Stroke risk mitigation. Prescribing and monitoring anticoagulation in atrial fibrillation
Atrial fibrillation heightens the risk of stroke. Current guidelines emphasise assessing stroke risk and promptly initiating oral anticoagulants for high-risk patients. Management should also include regular reviews to ensure medication adherence and persistence.
- People with nonvalvular atrial fibrillation (AF) have an increased risk of thromboembolic stroke.
- Patients with AF should have their stroke risk assessed, and high-risk patients should commence on nonvitamin K-dependent oral anticoagulants (NOACs) as the first-line antithrombotic agent.
- The benefits of anticoagulation for reducing stroke risk exceed bleeding risks in most cases. However, a patient’s reversible bleeding risk factors should be reviewed and specifically addressed.
- Antiplatelet agents, such as aspirin, do not reduce stroke risk due to AF but have similar bleeding profiles to NOACs, so therefore have no role in stroke risk reduction.
- Monitoring long-term patient adherence to, and persistence with, NOAC therapy is essential for effective reduction of stroke risk.
The incidence of atrial fibrillation (AF) is increasing with the ageing population, longer life expectancies and improved survival of patients with cardiovascular morbidities.1 It is estimated that about 10% of adults aged 75 years or older have AF.2 AF causes an unco-ordinated contraction of the atria of the heart, leading to thrombosis in the atrium and subsequently potential distal thromboembolism, increasing stroke risk fivefold. Additionally, AF-related thromboembolism can cause cognitive decline, renal impairment and mesenteric ischaemia.2
Over the past decade, there have been significant advancements in stroke risk mitigation for patients with AF in Australia. In 2009, a novel category of oral anticoagulants known as nonvitamin K antagonist oral anticoagulants (NOACs) gained approval in Australia and three NOACs were included in the PBS in 2013.3 In 2018, Australia saw the introduction of AF guidelines specifically addressing preventive care strategies in individuals with AF.1 These national guidelines introduced recommendations for proactive screening for AF, substantial modifications for evaluating stroke and bleeding risks, and evidence-based approaches to medication choices for stroke prevention anticoagulation that prioritise prescription of NOACs.
GPs have a central role in the proactive detection and management of AF, seeing more than 80% of adults aged 70 years and older at least once annually.4 The role of GPs in AF diagnosis via screening has been discussed previously.5 Following diagnosis, GPs also have a frontline role in initiating and monitoring patient adherence to and persistence with oral anticoagulant (OAC) therapy to reduce the risk of thromboembolism.
If managed with the appropriate antithrombotic therapy, stroke risk can be effectively reduced back to baseline levels.1 Ongoing adherence to, and persistence with, OAC therapy plays a central, but often overlooked, aspect of stroke preventive care. A large international study of ischaemic strokes in 2014, reported that about 20% occurred among patients with known AF who were inadequately anticoagulated, or inappropriately treated with antiplatelet drugs.6,7 Recent Australian general practice data showed that one in four high-risk patients were either receiving no anticoagulant treatment or inadequately anticoagulated, and therefore at higher risk of preventable stroke.3 Preventable cardioembolic stroke is a devastating and debilitating outcome for patients and the healthcare system and these data are a great reminder of the need to prioritise persistence of medication use as well as initiation.
Presentation of AF
In primary care, patients may present with AF in several ways. Symptomatic patients may attend with palpitations, dyspnoea, fatigue or reduced exercise tolerance. However, about one-third of patients with AF are asymptomatic. Australian guidelines recommend opportunistic screening by pulse palpation for all patients aged 65 years or older to increase the identification of this cohort.8 AF may also be an incidental finding when undertaking an ECG or while taking blood pressure. A small proportion of patients will be referred to their GP for ongoing management after AF has been detected during a recent medical procedure or hospital admission. Increasing numbers of patients are also self-presenting with alerts from their wearable devices that detect AF using a single lead ECG or photoplesmography.
