Fezolinetant – a new oral nonhormonal treatment for menopausal hot flushes

Vasomotor symptoms, including hot flushes and night sweats, affect a significant proportion of menopausal women. Fezolinetant, a neurokinin 3 receptor antagonist, is a newly approved nonhormonal therapy for menopausal vasomotor symptoms, offering a viable option for women who cannot, or prefer not to, use oestrogen-based therapies. Fezolinetant has been shown to be well tolerated, with minimal treatment-emergent adverse events and promising safety data.
- Fezolinetant is the first neurokinin 3 receptor antagonist approved by the TGA for the treatment of menopausal vasomotor symptoms (VMS), offering a new nonhormonal option for managing VMS in menopausal women, particularly those in whom oestrogen therapy is contraindicated or undesirable.
- Results to date have demonstrated rapid and substantial reduction in the frequency and severity of VMS in women taking fezolinetant.
- Fezolinetant may improve sleep quality. Little is known about the effects of fezolinetant on bone, cardiovascular, mental and sexual health at this stage.
- Clinical safety data thus far is promising, and ongoing research aims to shed light on its longer-term safety.
- Fezolinetant is contraindicated in women using CYP 1A2 inhibitors and in those with severe renal or liver disease or elevated liver transaminase levels.
- A baseline and subsequent periodic evaluation of liver function (at least once within the first three months of treatment) is recommended for women taking fezolinetant.
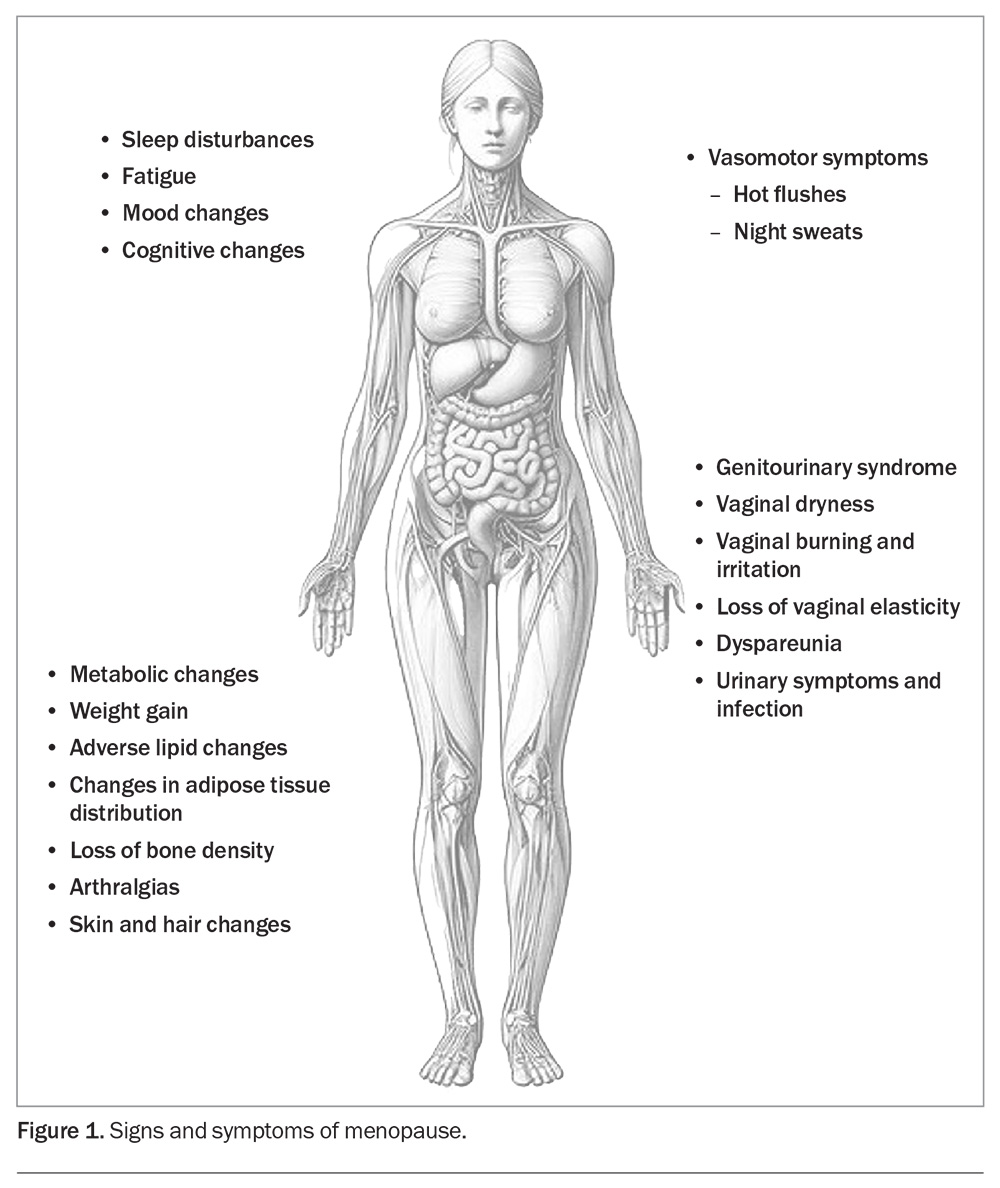
Menopause, defined as the permanent loss of menstruation caused by the loss of ovarian follicular activity, is, for most women, a physiological event. However, the symptoms associated with the menopause transition can be bothersome for many. Vasomotor symptoms (VMS), characterised by hot flushes (or flashes) and night sweats, affect up to 80% of women during the menopausal transition and are the most common menopause-associated symptoms for which women seek treatment (Figure 1).1
