Managing COVID-19 in children
SARS-CoV-2 infection, which can lead to severe disease known as COVID-19, differs in children compared with adults. The differences are not only in the clinical presentation and severity of disease, but also, consequently, in its management. When managing SARS-CoV-2 infection in paediatric patients in the community, it is important to be able to identify those children who may have an increased risk of severe disease and to be aware of the treatments that may be offered.
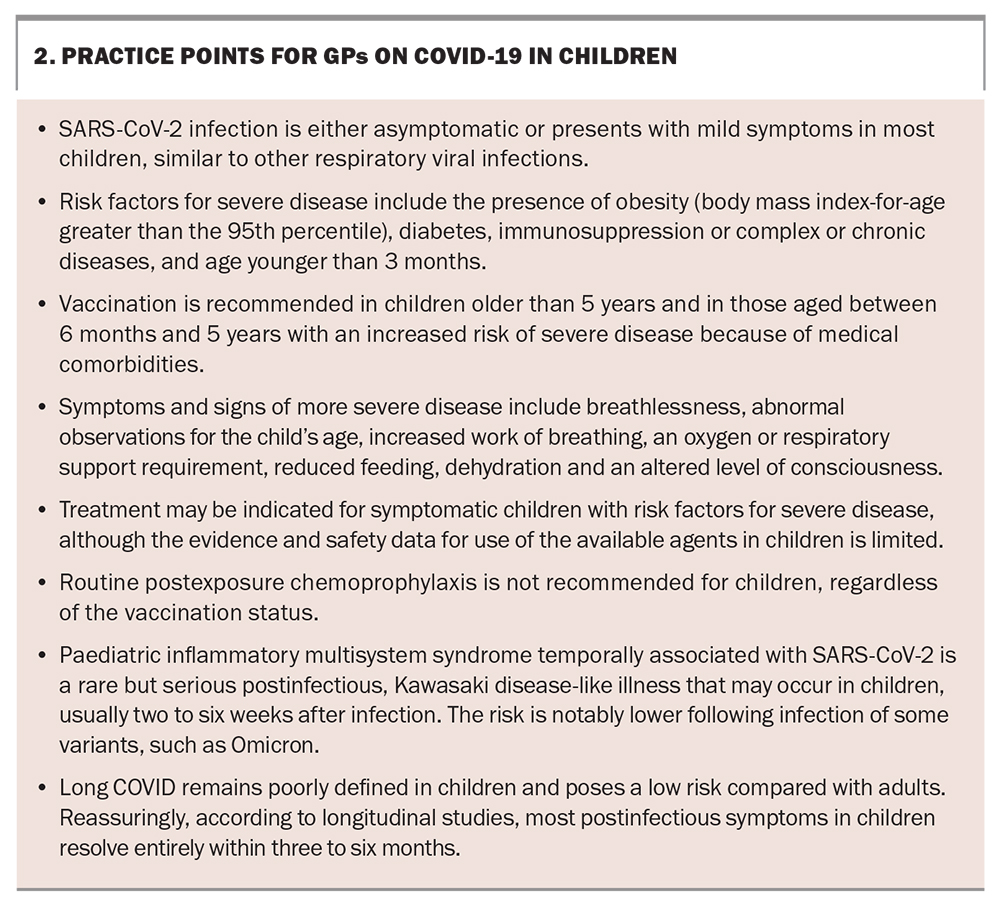
We now recognise that children and adults have an equivalent risk of being infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, unlike adults, over two-thirds of children who are infected with SARS-CoV-2 experience mild symptoms or are asymptomatic.1 If the need for hospitalisation is used as a marker of more severe or complicated infection, 98 to 99% of SARS-CoV-2 infections in children are mild or uncomplicated.2 In Australia, vaccination against SARS-CoV-2 is recommended in children older than 5 years of age or in at-risk groups (those with medical comorbidities) older than 6 months of age, primarily to reduce the frequency of severe disease.
Although uncommon, severe disease resulting in hospitalisation or death can still occur in children, especially in at-risk children for whom preventative therapies or early initiation of treatment may be beneficial. In addition, children are uniquely at risk of developing paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS)/multisystem inflammatory syndrome in children (MIS-C). A small proportion of children are also recognised to experience prolonged symptoms following infection.