Diagnosing AF: the first consultation
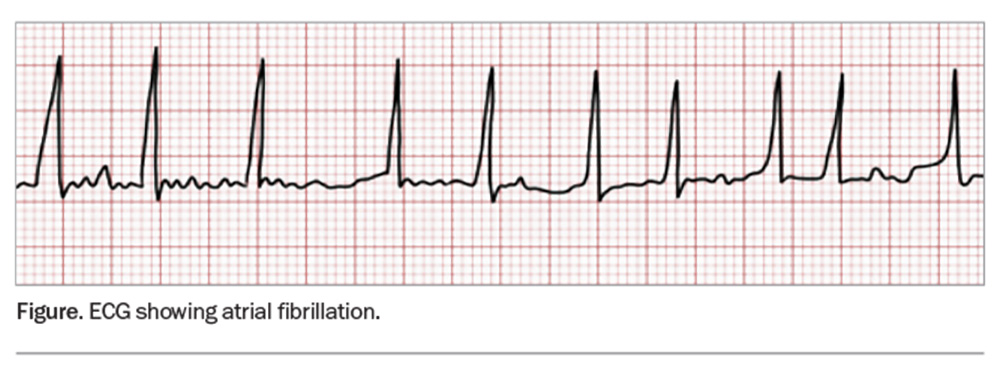
AF can be formally diagnosed from a 12-lead ECG or a 30-second rhythm strip from a handheld device demonstrating the absence of regular P waves and irregular R-R intervals (Figure).1 A 12-lead ECG is recommended to examine for conduction defects, ischaemia or structural issues. Newly diagnosed patients should have a full history and examination, focusing on symptoms, haemodynamic stability and presence of precipitating factors (e.g. infection, electrolyte disturbance or thyrotoxicosis).
Patients who are acutely unwell, dizzy, experiencing chest pain or haemodynamically unstable should be transferred to hospital immediately for further assessment and management.1
For stable patients, stroke risk and the need for anticoagulation needs to be assessed at this first consultation, as patients diagnosed with AF have up to 20% annual risk of stroke.6 Patients should be given referrals for blood tests to examine full blood count, electrolyte levels and renal and thyroid function, and a transthoracic echocardiogram to rule out structural abnormalities. Stable patients may also benefit from the introduction of ventricular rate-controlling medications (e.g. a beta blocker or digoxin).
Asymptomatic patients do not require routine referral to a cardiologist for review unless there are other clinical red flags or there is a potential role for a rhythm control strategy (including direct current cardioversion).
Stroke risk: assess at diagnosis and regularly thereafter
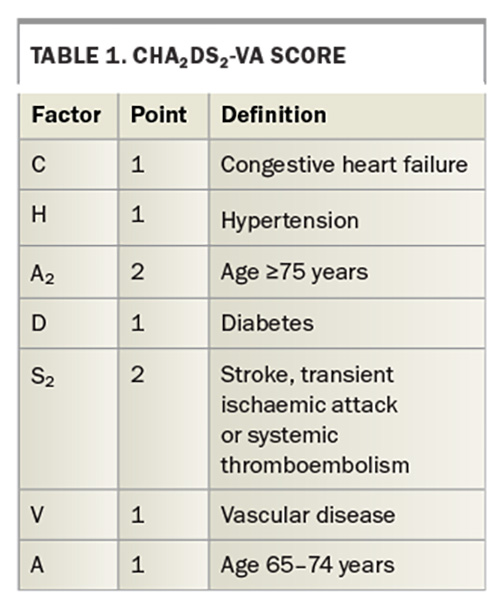
A patient’s stroke risk can be easily assessed using the CHA2DS2-VA score, as outlined in the Australian guidelines (Table 1). Female sex is no longer included in the CHA2DS2-VA stroke risk prediction score, in contrast to the previously recommended CHA2DS2-VASc score.9 Stroke risk factors change over time due to ageing or development of new comorbidities; hence, annual review of low-risk patients is recommended to ensure they are receiving appropriate treatment.1