VMS represent a disorder of hypothalamic thermoregulation and are characterised by transient intense sensations of heat accompanied by the activation of heat loss effectors causing skin vasodilatation and sweating. The hallmark is reddening of the face and upper body due to cutaneous vasodilatation. This may be accompanied by increased heart rate, lower blood pressure and reduced central blood flow.2
Importantly, it is not the absolute level of oestrogens that triggers the hot flush but the rate of oestrogen withdrawal or fluctuation. Hot flushes do not occur in prepubertal girls.
The most effective treatment for menopausal VMS is oestrogen, usually in the form of menopausal hormone therapy (MHT).1 MHT is generally considered first-line treatment for VMS, particularly for women under 60 years of age or within ten years of menopause. However, many women cannot take hormonal treatment either because of safety concerns related to their medical history or concomitant medical comorbidities or because of personal preference. For these women, options have been limited to therapies substantially less effective than MHT.
In recent years, a new class of nonhormonal medications, neurokinin 3 (NK3) receptor antagonists, have been developed which offer a new and effective option for the alleviation of menopausal VMS, particularly for women who cannot, or prefer not to, use oestrogen-based MHT.
Fezolinetant, a NK3 receptor antagonist, is a new oral nonhormonal therapy for the treatment of bothersome menopausal VMS. Although its long-term safety is still under scrutiny, preliminary clinical trial data indicate minimal treatment-emergent adverse events. This article discusses what fezolinetant is, how it works, when and how it should be used, and what precautions need to be taken in prescribing this medication.
What causes a hot flush?