This article provides an overview of the children who are at increased risk of severe disease, infection management approaches in children, treatments that may be indicated in children, when to use these treatments, and post-coronavirus disease (COVID-19) complications in children.
Vaccination in children
Since the introduction of vaccination against SARS-CoV-2, there has been a reduction in the incidence of severe COVID-19 in both children and adults. Vaccination remains the most effective preventative intervention against hospitalisation, severe disease and death. Vaccination not only further reduces the risk of severe COVID-19 in children but may also play a role in reducing transmission of the virus between children and between children and adults, although these benefits are notably limited in the Omicron variant era.3 COVID-19 vaccination may also be effective in protecting against the development of PIMS-TS/MIS-C.4,5
The Australian Technical Advisory Group on Immunisation (ATAGI) recommends vaccination with a COVID-19 vaccine approved for children in:
- all children aged 5 years and older
- children aged between 6 months and 5 years with an increased risk of severe disease who are severely immunocompromised or have a disability
- those who have complex or multiple health conditions that increase the risk of severe COVID-19.6-8
A single booster dose six months after infection or vaccination can be considered in at-risk children aged older than 5 years. A booster is currently not recommended in children aged younger than 5 years or children aged older than 5 years in Australia who do not have an increased risk of severe disease. Guidelines on the timing and number of booster vaccine doses are regularly reviewed and updated, with the most up-to-date information available from ATAGI.7 Where possible, COVID-19 vaccination in children should be separated by seven to 14 days from other vaccines to reduce the risk of adverse events, such as fever, but can be coadministered if required.9
COVID-19 vaccination is generally considered safe in children aged older than 6 months, although they may experience mild side effects at a similar frequency to that in adults; these include fever, fatigue and local injection site reactions or pain.10-13 An increase in myopericarditis following mRNA-based (e.g. Pfizer and Moderna vaccines) vaccination is well documented, especially after the second dose and usually in men aged 40 years or younger. In children, this risk seems to be highest in adolescents aged older than 12 years.14
There does not appear to be an increased risk of myopericarditis following vaccination in children aged between 6 months and 5 years compared with baseline population rates, and there is a small increase in risk in those aged between 5 and 11 years.15,16 Symptoms usually occur within seven days after vaccination, although they have been reported up to 14 days postvaccination. Adolescents and children presenting with chest pain, dyspnoea, palpitations, syncope or dizziness within 14 days of receiving an mRNA-based COVID-19 vaccine should be assessed thoroughly and further expert guidance should be sought.17 COVID-19 vaccination with mRNA-based vaccines has been shown to be safe in pregnant women, and evidence is emerging of associated protection in young infants via transplacental antibody transfer.18
Prevention beyond vaccines
Prevention of SARS-CoV-2 infection is ultimately the best approach to reduce morbidity and mortality. Beyond vaccination, other measures include limiting viral exposure through regular hand hygiene, physical distancing and mask wearing. However, as SARS-CoV-2 has continued to become more prevalent in society, the risk of incidental exposures increases.
Treatment designed to reduce the risk of infection before (pre-exposure prophylaxis) and after (postexposure prophylaxis) exposure, particularly with monoclonal antibodies, has been studied to varying degrees in adult populations.19 At the time of writing, there is no consensus on the recommendation for the use of any agent to prevent disease (either pre- or post-exposure) in children, regardless of the underlying risk factors. In part, this is because of evidence suggesting the limited ability of registered monoclonal antibodies in neutralising Omicron variants and the considerably lower rates of severe disease in children.20
Management of mild infection in children
Mild SARS-CoV-2 infection in children often presents with similar symptoms to those of other common upper respiratory tract infections, such as rhinorrhoea, fever, cough and headache. Children with mild infection have no or mild respiratory symptoms, including no or mild increased work of breathing (commonly in younger children) or breathlessness (commonly in older children). Those with mild infection should also be feeding normally or have slightly reduced food intake and have a normal state of consciousness. Given the similarities of SARS-CoV-2 infection in children to other common paediatric illnesses, diagnostic differentiation requires specific SARS-CoV-2 testing.
