SGLT-2 inhibitors: the gift that keeps on giving

Sodium-glucose cotransporter-2 (SGLT-2) inhibitors have shown benefits in both chronic heart failure and kidney disease, regardless of the presence of diabetes. These benefits are being recognised in evolving guideline recommendations for the use of SGLT-2 inhibitors for these conditions and subsidised availability on the PBS. GPs have an opportunity to incorporate SGLT-2 inhibitors early in the management of patients with heart failure and chronic kidney disease to improve outcomes.
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are an established therapy for the management of type 2 diabetes. In more recent years, their benefits beyond glucose lowering have been shown. Reductions in heart failure hospitalisation and renal benefits have been consistently reported in type 2 diabetes outcome studies, leading to studies in patients with heart failure and chronic kidney disease, which have reported clear benefits regardless of the presence or absence of diabetes. This article outlines the pivotal studies that have helped establish the benefits of SGLT-2 inhibitors beyond glycaemic control and the associated adverse effects that need consideration.
What are SGLT-2 inhibitors?
SGLT-2 inhibitors inhibit the reabsorption of glucose in the proximal tubule of the kidney, thereby lowering blood glucose levels by increasing its excretion in the urine. Sodium excretion is also increased, leading to natriuresis and osmotic diuresis. Additional mechanisms have been proposed to explain the benefits of SGLT-2 inhibitors in patients with cardiovascular disease, including reduced inflammation, oxidative stress and arterial stiffness, decreased uric acid levels, increased erythropoietin levels and beneficial effects on cellular energetics. Reductions in intraglomerular pressure may also contribute to the renal benefits of SGLT-2 inhibitors.1
What is the evidence?
Diabetes