Much of our current understanding of the physiological basis of VMS arose from work on ovariectomised animals in the 1990s through to the early 2000s by Rance and colleagues.2
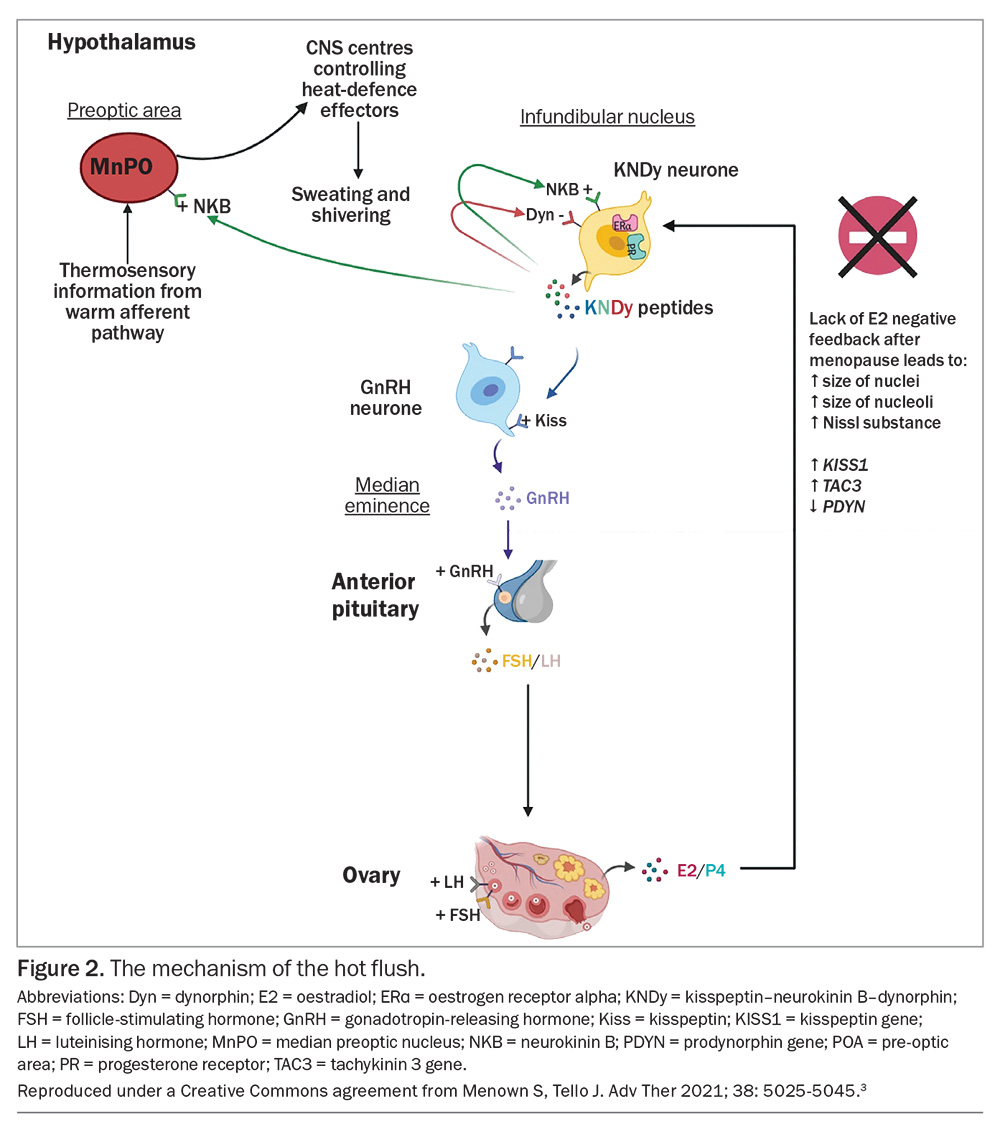
Human reproduction is centrally controlled through interplay between the neurotransmitters kisspeptin, neurokinin B (NKB) and dynorphin, found in KNDy neurons in the infundibular nucleus of the hypothalamus. Kisspeptin stimulates release of gonadotropin-releasing hormone (GnRH) and, in turn, GnRH stimulates release of luteinising hormone (LH) and follicle-stimulating hormone (FSH), ultimately causing the surge of LH which triggers ovulation. Kisspeptin itself is regulated by positive feedback from NKB and negative feedback from dynorphin and oestradiol. Following menopause, the oestrogenic negative feedback is lost. KNDy neurons hypertrophy and, while kisspeptin and NKB levels increase, dynorphin level declines slightly. This leads to a net increase in kisspeptin level and thus an increase in LH pulsatility, which is closely linked to the timing of hot flushes but is not causative (Figure 2).3 Research has shown that KNDy neurons also project to the thermoregulatory centre and play a key role in central regulation of core body temperature. Key to this control is the action of NKB. NKB is a tachykinin and, when infused into healthy premenopausal women, causes hot flushes.4,5
Recent research has demonstrated that an oral NKB receptor antagonist can effectively attenuate menopausal hot flushes by blocking release of NKB.6 Subsequent trials of other chemically distinct neurokinin receptor antagonists have demonstrated similar findings and, most recently, longer trials have also examined safety parameters. Importantly, neurokinin receptor antagonists do not increase serum oestradiol or FSH levels despite their effect on hot flushes. This is a finding of clinical significance for women who have a known or potential contraindication to oestrogen therapy.7
What is fezolinetant?