A positive SARS-CoV-2 PCR test result with sampling by an experienced healthcare worker is the gold standard for diagnosis; however, a reduction in the availability of PCR tests and the widespread increase in availability of commercial rapid antigen test (RAT) kits has led to a shift towards the use of RATs over PCR tests. It is important to note, however, that the sensitivity of RATs is considerably lower than that of PCR testing, and this may be impacted further by the limited user experience of sampling in children. If there is ongoing clinical concern for SARS-CoV-2 infection with a negative RAT result, consider repeat testing with a subsequent RAT or, preferably, a PCR test.
Children without risk factors
Mild SARS-CoV-2 infection in children without risk factors for severe disease can be managed in the community in most cases. Like other respiratory viral infections in children, mild SARS-CoV-2 infection in children often only requires supportive care in the form of ensuring adequate hydration with regular fluid intake, managing pain or discomfort with simple analgesics, and maintaining self isolation, especially from other at-risk individuals where possible. In most cases, this care can be achieved at home; however, it is important to consider social and geographical factors and how these may impact the care provided at home, follow up or escalation of care if required when managing SARS-CoV-2 infection in children in the community.
Children with risk factors
Children with risk factors have an increased risk of severe COVID-19 or death and thus need closer monitoring and follow up. In addition to being unvaccinated or partially vaccinated, factors that can increase the risk of COVID-19 in children include immunosuppression, a complex of severe chronic diseases, young age and the presence of obesity (Box 1). Infection in children who have these risk factors but have symptoms of mild disease can be managed at home or in the community with increased monitoring and support. The RACGP has developed home care guidelines for managing COVID-19 (https://www.racgp.org.au/clinical-resources/covid-19-resources/clinical-care/covid-19-home-care-guidelines/determining-the-appropriate-monitoring-protocol-1), and the National Clinical Evidence Taskforce for COVID-19 also has resources on caring for people with COVID-19 (https://clinicalevidence.net.au/covid-19/).
When to consider severe COVID-19
Severe COVID-19 in children often presents with similar symptoms to those of severe illness with other common respiratory infections. Children with more severe COVID-19 will often have abnormal vital signs for their age, including tachycardia, tachypnoea, moderately or severely increased work of breathing, subjective breathlessness in older children, reduced oxygen saturation and apnoea in infants. Fever lasting more than five days is an important additional red flag. There will often be a history of reduced feeding (less than half of the normal fluid intake) with the inability to maintain hydration without additional feeding or fluid support. Early recognition of warning signs of severe disease in children, with appropriate escalation to hospital-level support, is essential. Children with severe disease may have an altered state of consciousness, and in very severe cases, may present with shock, with haemodynamic instability and multiorgan involvement.
Treatments
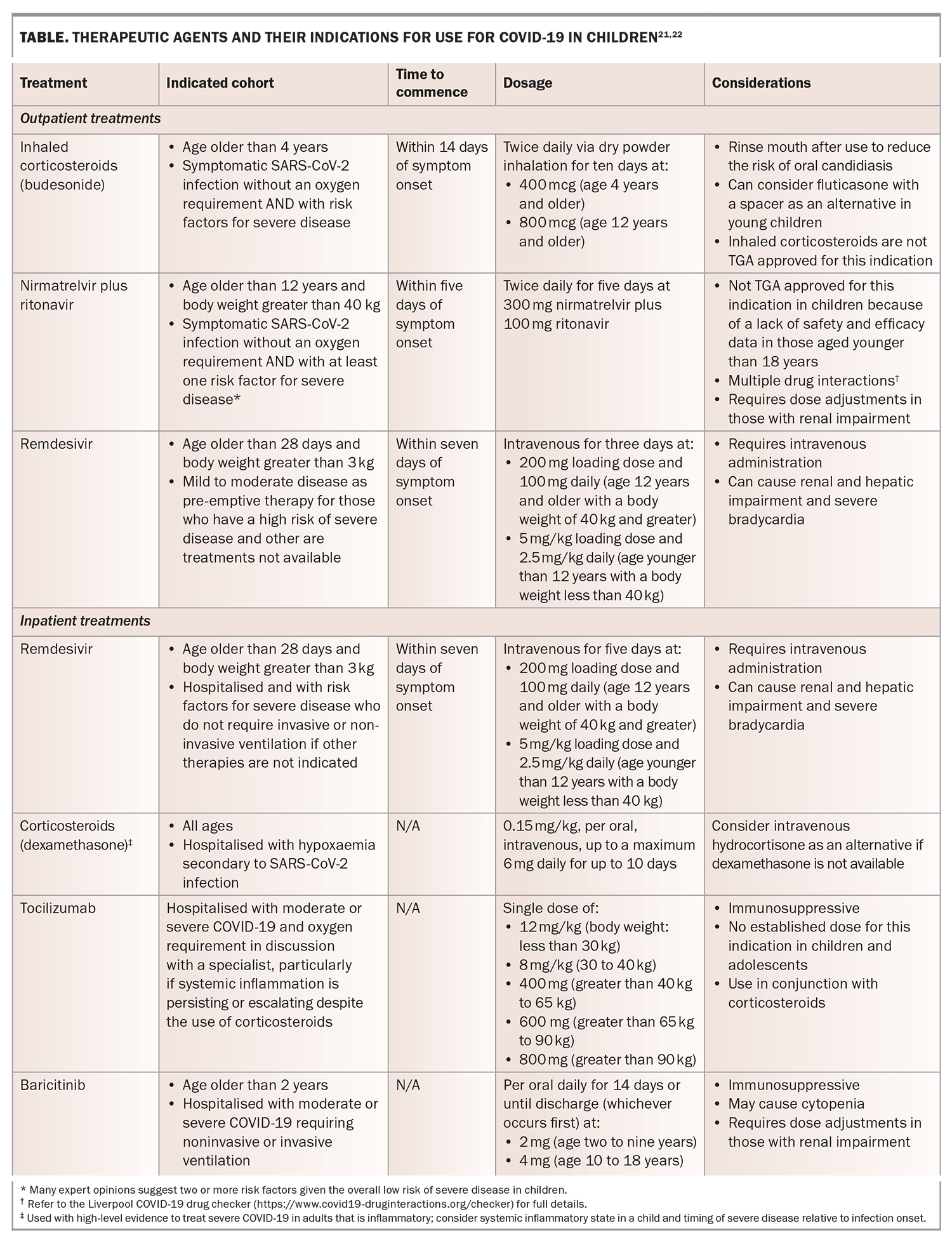
