of unrestrained lipolysis, as do serum levels of alanine because of muscle catabolism.
Thus, individuals using pump therapy should have an emergency management plan and a supply of long-acting insulin to use in the event of pump failure. Long-acting insulin should be taken as soon as a pump fails, and the dose repeated every 12 to 24 hours depending on the insulin type available to the individual, until a replacement pump is available. Typically, the dose of long-acting insulin should equal the pump’s basal insulin delivery history. If there is no history available, a basal insulin dose can be calculated using body weight, for example 0.25 units/kg body weight, although this will vary between individuals, particularly in the paediatric population. In children, the dose will usually be 0.5 units/kg of body weight, or even higher if they are pubertal.3 If no long-acting insulin is immediately available, short-acting insulin should be given every two to three hours via injection.
If the pump is functioning but ketones are detected in the blood or urine, insulin replacement must be delivered via an injection with an insulin pen or insulin syringe instead of via the pump. This is because the infusion set may be malfunctioning, causing ketones to develop.
A checklist for people using diabetes technology is shown in Box 1.
Continuous glucose monitoring systems
Traditionally, people with diabetes have needed to self-monitor blood glucose levels by pricking their fingers to obtain a capillary glucose sample. This can be painful and difficult to perform in some work and social situations. In the past decade, CGM technology has evolved.
What is continuous glucose monitoring?
Unlike conventional self-monitoring of blood glucose, which provides a snapshot of the blood glucose value at the time of sampling, CGM provides semicontinuous information about glucose levels. It does this indirectly, by extrapolating blood glucose levels from interstitial fluid glucose via an algorithm.
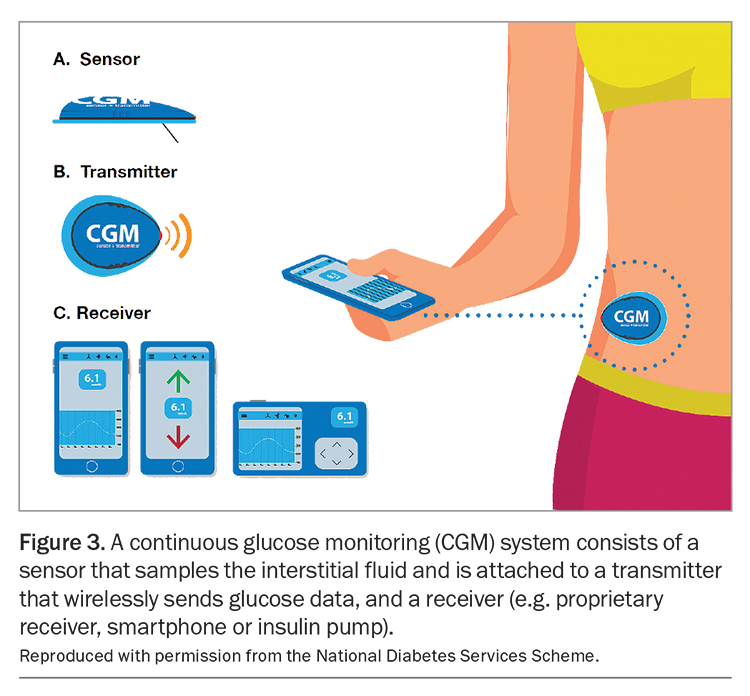
All CGM systems use sensors that are inserted by the user or their carer every seven to 14 days, depending on the system used. The sensor is attached to a transmitter that wirelessly sends glucose data to a receiver, most commonly a mobile phone or insulin pump (Figure 3).
In flash glucose monitoring, the glucose values are not shown constantly, but real-time interstitial glucose values can be obtained by placing a receiver in proximity to the sensor and scanning the sensor to display the result. It is important to ensure that these devices are correctly positioned to ensure accuracy and longevity of sensor use.
With both continuous and flash glucose monitoring, the receiver provides a report with the most recent sensor glucose reading, a graph of glucose levels over the past one to 24 hours, and trend arrows showing whether glucose levels are stable, rising or falling, along with the rate of change. Along with this information, CGM systems provide customisable hypo- and hyperglycaemia alarms, allowing people to make proactive treatment decisions.