Fezolinetant is one of several NK3 receptor antagonists under investigation for the treatment of VMS associated with the menopause. Adverse effects on liver enzymes led to the termination of trials for earlier drugs in this class.6 In clinical trials of fezolinetant, hepatic transaminase elevation was reported in up to 5% of patients and usually settled with ongoing use or on cessation of treatment.8
Fezolinetant 45 mg was approved by the TGA in March 2024 for the treatment of moderate to severe VMS associated with the menopause. In the US, it was approved as a nonhormonal therapy for the treatment of VMS associated with the menopause in May 2023 and in the European Union and the UK in December 2023.
Fezolinetant acts by blocking the effects of neurokinin peptides on the central thermoregulatory centre. Its use is associated with a drop in LH levels and no change in FSH or oestradiol levels.
Efficacy of fezolinetant
The safety and efficacy of fezolinetant has been tested in several clinical trials.8,9 Fezolinetant has been shown to be well tolerated in both single and multi-daily dosing regimens (30 mg once daily through to 120 mg four times daily). There were no clinically significant changes in laboratory parameters, vital signs or ECG measurements across dose groups.
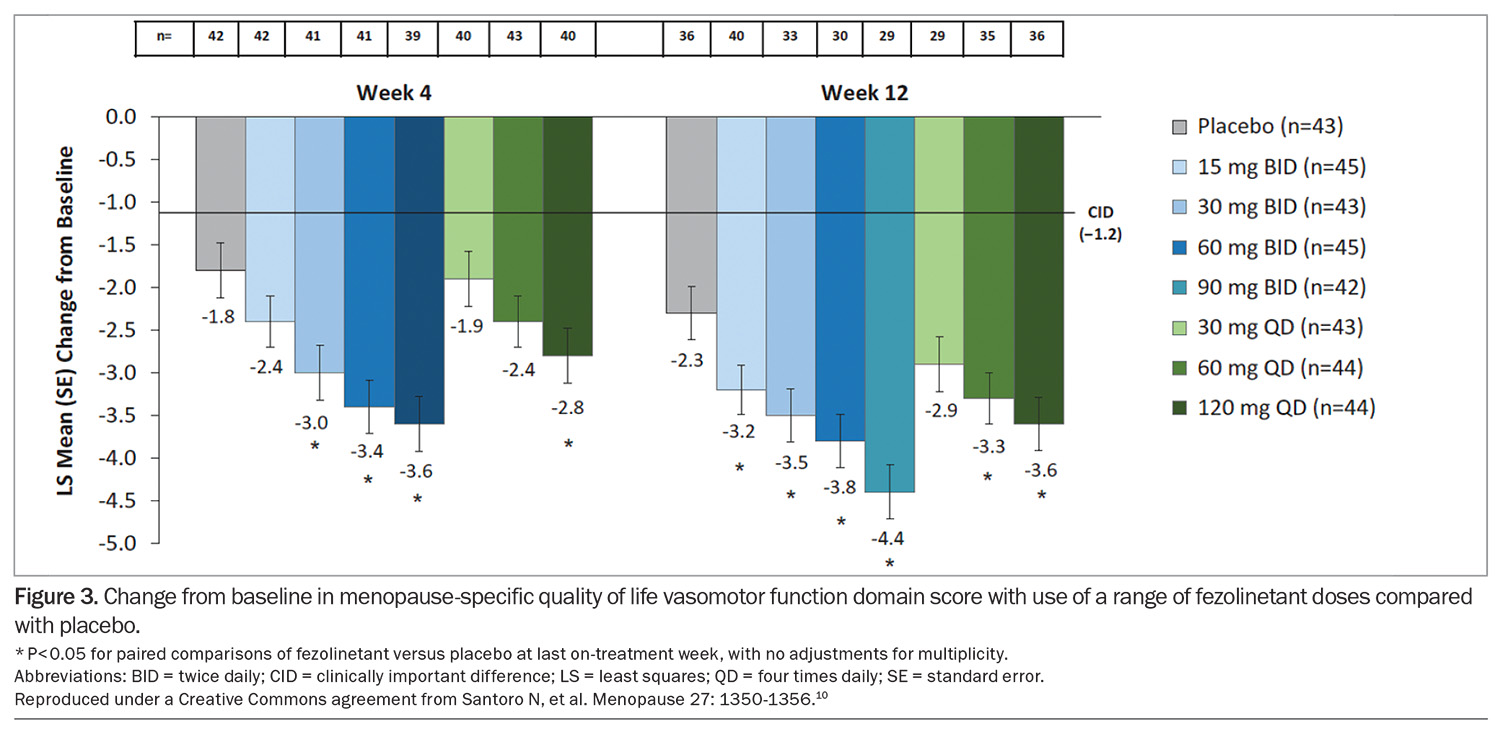
In a 12-week, placebo-controlled, dose-ranging study of the effects of fezolinetant on menopausal VMS, most women who received fezolinetant experienced a 50 to 60% reduction in hot flush frequency and an improvement in menopause-specific quality of life at weeks 4 and 12 (Figure 3).10
A subsequent phase 3 trial of 52 weeks’ duration demonstrated a significant improvement in VMS at four weeks and 12 weeks for women taking fezolinetant 30 mg or 45 mg compared with placebo. These effects on VMS were maintained for the duration of the trial and amounted to an approximate 50% reduction in severity and frequency of moderate to severe VMS compared with a reduction of 30 to 35% in women assigned to placebo.9
How does fezolinetant compare with other agents used to treat menopausal VMS?