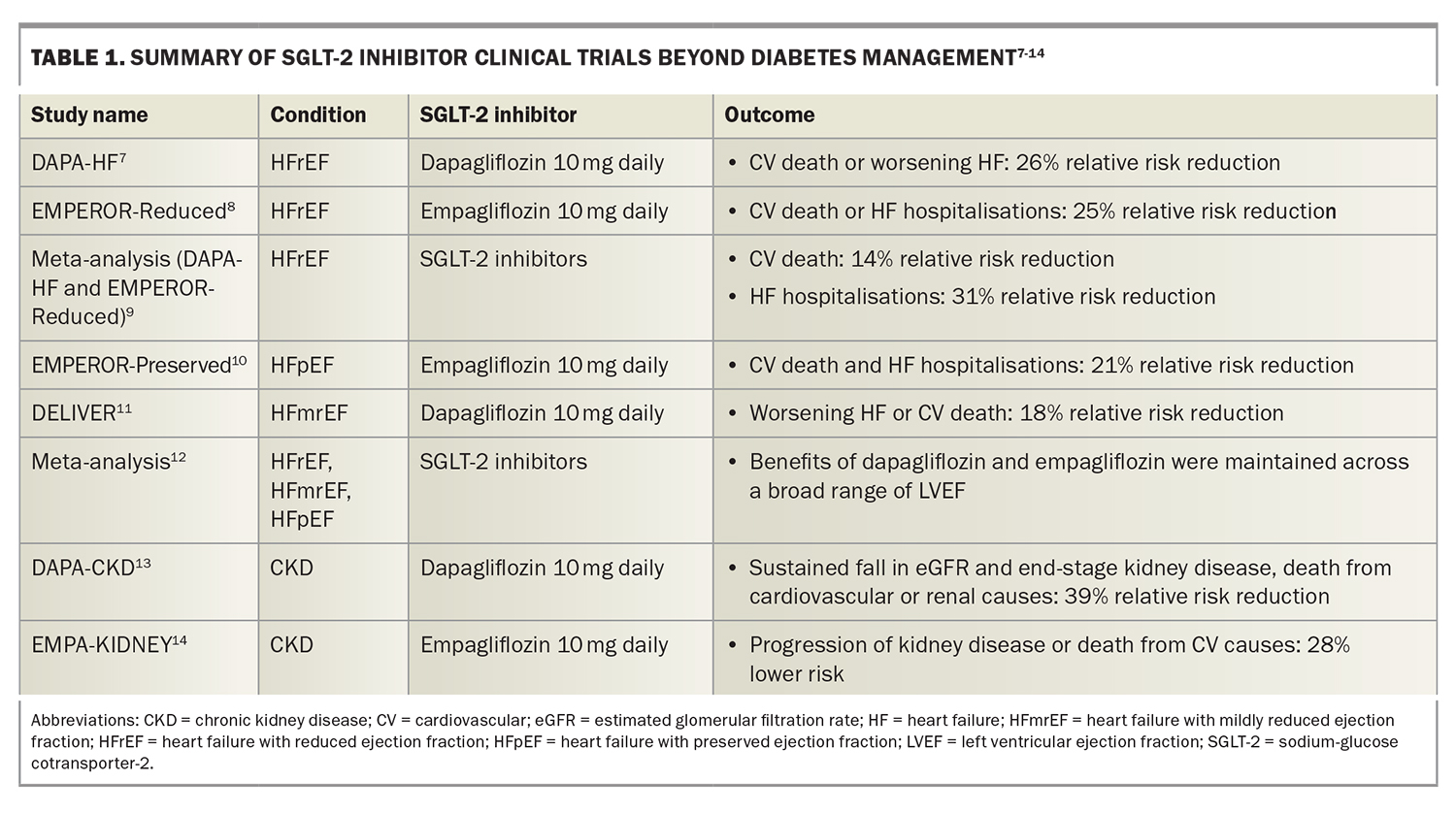
Australian guidelines recommend the use of SGLT-2 inhibitors as second line-therapy, after metformin, for the management of adults with type 2 diabetes, with modest reductions in glycated haemoglobin (HbA1c) levels (0.4 to 0.8%) reported in clinical trials.2 Trials of SGLT-2 inhibitors in patients with type 2 diabetes associated with cardiovascular disease or cardiovascular risk factors reported benefits beyond their glucose-lowering effect, including reductions in heart failure hospitalisations and deterioration of renal function.3-5 A meta-analysis of placebo-controlled trials of SGLT-2 inhibitors in patients with diabetes reported a 22% relative risk reduction in cardiovascular death or heart failure hospitalisation and a 38% relative risk reduction in a composite renal outcome comprising worsening estimated glomerular filtration rate (eGFR) or creatinine level, end-stage kidney disease, kidney death or cardiovascular death.6 These findings have led to trials of SGLT-2 inhibitors in patients with heart failure and chronic kidney disease (Table 1).7-14
Heart failure
Two landmark studies in patients with heart failure with a reduced left ventricular ejection fraction (LVEF) of 40% or below (HFrEF) showed a reduction in heart failure hospitalisations and cardiovascular death regardless of diabetes status.7-8 The large placebo-controlled Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial evaluating the efficacy of dapagliflozin 10 mg daily in addition to standard heart failure treatments, including renin angiotensin system inhibitors, beta-blockers and mineralocorticoid receptor antagonists, showed a 26% relative risk reduction in cardiovascular death or worsening heart failure in patients randomised to receive dapagliflozin.7