Guidelines for managing stroke risk with oral anticoagulation
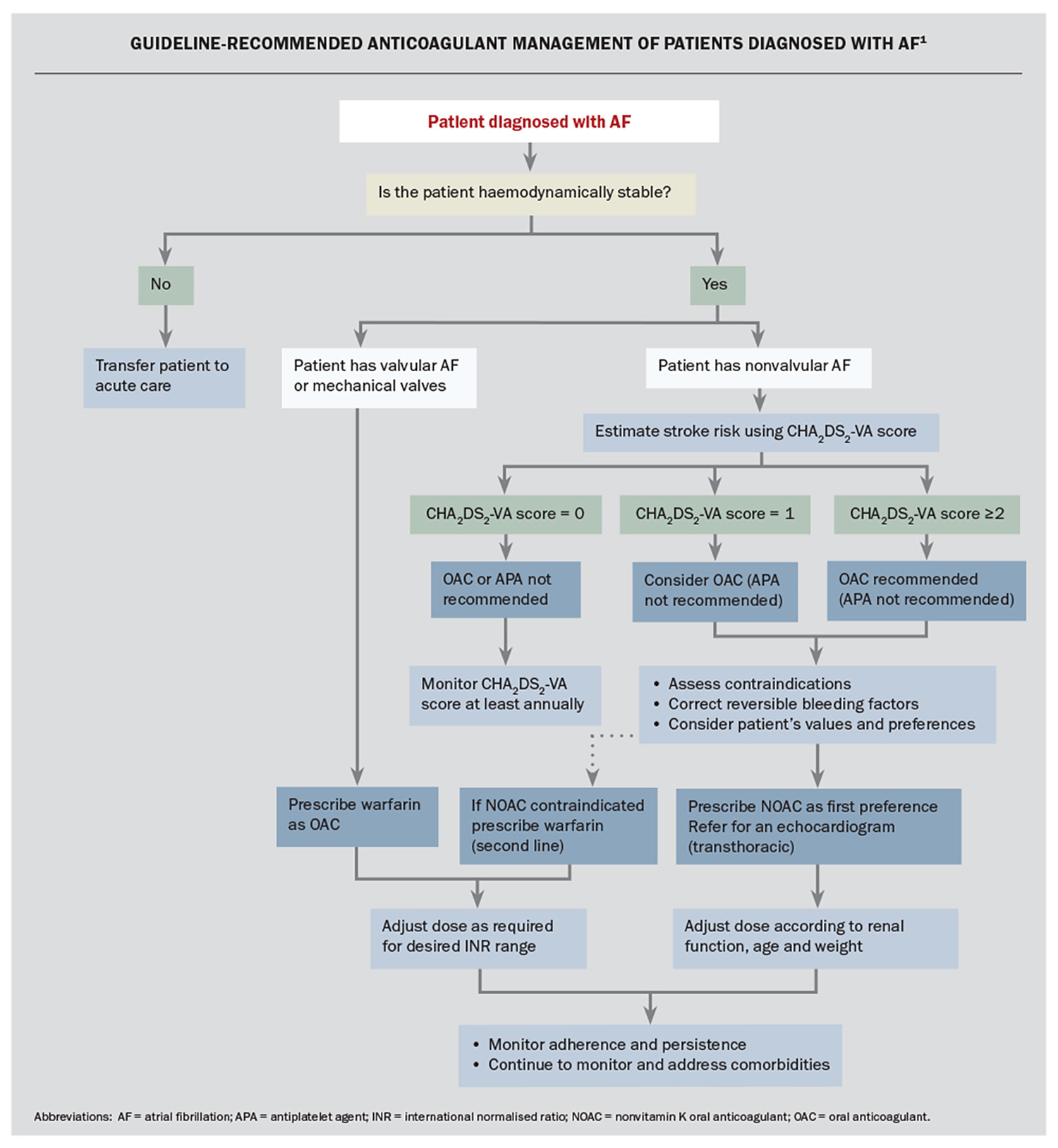
The Flowchart summarises the current guidelines for anticoagulation management of patients diagnosed with AF.1 OAC therapy to prevent stroke is not recommended in patients whose CHA2DS2-VA score is 0, but should be considered in patients with a score of 1 and is recommended for those with a score 2 or above unless there are contraindications to anticoagulation.1 We note that all patients aged 75 years or older and most aged over 65 years (>90%) will have a CHA2DS2-VA score of 2 or more.10
After assessment and modification of bleeding risk factors (see below), and discussion with the patient about management options, OAC therapy should be commenced by the GP. Specialist review is not needed to initiate this treatment, and timely commencement of an OAC by the GP may avoid a stroke, which can occur soon after AF diagnosis.
Concerns about bleeding are the main barrier for prescribing an OAC.11,12 However, the net clinical benefit almost always favours stroke prevention over major bleeding, so bleeding risk scores should not be used to avoid anticoagulation in patients with AF.1 The recent Australian guidelines recommend that reversible bleeding factors should be identified and corrected in patients for whom anticoagulation is indicated.1 Bleeding risk can be estimated by various clinical scores such as HEMORR2HAGES and HAS-BLED. Higher scores might alert the clinician to a greater need to attend to any modifiable bleeding risk factors. The risk associated with recurrent falls may be reduced by implementing a falls prevention program, and a more detailed discussion may be needed with patients regarding the risks associated with commencing or not commencing anticoagulation.