No comparator trials have been conducted between fezolinetant and MHT or other nonhormonal pharmaceutical agents used to treat VMS. The magnitude of change in VMS reported by women after fezolinetant therapy is somewhat less than that reported in a systematic review of response to MHT.11 A systematic review examined the effects of seven serotonin and norepinephrine reuptake inhibitors and four NK3 receptor antagonists on VMS and concluded that NK3 receptor antagonists may be more effective than serotonin and norepinephrine reuptake inhibitors in alleviating VMS.3 Further long-term trials are required to verify these findings.
Side effects and adverse events
The safety of fezolinetant 30 mg and 45 mg daily was evaluated in a multicentre double-blind, placebo-controlled, randomised trial over 52 weeks.8 Women recruited were postmenopausal, aged between 40 and 65 years and seeking treatment for VMS associated with the menopause. Primary endpoints were treatment-emergent adverse events and percentage of participants with endometrial hyperplasia or malignancy. Secondary endpoints included change in bone mineral density. Treatment-emergent adverse events were similar between the 30 mg, 45 mg and placebo groups.
In the fezolinetant 45 mg group, one of 203 participants had endometrial hyperplasia (0.5%, one-sided confidence interval upper limit, 2.3%) and there were no cases in the placebo (n = 186) or fezolinetant 30 mg (n = 210) groups. Endometrial malignancy occurred in one of 210 in the fezolinetant 30 mg group (0.5%; 95% one- sided upper limit confidence interval, 2.2%) with no cases in the other groups.8 There were no differences between groups in bone mineral density.
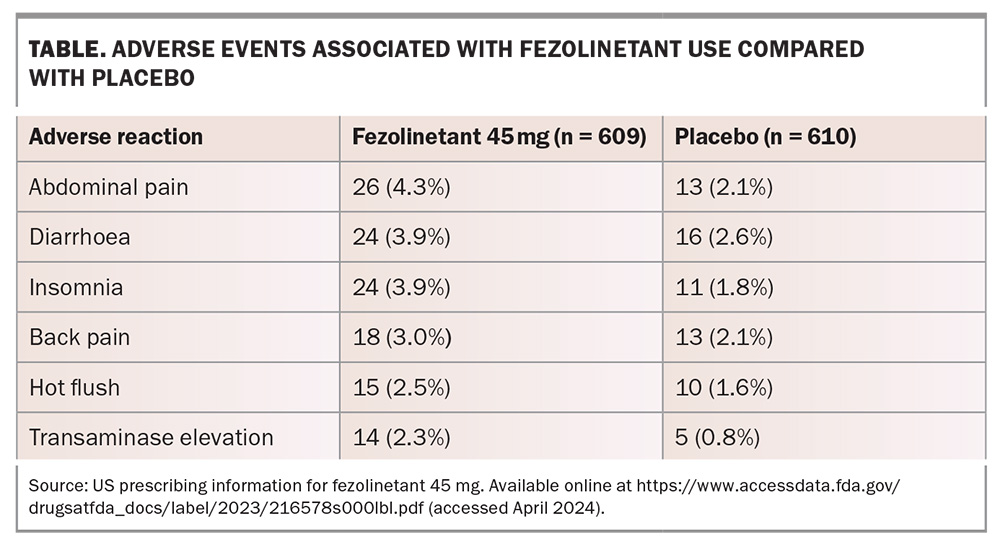
The most common side effects reported were headache, abdominal pain, diarrhoea and insomnia. Elevations in serum transaminase levels greater than three times the upper limit of normal occurred in 2.3% of women receiving fezolinetant 45 mg and 0.8% of women receiving placebo (Table). Increases in alanine aminotransferase or aspartate aminotransferase levels were generally asymptomatic and isolated, intermittent or transient, and generally returned to baseline while on treatment or soon after discontinuation.