The subsequent Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced) reported a significant reduction in cardiovascular death or heart failure hospitalisations in patients randomised to receive empagliflozin 10 mg daily compared with placebo on top of similar background therapy.8 These findings were confirmed in a meta-analysis combining the DAPA-HF and EMPEROR-Reduced studies that reported a 14% relative risk reduction in cardiovascular death and a 31% relative risk reduction in heart failure hospitalisations.9
Recent studies have also reported clinical benefits of SGLT-2 inhibitors in patients with heart failure associated with either a preserved LVEF of 50% or more (HFpEF), or a mildly reduced LVEF of 41 to 49% (HFmrEF). The Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) achieved its primary endpoint, with a 21% relative risk reduction in cardiovascular death or heart failure hospitalisations in patients randomised to receive empagliflozin 10 mg daily compared with placebo.10 This benefit was mostly driven by a 29% relative risk reduction in heart failure hospitalisations.10 The Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) trial also achieved its primary endpoint, with an 18% relative risk reduction in worsening heart failure or cardiovascular death.11 A subsequent meta-analysis showed that the benefits of dapagliflozin and empagliflozin were maintained across the broad range of LVEF.12
Chronic kidney disease
A meta-analysis combining five studies evaluating SGLT-2 inhibitor use in patients with diabetes who were at high risk of cardiovascular events (either because of documented cardiovascular disease, multiple cardiovascular risk factors or chronic kidney disease), reported a 38% relative risk reduction in the progression of kidney adverse outcomes.6