Warfarin: previously the mainstay for stroke risk reduction
Warfarin is a vitamin K antagonist and has been an often-used medication by GPs to treat or prevent thrombosis in a range of clinical settings.13 When initiated among high-risk patients with AF, warfarin reduces stroke risk by 64%.14 However, it is also associated with higher risks for intracranial haemorrhage (ICH) compared with NOACs and aspirin; a meta-analysis of more than 20 trials involving about 140,000 patients showed that warfarin was associated with higher ICH rates compared with aspirin and all NOACs.15 Other studies have shown comparable rates of major gastrointestinal bleeds among patients taking either warfarin or a NOAC.16
Warfarin is a notoriously difficult medication to prescribe safely, due to the large variation around the dose needed to achieve the target international normalised ratio (INR) and the narrow therapeutic range. Various factors can impact on the daily dose required (including food and other medications) and it takes at least five to seven days for the full anticoagulant effect to be reached. Warfarin is contraindicated if patients have a tendency to haemorrhage (inherited or otherwise), severe liver disease (including oesophageal varices) or aneurysms. It is not advised if the patient is unwilling or unable to comply with regular monitoring due to cognitive impairment, alcoholism, psychosis or problems with accessing services.
Prescribing of warfarin for high-risk patients with AF has been supplanted over the past decade in Australia by NOACs, with NOACs now being the preferred OAC for stroke prevention in nonvalvular AF.3 This article pertains to patients with nonvalvular AF. Nonvalvular AF is AF that occurs without concomitant valvular disease, whereas valvular AF is AF that occurs in patients with mechanical heart valves and rheumatic mitral valve disease (primary mitral stenosis). Current Australian guidelines have different stroke risk reduction recommendations for patients with valvular AF, as valvular AF is already high risk for thromboembolism and affected patients will need an OAC without the need to do a CHA2DS2-VA score. Because mechanical valves have a high thrombogenic potential, it is likely that is the reason why vitamin K antagonists like warfarin are preferred. In clinical trials warfarin was superior for stroke risk reduction to NOAC in prosthetic valve AF; therefore, warfarin is recommended in this specific subgroup of patients and not NOACs.17
NOACs: first-line anticoagulants for nonvalvular AF
NOACs are direct-acting medications that are selective for one specific coagulation factor: thrombin (IIa) or activated factor X (Xa). The following NOACs are currently available in Australia: dabigatran (a direct inhibitor of factor IIa), rivaroxaban and apixaban (direct inhibitors of factor Xa).
There are strong data to show that NOACs are at least as good as, or better than, warfarin in reducing stroke and that bleeding rates are less than or similar to that of warfarin. Anticoagulation with NOACs reduces the risk of stroke by about 70% and mortality by about 30%.18 ICH is significantly reduced with all NOACs compared with warfarin, and the incidence of major gastrointestinal bleeds is similar for warfarin and NOACs.16 NOACs offer some advantages in clinical practice because they do not involve multiple food and drug interactions, or the need for frequent monitoring.
Full anticoagulation is achieved within one to two hours of dosing for all NOACs due to their rapid onset. Consequently, they also have a rapid offset of action, so that within 24 hours of taking the last dose, minimal anticoagulant effect remains. Therefore, missed doses are problematic and could lead to strokes. This is less of an issue with warfarin, which has a much longer half-life.