The treatment options for COVID-19 in children are somewhat limited compared with those for adults, given the lack of high-quality trials involving children for many of the available medications. The benefits and safety profile for most medications have been extrapolated from adult data; therefore, caution should be used when considering these available treatments. Therapeutic options for children are reserved for those who are symptomatic with risk factors for severe disease, and usually if more than one risk factor is present. The available therapeutic agents for use in children are outlined in the Table.21,22 Both inhaled corticosteroids and nirmatrelvir plus ritonavir could be prescribed by GPs for use in the community where indicated. Treatment with remdesivir will likely involve further consultation with local hospital services as it requires intravenous administration. Postexposure chemoprophylaxis is not recommended routinely in asymptomatic children regardless of their vaccination status.
Postinfectious complications in children
Paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2/multisystem inflammatory syndrome in children
PIMS-TS/MIS-C is a rare but important post-SARS-CoV-2 complication that occurs in less than one in 3000 children (although this risk seems to be lower again with the Omicron variant), usually two to six weeks after infection with SARS-CoV-2. PIMS-TS/MIS-C should be considered in unwell children (especially those with known recent SARS-CoV-2 infection) who have persistent fever (persisting for more than three days), systemic inflammation (raised levels of inflammatory markers), rash, gastrointestinal symptoms (e.g. abdominal pain, vomiting, diarrhoea) or evidence of organ dysfunction.23
There may be some overlap in the clinical presentations of PIMS-TS/MIS-C, Kawasaki disease and toxic shock syndrome. Specialist opinion should be sought early for suspected cases of PIMS-TS/MIS-C, as therapy includes admission to a hospital, close inpatient monitoring and the administration of corticosteroids alone or in combination with human intravenous immunoglobulin, aspirin or other anticoagulants.24,25
Long COVID
Long COVID (also known as post-COVID condition or post-acute sequelae of COVID-19) is being recognised increasingly among children and adolescents. Prolonged and even nonspecific symptoms following SARS-CoV-2 infection should not be ignored. A number of definitions for long COVID in children have been published and are the subject of ongoing discussions.26,27 Overall, children and adolescents likely have a lower risk (estimated around fivefold lower) of developing long COVID than adults, but the exact prevalence remains unclear.28 Fatigue, headache, mood changes and sleep disturbance appear to be the most frequent symptoms in children.29 Despite this, the symptoms of long COVID in children and adolescents seem to resolve within six months of onset in most cases.30,31 The potential implications of recurrent infections of SARS-CoV-2 in children and the impacts of these on developing long COVID or other post-COVID sequelae are unknown and remain an area for further research. Resources are being developed in Australia to support GPs and paediatricians in assessing and managing long COVID in children and adolescents. Currently, resources from the UK’s National Health Service can be used as a good starting point (https://www.yourcovidrecovery.nhs.uk/children-and-young-people-with-covid/).