Practical considerations with CGM
People using CGM devices still need to self-monitor their blood glucose level in some situations. For example, some CGM systems need to be calibrated with blood glucose levels, typically twice daily. Glucose levels in interstitial fluid also lag behind the glucose levels in blood.4 Consequently, CGM results may be inaccurate, particularly when glucose levels are changing rapidly. For example, sensor glucose levels can continue to register as low for up to 20 to 30 minutes after food is ingested to treat hypoglycaemia. People with diabetes should be taught to check a capillary glucose level before repeating hypoglycaemia treatment to reduce the risk of rebound highs.
Movement of the sensor can also lead to inaccurate results, typically false-low readings. False-high readings can also occur because of interference by paracetamol, ascorbate and other agents that are active in glucose-oxidase based electrochemical sensors.5 The capillary glucose level should always be measured before making management decisions if the sensor glucose reading does not ‘match’ the clinical scenario. This means that individuals should be taught that, if they are feeling unusual symptoms, they should check their blood glucose: ‘if in doubt, get your meter out!’
Sensor attachment difficulties
Further issues with CGM include difficulties keeping sensors attached, particularly for people who spend a lot of time in water or sweat a lot during exercise or work. Sensors can also be displaced during play or sport. After a sensor comes off, it cannot be reused.
Strategies to help prevent sensors being displaced include placement of the sensor at a site that is less likely to be knocked, for example the back of the arm, and overtaping. The two primary types of overtapes are transparent hypoallergenic films and kinesiology tape-like products. These come in a variety of pre-cut sizes and shapes, as well as rolls for custom cutting. Application of antiperspirant spray (unscented) before applying the sensor may also help sensor adherence to skin that is prone to sweating, as can the use of skin adhesive wipes.
Skin reactions and hypersensitivity
Occasionally, people with diabetes can develop hypersensitivity to CGM sensors, overtapes or pump infusion sets. This is especially significant for individuals with skin sensitivities, younger patients and those who use the devices long term. Good skin hygiene, cleaning the area with antibacterial soap and rotating the sensor through multiple sites can help prevent hypersensitivity.6 Tips for looking after your skin while using diabetes technology are shown in Box 2.
A common solution to previously known hypersensitivity reactions has been off-label use of nasal corticosteroid sprays (e.g. mometasone), applied topically to the skin. Liquid barriers, such as barrier wipes or sprays, can also protect the skin from adhesive glues. Creams and oil-based products should be avoided before applying adhesives as they will affect the adhesive’s effectiveness.
Careful removal techniques can reduce the likelihood of skin irritation. In general, adhesive tapes should be removed slowly. There are a variety of removal aids on the market that help loosen the adhesive glue from the skin, including adhesive wipes. Household oils, such as baby oil, eucalyptus oil or olive oil, can also be used to aid removal of adhesives.
Accessing diabetes technologies
The Australian Government provides access to subsidised insulin pump consumables (insulin cartridges, reservoirs and infusion sets) and CGM supplies through the National Diabetes Services Scheme (NDSS). These subsidised consumables and supplies are available only to people with type 1 diabetes and some people with conditions very similar to type 1 diabetes who require insulin. These include specific genetic conditions, infections and drugs or chemicals affecting pancreatic or insulin function (www.ndss.com.au/about-the-ndss/cgm-access/other-eligible-conditions-age-under-21-years/).
People aged under 21 years, those who are pregnant or planning a pregnancy and those with a health benefits card can access fully subsidised continuous and flash glucose monitoring products, with no copayment required. Other people are required to make a small copayment ($1.07 daily at the time of writing). At present, only diabetes medical or nursing specialists, including nurse practitioners and credentialled diabetes educators, can authorise NDSS access to these products.
Public funding for insulin pumps is extremely limited and is administered by the Juvenile Diabetes Research Foundation. Access is means tested, and the program is restricted to people up to the age of 21 years who meet strict eligibility criteria. For most people, funding of insulin pumps requires the individual to have private health insurance, although some people may choose to self-fund, with the cost of insulin pumps ranging from about $6500 to $8500.
Diabetes technologies during medical and surgical procedures
Manufacturers of insulin pumps and CGM devices recommend that they be removed before the user undergoes x-rays, CT or PET scans. Although there is minimal evidence that the devices can be damaged by this imaging, their removal when possible is considered best practice. Insulin pumps and CGM devices may also need to be removed for surgical procedures, particularly if imaging will be performed during the procedure, or high-frequency electrical heat (diathermy) will be required.