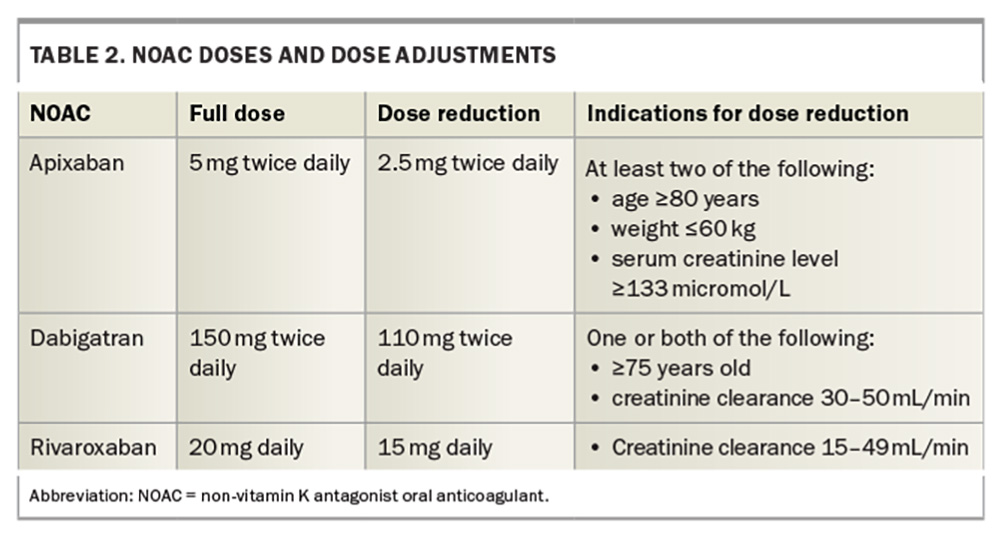
The NOACs are all renally excreted therefore may not be suitable for patients with stage 4 or 5 kidney disease. Dosage adjustments should be made for patients with milder renal dysfunction, and adjustments for age and weight are also indicated for some NOACs (Table 2). Once-daily dosing is also available for rivaroxaban, which may lead to better adherence and compliance for some patients.
Antiplatelet agents: ineffective for stroke risk reduction and high bleeding risks
Antiplatelet agents (APAs) are associated with high bleeding risks, with little or no reward for stroke risk reduction in patients with AF. Guidelines for managing patients with AF no longer recommend APAs, including aspirin, for reducing stroke risk. The accumulated evidence for stroke prevention with aspirin is weak, and, in terms of bleeding risk, it is not as benign as once thought, with data suggesting that bleeding rates are similar to those of some NOACs.1 Multiple randomised clinical trials now provide convincing evidence that oral anticoagulants, such as warfarin or NOACs, are markedly superior to aspirin in preventing stroke in those patients who are at moderate to high risk.1 Aspirin reduces stroke risk by only about 30% compared with no therapy in high-risk patients, but increases the risk of major bleeding by over 50%.19
A recent study using Australian general practice data showed the rates of APA monotherapy use for high-risk patients with AF had halved over the past decade in Australia from about 30% to 15%.20 However, about 15% of high-risk patients still remain on an APA, which offers little in terms of stroke risk reduction and increases the risk of major bleeding. Similar trends of declining APA use have been seen in the UK and Europe.21,22
Anticoagulation: lifelong therapy for high-risk patients
Anticoagulation treatment is lifelong for high-risk patients. The effectiveness of the treatment also relies on patients taking the medication as prescribed (adherence) and continuing the treatment long term (persistence). The consequence of poor persistence and adherence is stroke. Once prescribed, a large proportion of patients fill their initial anticoagulation prescription, but persistence declines over time, with about only half of patients still taking their prescribed therapy by two years.23 Discontinuation often seems to occur quite early after initial prescription. US data (on 16,253 individuals) have shown the mean time to discontinuation occurred at about 3.7 months, and after two-years’ follow up persistence with warfarin therapy was only 49%.24
Adherence rates are also low. Another US study found that at 12 months adherence to anticoagulant treatment among patients with AF was 48% using prescription refill data and only 37% when measured using patient-reported adherence.25 Several factors have been associated with poorer persistence and adherence to anticoagulants, such as younger age, lower health or medication literacy, busy lifestyles, no previous background of stroke or transient ischaemic attacks and fewer comorbidities.26
It was anticipated that the availability of NOACs would result in better adherence and persistence, in part due to the reduced need for monitoring, INR testing and dietary restrictions. Unfortunately, although many studies have reported higher persistence rates for NOACs, some recent large studies and meta-analyses have shown no significant differences in persistence rates between warfarin and NOACs.26
Strategies to improve OAC therapy adherence and persistence
GPs have a pivotal role in assessing and maximising medication adherence and persistence in their patients. A good GP–patient relationship and communication is key to improved patient adherence and persistence. Patients want to be involved in the decision-making process and wish to feel reassured about the diagnosis, comprehend their condition sufficiently and understand the possible side effects of OACs.26
At each follow-up visit, the GP has the opportunity to assess patient knowledge of AF, anticoagulant risk–benefit and bleeding risk and check on the patient’s current priorities and concerns and how treatment may be impacting on their quality of life. It is important to determine any barriers for taking medications, including the patient’s cognition and memory, and understand the facilitators and assistance available to them, such as family or carer support. Establishing an appropriate medication routine or system is also important, as this can improve both adherence and persistence.26 Stroke is a continuing penalty of nonpersistence.27
The critical role of monitoring OAC persistence has recently been highlighted by the European Heart Rhythm Association (EHRA).28 EHRA’s practical guide for OACs in AF advocates for three-monthly follow up to review persistence. The guide recommends multiple strategies to achieve optimal adherence and persistence, as shown in the Box.28
The Australian AF management guidelines also recommend similar strategies and note that specific attention should be paid to patient persistence.1 Accessing the patient My Health Record medications portal can provide the GP some insights into when the last script for an OAC was dispensed, and the dispensing interval between scripts, which may be a crude but valuable indicator of patient compliance and adherence.