Conclusion
In most children, SARS-CoV-2 infection will be asymptomatic or present with mild symptoms, similar to other common viral upper respiratory infections. The disease in these children can almost always be managed at home. Children who have risk factors for severe disease, such as those who are immunocompromised, have chronic conditions, are obese or are very young infants, are most likely to have mild disease. These children require additional support and monitoring, wherein GPs play a pivotal role. Children with mild infection and the presence of one or more risk factors for severe disease, especially if they are unvaccinated, should be considered for for treatment with either nirmatrelvir plus ritonavir, inhaled corticosteroids or, rarely, early use of remdesivir if they fulfil the other criteria for these treatments.Children with symptoms of severe disease should be referred to a hospital for assessment and support.
PIMS-TS/MIS-C remains a rare but important diagnosis to consider in children presenting with fever and signs of systemic inflammation, usually two to six weeks after SARS-CoV-2 infection. Additionally, children and adolescents are increasingly being reported to develop persistent and prolonged symptoms following SARS-CoV-2 infection, often called long COVID; reassuringly, although some children and adolescents may be significantly impacted, these symptoms do not often seem to last for more than six months.
Vaccination remains the best method of prevention of severe disease and other manifestations of COVID-19 and is recommended in children older than 6 months of age with risk factors for severe disease, and in all children older than 5 years of age. Vaccination is also recommended and safe to use in pregnancy, which protects infants younger than 6 months of age. There is no consensus for recommendations of the use of pre- or postexposure prophylaxis. Practice points for GPs are presented in Box 2. MT
COMPETING INTERESTS: None.
References
1. Assaker R, Colas AE, Julien-Marsollier F, et al. Presenting symptoms of COVID-19 in children: a meta-analysis of published studies. Br J Anaesth 2020; 125: e330-e2.
2. Williams P, Koirala A, Saravanos GL, et al. COVID-19 in New South Wales children during 2021: severity and clinical spectrum. Med J Aust 2022; 217: 303-310.
3. Koirala A, Winkler NE, Quinn HE, et al. Understanding SARS-CoV-2 Delta and Omicron variant transmission and vaccine impact in schools and child-care settings in Australia: a population-based study. Lancet Reg Health West Pac 2023 Mar 22; e-pub (https://doi.org/10.1016/j.lanwpc.2023.100736).
4. Price AM, Olson SM, Newhams MM, et al. BNT162b2 Protection against the Omicron variant in children and adolescents. N Engl J Med. 2022; 386: 1899-1909.
5. Nygaard U, Holm M, Hartling UB, et al. Incidence and clinical phenotype of multisystem inflammatory syndrome in children after infection with the SARS-CoV-2 delta variant by vaccination status: a Danish nationwide prospective cohort study. Lancet Child Adolesc Health 2022; 6: 459-465.
6. Australian Technical Advisory Group on Immunisation (ATAGI). Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://www.health.gov.au/committees-and-groups/australian-technical-advisory-group-on-immunisation-atagi (accessed May 2023).
7. Department of Health and Aged Care. COVID-19 vaccines for children. Canberra: Department of Health and Aged Care; 2023. Available online at: https://www.health.gov.au/our-work/covid-19-vaccines/who-can-get-vaccinated/children?gclid=CjwKCAjwxr2iBhBJEiwAdXECw4xW_jlM6AnGu4H51AT3dB9kBeva4EsdJcxfNneDCjANe6T-Yc2EKRoCHoMQAvD_BwE&gclsrc=aw.ds (accessed May 2023).