In the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial, patients with chronic kidney disease, with or without diabetes, were randomised to either dapagliflozin 10 mg daily or placebo.13 Chronic kidney disease was defined as an eGFR of 25 to 75 mL/min/1.73 m2 and a urinary albumin to creatinine ratio (uACR) of 500 to 2000 mg/g (22.6 to 565 mg/mmol). A 39% relative risk reduction in a sustained fall in eGFR, end-stage kidney disease, or death from cardiovascular or renal causes was reported in the cohort receiving dapagliflozin, and the magnitude of benefit was similar regardless of diabetic status.13
The Study of Heart and Kidney Protection with Empagliflozin (EMPA-KIDNEY) trial included patients with an eGFR of 20 to 45 mL/min/1.73 m2 or an eGFR of 45 to 90 mL/min/1.73 m2 with a uACR of at least 200 mg/g.14 Patients treated with empagliflozin 10 mg daily had a 28% lower risk of progression of kidney disease or death from cardiovascular causes compared with those treated with placebo, regardless of the patient’s diabetic status. Patients in the empagliflozin cohort were also 14% less likely to be hospitalised for any cause.14
Guideline recommendations
The number of clinical trials published since the Guidelines for the Prevention, Detection, and Management of Heart Failure in Australia 2018 has prompted an updated ‘evidence to practice’ consensus statement on the current pharmacological prevention and management of heart failure.15,16 This consensus statement, published in August 2022, recommends an SGLT-2 inhibitor (dapagliflozin or empagliflozin) for all patients with HFrEF, HFmrEF and HFpEF.16
Recent international heart failure guidelines provide recommendations for the use of SGLT-2 inhibitors in patients with heart failure. Both the European Society of Cardiology (ESC) and American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Failure Society of America (HFSA) heart failure guidelines recommend using SGLT-2 inhibitors, in conjunction with standard heart failure therapies, for patients with HFrEF to reduce the risk of heart failure hospitalisations and death.17,18 Furthermore, the AHA/ACC/HFSA heart failure guidelines recommend an SGLT-2 inhibitor for patients with HFpEF to reduce heart failure hospitalisations and cardiovascular mortality.18