Addressing comorbidities, such as hypertension, diabetes, heart failure, valvular heart disease, alcohol excess, obesity and physical activity, are also recommended in the Australian guidelines.1
When is referral to a cardiologist warranted?
Most patients with asymptomatic AF can be managed by a GP, and referral is not required. However, input from a cardiologist may be needed:
- if there is underlying structural heart disease, are contraindications to OACs, are other cardiovascular comorbidities, are difficulties achieving adequate rate control, is a high symptom burden
- to perform an echocardiogram
- if advice or input is needed on the choice of rate versus rhythm control, which may include antiarrhythmic medications, direct current cardioversion and consideration of AF ablation.
Conclusion
AF is a common condition that is safely managed by GPs. Strokes can be effectively prevented when oral anticoagulation is prescribed and monitored. A patient’s stroke risk should be calculated when diagnosed with AF and reassessed at least annually thereafter. High-risk patients (CHA2DS2-VA ≥2) should be immediately commenced on an OAC, and an OAC should be considered for those at intermediate risk (CHA2DS2-VA = 1).
NOACs are recommended as first-line treatment, unless contraindicated. Aspirin and other APAs do not effectively reduce stroke risk due to AF and are no longer recommended for anticoagulation in patients with AF. Stroke risk is higher than bleeding risk; therefore, bleeding risk should not over-ride the decision to anticoagulate. However, bleeding risk factors should be reviewed and actively addressed.
Once diagnosed, AF is a lifelong condition and GPs have an important role in reviewing and maintaining patients’ adherence and persistence to OAC management. MT
COMPETING INTERESTS: Dr Giskes has received honoraria from Bristol Myers Squibb and Pfizer for presenting lectures. Dr Lowres: None. Professor Hespe: None. Professor Freedman has served as a speaker for Omron and an advisory board member for Bristol-Myers Squibb-Pfizer Alliance, and his institution has received a NSW Health research grant.
References
1. Brieger D, Amerena J, Attia JR, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Med J Aust 2018; 209: 356-362.
2. Diouf I, Magliano DJ, Carrington MJ, Stewart S, Shaw JE. Prevalence, incidence, risk factors and treatment of atrial fibrillation in Australia: The Australian Diabetes, Obesity and Lifestyle (AusDiab) longitudinal, population cohort study. Int J Cardiol 2016; 205: 127-132.
3. Bezabhe WM, Bereznicki LR, Radford J, et al. Ten-year trends in the use of oral anticoagulants in Australian general practice patients with atrial fibrillation. Front Pharmacol 2021; 12: 586370.
4. Royal Australian College of General Practitioners. General Practice: Health of the Nation 2023. East Melbourne: RACGP; 2023.
5. Giskes KH, Hespe C, Freedman B. Screening for atrial fibrillation: the essential role of GPs. Med Today 2019; 20(11): 10-16.
6. Friberg L, Rosenqvist M, Lindgren A, Terent A, Norrving B, Asplund K. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke 2014; 45: 2599-2605.
7. Freedman B. Major progress in anticoagulant uptake for atrial fibrillation at last: does it translate into stroke prevention? Eur Heart J 2018; 39: 2984-2986.
8. Royal Australian College of General Practitioners. Guidelines for Preventive Activities in General Practice. 9th ed, updated. East Melbourne: RACGP; 2018.
9. Renoux C, Coulombe J, Suissa S. Revisiting sex differences in outcomes in non-valvular atrial fibrillation: a population-based cohort study. Eur Heart J 2017; 38: 1473-1479.
10. Orchard JJ, Giskes K, Orchard JW, et al. In a large primary care data set, the CHA2DS2-VASc score leads to an almost universal recommendation for anticoagulation treatment in those aged >/=65 years with atrial fibrillation. Eur J Cardiovasc Nurs 2023; 22: 769-772.