There appeared to be no clinically relevant changes in haematology, biochemistry, coagulation or urinalysis across all groups. The researchers deemed there to be no serious treatment-emergent adverse events in any of the studies. However, Australian regulators recommend that a baseline and subsequent evaluation of liver function (within three months) be performed in women prescribed fezolinetant.
Important drug interactions
Fezolinetant is a substrate of CYP 1A2 and is contraindicated in individuals using moderate or strong CYP 1A2 inhibitors, for example, ethinyl estradiol-containing contraceptives, mexiletine and fluvoxamine.
Cautions when prescribing fezolinetant
There are no data on the effects of fezolinetant use in pregnant or lactating women or a breastfed child. There is also no evidence for use of fezolinetant in women under the age of 18 years or over the age of 65 years.
Fezolinetant is contraindicated in women with severe renal impairment (estimated glomerular filtration rate <30 mL/min/1.73m2). No dose adjustment is required for women with mild to moderate renal impairment. Patients with mild to moderate liver disease may have increased exposure to fezolinetant. Fezolinetant should not be started in individuals with elevated liver transaminase levels and is contraindicated in women with cirrhosis.12
Conclusion
Fezolinetant 45 mg is a new, first-in-class, nonhormonal, oral treatment approved by the TGA for treatment of menopausal VMS (see Box on practice points).7 Clinical trial data have shown a promising reduction in the frequency and severity of VMS and a reduction in the frequency of sleep disturbances. Side effects have been minor, with just over 4% of women experiencing gastrointestinal side effects and 5% elevated liver enzymes that returned to baseline during or upon completion of treatment. MT
COMPETING INTERESTS: Dr Mathur: None. Professor Baber has served on the medical advisory board for Astellas Pty Ltd.
This article is for general information purposes only, and the full product information should be consulted before prescribing any of the mentioned medications.
References
1. Baber R, Panay N, Fenton A. IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016; 9: 109-150.
2. Rance N, Dacks P, Mittelman-Smith M, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol 2013; 34: 211-227.
3. Menown S, Tello J. Neurokinin 3 receptor antagonists compared with serotonin norepinephrine reuptake inhibitors for non-hormonal treatment of menopausal hot flushes: a systematic review. Adv Ther 2021; 38: 5025-5045.
4. Davis S, Baber R. Treating menopause – MHT and beyond. Nature Rev Endocrinol 2022; 18: 490-502.
5. Jayasena C, Comninos A, Stefanopoulou E, et al. Neurokinin B administration induces hot flushes in women. Sci Rep 2015; 16: 8466.
6. Prague J, Roberts R, Comninos A, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2 randomized double-blind placebo-controlled trial. Lancet 2017; 389: 1809-1820.
7. Prague J, Abbara A, Comninos A, et al. Neurokinin 3 receptor antagonists do not increase FSH or estradiol secretion in menopausal women. J Endocrine Society 2019; 14: 1-10.
8. Neal-Perry G, Cano A, Lederman S, et al. Safety of fezolinetant for vasomotor symptoms associated with menopause: a randomized controlled trial. Obstet Gynecol 2023; 141: 737-747.
9. Lederman S, Ottery F, Cano A, et al. Fezolinetant for treatment of moderate to severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomized controlled study. Lancet 2023; 401: 1091-1102.
10. Santoro N, Waldbaum A, Lederman S. Effect of the neurokinin 3 receptor antagonist fezolinetant onpatient reported outcomes in postmenopausal women with vasomotor symptoms: results of a randomized, placebo controlled double blind, dose ranging study. Menopause 2020; 27: 1350-1356.
11. MacLennan A, Broadbent J, Lester S, Moore V. Oral estrogen and combined estrogen and progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev 2004; 2004(4): CD002978.
12. Onge E, Phillips B, Miller L. Fezolinetant: a new non hormonal treatment for vasomotor symptoms. J Pharmacy Technology 2023; 39: 291-297.