Current international guidelines for chronic kidney disease largely focus on the role of SGLT-2 inhibitors in patients with diabetes.19 Although they acknowledge the evolving evidence, recommendations beyond that of patients with diabetes are not specified. The UK Kidney Association recommends SGLT-2 inhibitors for patients with type 2 diabetes and an eGFR 25 mL/min/1.73 m2 or higher if their uACR is 25 mg/mmol or more and attributed to diabetic nephropathy, or they have established coronary disease or stable symptomatic heart failure.20
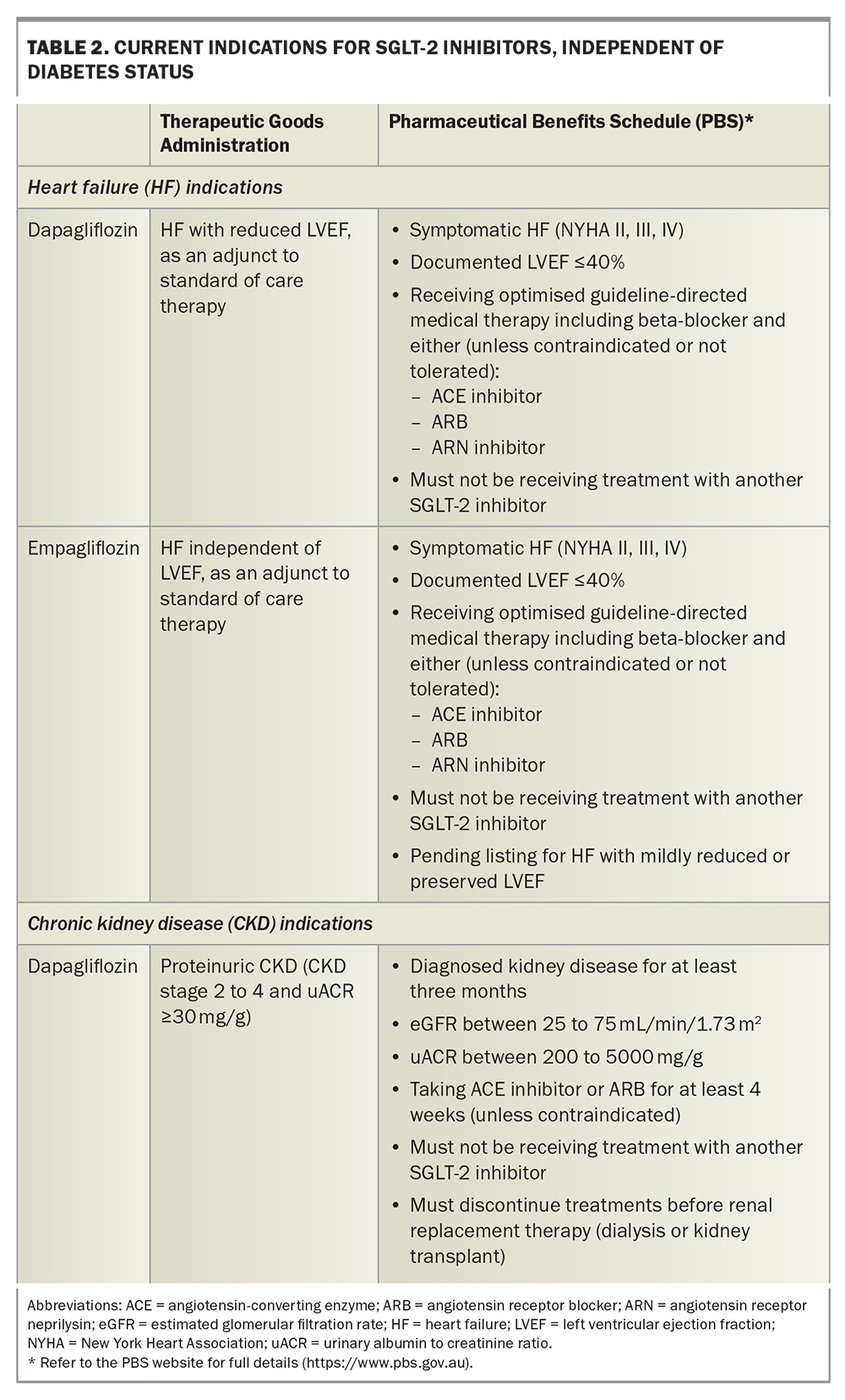
Current indications for SGLT-2 inhibitors in Australia beyond diabetes
Based on the clinical data described above, recent indications for SGLT-2 inhibitors, regardless of diabetes status, have been approved in Australia. The TGA and PBS listings are summarised in Table 2. Dapagliflozin and empagliflozin are listed on the PBS for patients with symptomatic chronic heart failure (NYHA classes II, III or IV) with LVEF 40% or below. Empagliflozin has been recently approved by the TGA for the treatment of symptomatic heart failure independent of LVEF as an adjunct to standard of care therapy and has received an out-of-session approval for PBS listing for patients with heart failure and a mildly reduced or preserved LVEF. Dapagliflozin is approved by the TGA and recently listed on the PBS for CKD. Eligible patients must have an eGFR of 25 to 75 mL/min/1.73 m2 and a uACR of 200 to 5000 mg/g (22.6 to 565 mg/mmol) inclusive before initiating treatment with this drug.
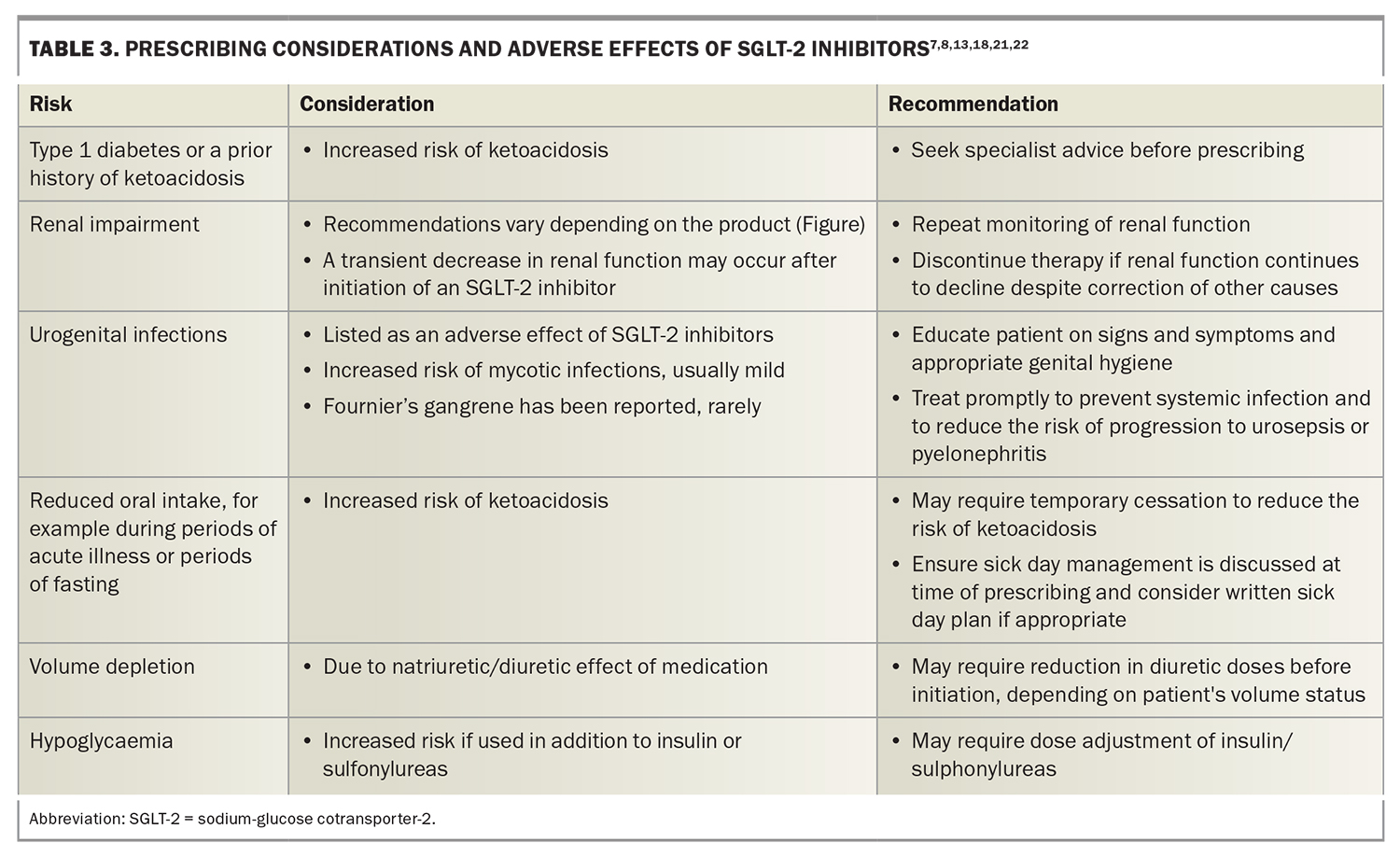
Prescribing considerations and adverse effects of SGLT-2 inhibitors
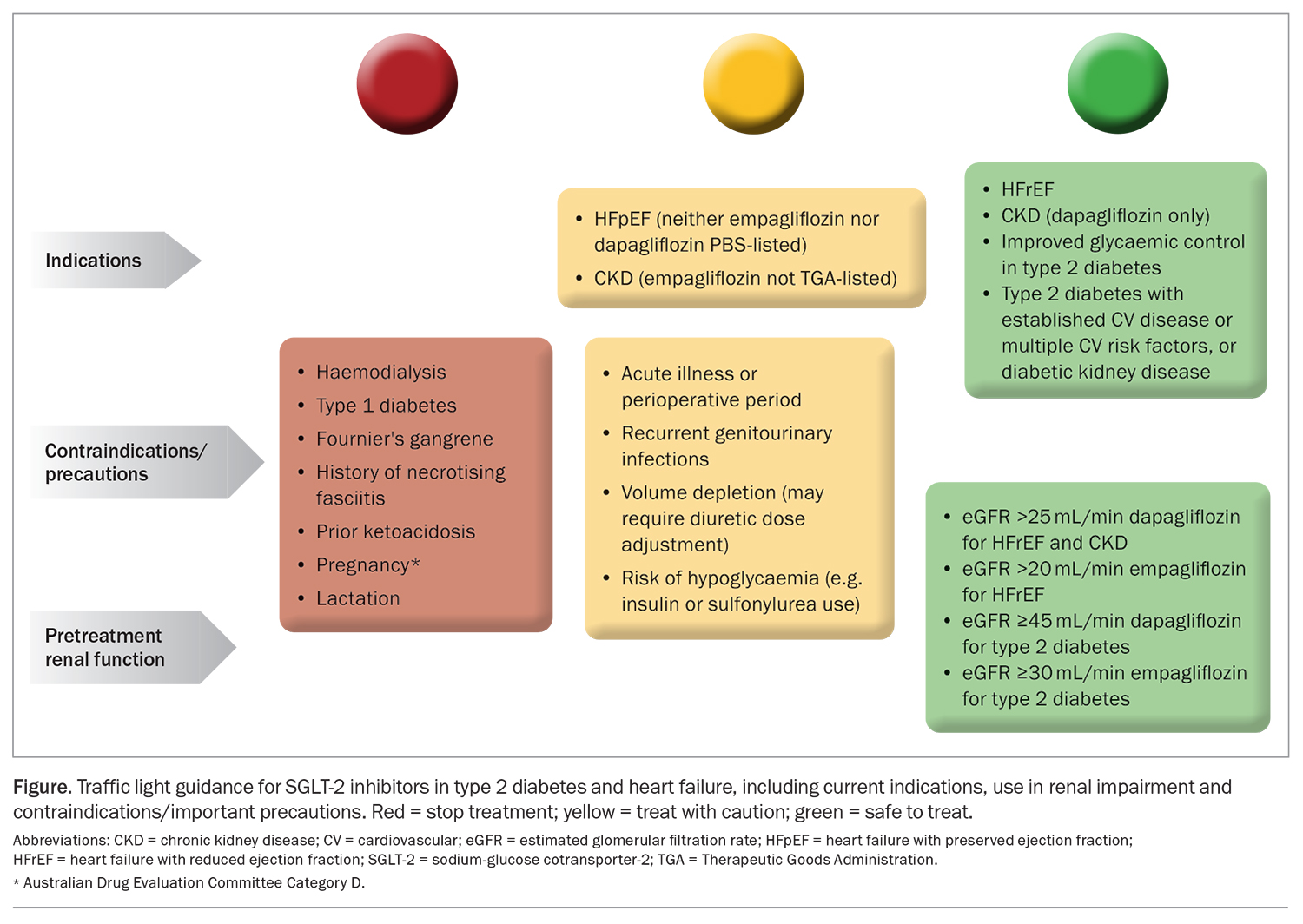