8. Australian Technical Advisory Group on Immunisation (ATAGI). ATAGI recommendations on COVID-19 vaccine use in children aged 6 months to <5 years. Canberra: Department of Health and Aged Care: 2022. Available online at: https://www.health.gov.au/news/atagi-recommendations-on-covid-19-vaccine-use-in-children-aged-6-months-to (accessed May 2023).
9. Department of Health and Aged Care. Clinical recommendations for COVID-19 vaccines. Canberra: Department of Health and Aged Care; 2023. Available online at: https://www.health.gov.au/our-work/covid-19-vaccines/advice-for-providers/clinical-guidance/clinical-recommendations (accessed May 2023).
10. Department of Health. Australian Public Assessment Report for Elasomeran. Canberra: Therapeutic Goods Administration; 2022. Available online at: https://www.tga.gov.au/sites/default/files/auspar-elasomeran-220221.pdf (accessed May 2023).
11. Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021; 385: 239-250.
12. Ali K, Berman G, Zhou H, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med 2021; 385: 2241-2251.
13. Department of Health. Australian Public Assessment Report for Nuvaxovid. Canberra: Therapeutic Goods Administration; 2022. Available online at: https://www.tga.gov.au/sites/default/files/2022-08/auspar-nuvaxovid-220726.pdf (accessed May 2023).
14. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. J Am Med Assoc 2022; 327: 331-340.
15. Hause AM, Shay DK, Klein NP, et al. Safety of COVID-19 vaccination in United States children ages 5 to 11 years. Pediatrics 2022; 150: e2022057313.
16. Hause AM, Baggs J, Marquez P, et al. Safety monitoring of Pfizer-BioNTech COVID-19 vaccine booster doses among children aged 5-11 years - United States, May 17-July 31, 2022. Morb Mortal Wkly Rep 2022; 71: 1047-1051.
17. Department of Health and Aged Care. Guidance on myocarditis and pericarditis after COVID-19 vaccines. Canberra: Department of Health and Aged Care: 2022. Available online at: https://www.health.gov.au/sites/default/files/documents/2022/11/covid-19-vaccination-guidance-on-myocarditis-and-pericarditis-after-covid-19-vaccines.pdf (accessed May 2023).
18. Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med 2021; 384: 2273-2282.
19. Vita S, Rosati S, Ascoli Bartoli T, et al. Monoclonal antibodies for pre- and post‑exposure prophylaxis of COVID-19: review of the literature. Pathogens 2022; 11: 882.
20. Takashita E, Yamayoshi S, Simon V, et al. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med 2022; 387:468-470.
21. Boast A, Curtis N, Holschier J, et al. An approach to the treatment of children with COVID-19. Pediatr Infect Dis J 2022; 41: 654-662.
22. Drug treatments for children and adolescents with COVID-19. Melbourne: National Clinical Evidence Taskforce, COVID-19; 2023. Available online at: https://clinicalevidence.net.au/wp-content/uploads/FLOWCHART-DT-FOR-PAEDIATRICS.pdf?=221207-05648 (accessed May 2023).
23. Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19. UK: Royal College of Paediatrics and Child Health; 2020. Available online at: https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf (accessed May 2023).
24. Channon-Wells S, Vito O, McArdle AJ, et al. Immunoglobulin, glucocorticoid, or combination therapy for multisystem inflammatory syndrome in children: a propensity-weighted cohort study. Lancet Rheumatol 2023; 5: e184-e99.
25. Welzel T, Atkinson A, Schöbi N, et al. Methylprednisolone versus intravenous immunoglobulins in children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS): an open-label, multicentre, randomised trial. Lancet Child Adolesc Health 2023; 7: 238-248.
26. Stephenson T, Allin B, Nugawela MD, et al. Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch Dis Child 2022; 107: 674-680.
27. A clinical case definition for post COVID-19 conditions in children and adolescents by expert consensus, 16 February 2023. Geneva: WHO; 2023. Available online at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post-COVID-19-condition-CA-Clinical-case-definition-2023-1 (accessed May 2023).
28. Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J 2021; 40: e482-e487.
29. World Health Organisation (WHO). Roessler M, Tesch F, Batram M, et al. Post-COVID-19-associated morbidity in children, adolescents, and adults: A matched cohort study including more than 157,000 individuals with COVID-19 in Germany. PLoS Med 2022; 19: e1004122.
30. Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health 2021; 5: e22-e23.
31. Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health 2021; 5: 708-718.