11. Breen AM, Burton-Houle T, Aron DC. Applying the theory of constraints in health care: Part 1--The philosophy. Qual Manag Health Care 2002; 10: 40-46.
12. Kjerpeseth LJ, Ellekjaer H, Selmer R, Ariansen I, Furu K, Skovlund E. Risk factors for stroke and choice of oral anticoagulant in atrial fibrillation. Eur J Clin Pharmacol 2018; 74: 1653-1662.
13. Tadros R, Shakib, S. Warfarin indications, risks and drug interactions. Aust Fam Physician 2010; 39: 476-479.
14. Xian Y, Wu J, O’Brien EC, Fonarow GC, et al. Real world effectiveness of warfarin among ischemic stroke patients with atrial fibrillation: observational analysis from Patient-Centered Research into Outcomes Stroke Patients Prefer and Effectiveness Research (PROSPER) study. BMJ 2015; 351: h3786. doi: 10.1136/bmj.h3786.
15. Ma T, Liu C, Jiang T, Qin H, Wu R, Zhou P. Comparative risk for intracranial hemorrhage related to new oral anticoagulants: A network meta-analysis. Medicine (Baltimore) 2021; 12: e24522.
16. Saviano A, Brigda, M, Petruzziello C, Candelli M, Gabrielli M, Ojetti V. Gastrointestinal bleeding due to NOACs use: exploring the molecular mechanisms. Int J Mol Sci 2022; 22: 13955.
17. Connolly SJ, Karthikeyan G, Ntsekhe M, et al, for the INVICTUS Investigators. Rivaroxaban in rheumatic heart disease-associated atrial fibrillation. N Engl J Med 2022; 387: 978-988.
18. Shi M, Liu L, Wafa H, Curcin V, Wang Y. Effectiveness and safety of non-vitamin K oral anticoagulants versus warfarin in patients with atrial fibrillation and previous stroke: A systematic review and meta-analysis. Neuroepidemiol 2023; 58: 1-14.
19. Freedman BS, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36: 653-656.
20. Giskes K, Lowres N, Orchard J, Hyun K, Hespe C, Freedman B. Time trends in stroke risk management among high-risk patients with non-valvular atrial fibrillation in Australia between 2011-2019. Int J Cardiol Heart Vasc 2024; 55: 101443.
21. Apenteng PN, Gao H, Hobbs FR, Fitzmaurice DA, UK GARFIELD-AF Investigators and GARFIELD-AF Steering Committee. Temporal trends in antithrombotic treatment of real-world UK patients with newly diagnosed atrial fibrillation: findings from the GARFIELD-AF registry. BMJ Open 2018; 8(1): e018905.
22. Maura G, Billionnet C, Drouin J, Weill A, Neumann A, Pariente A. Oral anticoagulation therapy use in patients with atrial fibrillation after the introduction of non-vitamin K antagonist oral anticoagulants: findings from the French healthcare databases, 2011-2016. BMJ Open 2019; 9(4): e026645.
23. Johnson ME, Lefevre C, Collings SL, et al. Early real-world evidence of persistence on oral anticoagulants for stroke prevention in non-valvular atrial fibrillation: a cohort study in UK primary care. BMJ Open 2016; 6(9): e011471.
24. Deitelzweig SB, Buysman E, Pinsky B, et al. Warfarin use and stroke risk among patients with nonvalvular atrial fibrillation in a large managed care population. Clin Ther 2013; 35: 1201-1210.
25. Stephenson JJ, Shinde MU, Kwong WJ, Fu AC, Tan H, Weintraub WS. Comparison of claims vs patient-reported adherence measures and associated outcomes among patients with nonvalvular atrial fibrillation using oral anticoagulant therapy. Patient Prefer Adherence 2018; 12: 105-117.
26. Lowres N, Giskes K, Hespe C, Freedman B. Reducing stroke risk in atrial fibrillation: adherence to guidelines has improved, but patient persistence with anticoagulant therapy remains suboptimal. Korean Circ J 2019; 49: 883-907.
27. Martinez C, Wallenhorst C, Rietbrock S, Freedman B. Ischemic stroke and transient ischemic attack risk following vitamin K antagonist cessation in newly diagnosed atrial fibrillation: a cohort study. J Am Heart Assoc 2020; 9(2): e014376.
28. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace 2018; 20: 1231-1242.