SGLT-2 inhibitors have been shown to reduce the risk of cardiovascular death and hospitalisation in people with heart failure and chronic kidney disease. However, GPs need to be aware of the increased risk of adverse events with SGLT-2 inhibitor use. These include ketoacidosis, volume depletion and hypoglycaemia, especially if used in addition to insulin or sulfonylureas. Important factors and potential adverse effects that need to be considered before prescribing SGLT-2 inhibitors are outlined in Table 3 (Figure).7,8,13,18,21,22
After initiating an SGLT-2 inhibitor, a transient decrease in renal function may occur. If renal function continues to decline despite correction of other causes, therapy should be discontinued.
Urinary tract infections are listed as an adverse effect of SGLT-2 inhibitors; however, recent large, randomised control trials have not reported a significant excess risk compared with placebo.8,13 Cases of Fournier’s gangrene, an acute necrotic infection of the scrotum, penis or perineum, have been rarely reported. Suspected urogenital infections should be treated promptly to reduce the risk of progression to urosepsis or pyelonephritis and patients educated on the signs and symptoms of infection as well as on appropriate genital hygiene measures.
Patients with reduced oral intake due to acute illness or those undergoing periods of fasting may require temporary cessation to reduce the risk of ketoacidosis.18,21 Discuss sick-day management with all patients and consider a written sick-day plan at the point of prescription. An example of sick day advice can be found on the Queensland Heart Failure Support Service website (https://www.health.qld.gov.au/__data/assets/pdf_file/0022/1154380/SGLT2-inhibitor-Patient-Information.pdf). GPs can also refer to their local guidelines for perioperative advice regarding patients prescribed SGLT-2 inhibitors. General advice can be found on Australian and New Zealand College of Anaesthetists (ANZCA) website (https://www.diabetessociety.com.au/downloads/20220726%20ADS%20ADEA%20ANZCA%20NZSSD_DKA_SGLT2i_Alert_Ver%20July %202022.pdf).