Insulin pump infusion sets are changed every two to three days, to prevent problems from reduced insulin absorption at the insertion site. People scheduled to undergo surgery are recommended to change their infusion set in the morning or afternoon of the day before surgery, allowing enough time to ensure that the infusion set is working correctly. Consideration should be given to both the type and placement of the pump infusion set. A plastic infusion set is preferred because of the risks caused by subcutaneous metals with diathermy. The site of insulin infusion needs to be away from the site of the surgery and from the field of diathermy. The pump should also be readily accessible to the anaesthetist.
Basal insulin delivery should continue for the duration of fasting and surgery. To reduce the risk of hypoglycaemia, a lower basal rate may be used. However, as surgery represents a time of metabolic stress and a predisposition to hyperglycaemia, continuing the normal basal rate during short surgical procedures may be appropriate. CGM greatly assists with determining the effectiveness of the insulin rates during this time.
Travelling with diabetes technologies
Insulin pumps and CGM can help to maintain glycaemic stability in different situations, such as during travel. Tips for travelling with a diabetes technology are listed in Box 3. Provided that the basal rate is appropriately set, varying levels of activity and time-zone changes can be accommodated with relative ease. Users should change the time and date on the insulin pump to the local time on arrival at their destination to adjust their settings accordingly.
Both insulin pumps and CGM devices are designed to withstand common electromagnetic interference, including airport security systems. They can be worn while the user goes through airport metal detectors, as this will not harm the devices or trigger an alarm. However, it is advised not to put insulin pumps or CGM devices through x-ray machines or full-body scanners.
People with diabetes are recommended to carry a letter from their treating team or specialist while travelling, to inform airport customs and security staff of their need to carry insulin, insulin injection devices and blood glucose monitoring equipment with them at all times. The letter should also state that the person is wearing an insulin pump or CGM device and that removal of the pump for extended periods could potentially be life-threatening.
What’s coming in diabetes technologies?
In 2017, the US Food and Drug Administration approved the first smart pen for insulin delivery for use in the USA. Smart pens can record the amount and timing of each insulin dose and wirelessly transmit the information via bluetooth to a mobile phone app. The associated app makes dosing recommendations, with suggested doses adjusted for insulin ‘on board’ (the insulin still active from a previous insulin dose). A smart insulin pen was recently launched in Australia. Used in combination with CGM, these insulin pens may help people with diabetes become more actively engaged with their diabetes care.
Conclusion
Diabetes technologies are advancing at an extraordinary pace and have the potential to improve diabetes outcomes for people of all ages living with type 1 diabetes. However, initial education, regular follow up and ongoing education and support are required and highly recommended to achieve success with these technologies. Those caring for people with type 1 diabetes also have a duty to maintain up-to-date skills and knowledge about diabetes technology. GPs and practice nurses, together with diabetes specialist teams, have an important role to play in this regard. Some tips for GPs with patients who use diabetes technology are shown in Box 4. MT
COMPETING INTERESTS: Ms Casson has received consulting fees from AMSL, Insulet Australia, Medtronic and Ypsomed; and honoraria for presentations and article review from Insulet Australia and Medtronic. Dr Overland has received research grants from Roche and Sanofi Aventis; and honoraria for presentations from Abbott, AMSL, AstraZeneca, Eli Lilly, Medtronic, Novo Nordisk, Roche and Ypsomed, and for manuscript writing from Novo Nordisk.
References
1. Craig ME, Twigg SM, Donaghue KC, et al for the Australian Type 1 Diabetes Guidelines Expert Advisory Group. National evidence-based clinical guidelines for type 1 diabetes in children, adolescents and adults. Canberra: Australian Department of Health and Ageing; 2011.
2. Lopez PE, Smart CE, McElduff P, et al. Optimizing the combination insulin bolus split for a high-fat, high-protein meal in children and adolescents using insulin pump therapy. Diabet Med 2017; 34: 1380-1384.
3. Mitsui Y, Kuroda A, Ishizu M, et al. Basal insulin requirements in patients with type 1 diabetes depends on the age and body mass index. J Diabetes Investig 2022; 13: 292-298.
4. Basu A, Dube S, Slama M, et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes 2013; 62: 4083-4087.
5. Basu A, Veettil S, Dyer R, et al. Direct evidence of acetaminophen interference with subcutaneous glucose sensing in humans: a pilot study. Diabetes Technol Ther 2016; 1: S2-43-S2-47.
6. Messer LH, Berger C, Beatson C, et al. Preserving skin integrity with chronic device use in diabetes. Diabetes Technol Ther 2016; S2-54-S2-64.