An increased incidence of lower limb amputations was observed in a trial of canagliflozin in patients with type 2 diabetes and a high cardiovascular risk; however, canagliflozin is not available in Australia.4 Additionally, these findings have not been observed in other studies evaluating the safety of SGLT-2 inhibitors.7,8,13
Conclusion
The evidence beyond the glucose-lowering benefits of SGLT-2 inhibitors is now well established. International guidelines have included these drugs as part of first-line management for heart failure with a reduced or preserved LVEF, in addition to standard therapy, regardless of diabetic status. Given the outcomes of trials in patients with chronic kidney disease (DAPA-CKD and EMPA-KIDNEY studies), it is likely that future guidelines will also recommend SGLT-2 inhibitors in patients with proteinuric chronic kidney disease, regardless of diabetic status. MT
COMPETING INTERESTS: Ms Rofail: None. Ms Williams has received honoraria for a speaking engagement from Astra Zeneca. Professor Atherton is on the Cardiac Society of Australia and New Zealand Education Trust Board of Trustees and National Cardiac Registry Board; and has received consultancy fees from Amgen and Eli Lilly; and travel support from Bayer, Eli Lilly and Novartis.
References
1. Rasalam R, Atherton JJ, Deed G, Molloy-Bland M, Cohen N, Sindone A. Sodium-glucose cotransporter 2 inhibitor effects on heart failure hospitalization and cardiac function: systematic review. ESC Heart Fail 2021; 8: 4093-4118.
2. Sodium-glucose co-transporter 2 inhibitors for adults with type 2 diabetes [Published Jan 2019, amended Dec 2020]. In: Therapeutic Guidelines. Melbourne: Therapeutic Guidelines Limited; 2022.
3. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117-2128.
4. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644-657.
5. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347-357.
6. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021; 6: 148-158.
7. McMurray JJ, Solomon SD, Inzucchi SE, et al; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995-2008.
8. Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413-1424.
9. Zannad F, Ferreira JP, Pocock SJ, et al; SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020; 396: 819-829.
10. Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021; 385: 1451-1461.
11. Solomon SD, McMurray JJV, Claggett B, et al; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022; 387: 1089-1098.
12. Jhund PS, Kondo T, Butt JH, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med 2022; 28: 1956-1964.
13. Heerspink HJ, Stefánsson BV, Correa-Rotter R, et al; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436-1446.
14. EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, Wanner C, et al. N Engl J Med 2023; 388: 117-127.
15. NHFA CSANZ Heart Failure Guidelines Working Group; Atherton JJ, Sindone A, De Pasquale CG, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ 2018; 27: 1123-1208.
16. Sindone AP, De Pasquale C, Amerena J, et al. Consensus statement on the current pharmacological prevention and management of heart failure. Med J Aust 2022; 217: 212-217.
17. McDonagh TA, Metra M, Adamo M, et al; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599-3726.
18. Heidenreich PA, Bozkurt B, Aquilar D, et al. AHA/ACC/HFSA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022; 18: e895-e1032.
19. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2022; 102(5S): S1-S127.
20. UK Kidney Association. UK Kidney Association clinical practice guideline: sodium-glucose co-transporter-2 (SGLT-2) inhibition in adults with kidney disease. Bristol; UK Kidney Association, 2021. Available online at: https://ukkidney.org/sites/renal.org/files/UKKA%20guideline_SGLT2i%20in%20adults%20with
%20kidney%20disease%20v1%2020.10.21.pdf (accessed March 2023).
21. Australian Medicines Handbook. Adelaide: Australian Medicines Handbook Pty Ltd; 2022 July. Available online at: https://amhonline.amh.net.au/ (accessed March 2022).
22. Maddox TM, Januzzi JL Jr, Allen LA, et al; Writing Committee. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021; 77: 772-810.
FURTHER READING
Dapagliflozin Product information. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/PICMI?OpenForm&t=&q=Dapagliflozin&r=https://www.pbs.gov.au/ (accessed March 2023).
Empagliflozin Product information. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/PICMI?OpenForm&t=&q=Empagliflozin&r=/ (accessed March 2023).
Queensland Health. Sodium-glucose co-transporter-2 (SGLT2) inhibitors for heart failure. Patient Information Sheet. Available online at: https://www.health.qld.gov.au/__data/assets/pdf_file/0022/1154380/SGLT2-inhibitor-Patient-Information.pdf (accessed March 2023).
Diabetes Australia. Alert update January 2020 periprocedural diabetic ketoacidosis (DKA) with SGLT2 inhibitor use. Sydney; Diabetes Australia, 2020. Available online at: https://www.diabetessociety.com.au/documents/ADS_DKA_SGLT2i_Alert_update_2020.pdf (accessed March 2023).