A new paradigm of PBS-based diabetes care: what is the first-line therapy for type 2 diabetes in 2025?

For most of the 20th century, type 2 diabetes treatment focused on glycaemic control using insulin, metformin and sulfonylureas. Sodium-glucose cotransporter-2 inhibitors are now the preferred option because of their cardiovascular and renal benefits. Recent PBS updates allow their use in combination with metformin as first-line therapy for patients at high cardiovascular risk, with established cardiovascular disease or of Aboriginal or Torres Strait Islander origin, regardless of glycated haemoglobin levels. This represents a significant shift in diabetes care, broadening treatment access for many patients.
- There have been three changes to the PBS for the treatment of type 2 diabetes in less than a year.
- Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are now prominently emphasised for earlier use in the course of type 2 diabetes, over dipeptidase-4 inhibitors and glucagon-like peptide receptor agonists.
- The lastest PBS change allows SGLT-2 inhibitors to be used as first-line therapy (in combination with metformin) in people with established cardiovascular disease, at high risk of a cardiovascular event or of Aboriginal or Torres Strait Islander origin, regardless of glycated haemoglobin levels.
- These changes have come about because of overwhelming evidence of the benefit of the SGLT-2 inhibitor class on cardiovascular disease, heart failure and renal disease.
- The PBS changes are in line with key overseas guidelines. Local guidelines will need to be adapted in the near future.
For the vast majority of the 20th century, the care of type 2 diabetes did not change: the aim was for reasonable glycaemic control. Although what constituted ‘reasonable’ may have changed over time, what remained constant was the triad upon which we depended to achieve this: insulin, metformin and sulfonylureas. Around the turn of the century, an entire panoply of options was introduced in a seeming avalanche of novel options that perplexed clinicians and regulatory bodies alike. Alpha-glucosidase inhibitors, novel insulin analogues, thiazolodinediones, insulin secretagogues, amylin analogues, dipeptidase-4 (DPP-4) inhibitors, glucagon-like peptide receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 (SGLT-2) inhibitors were all added to the armamentarium in very rapid succession.
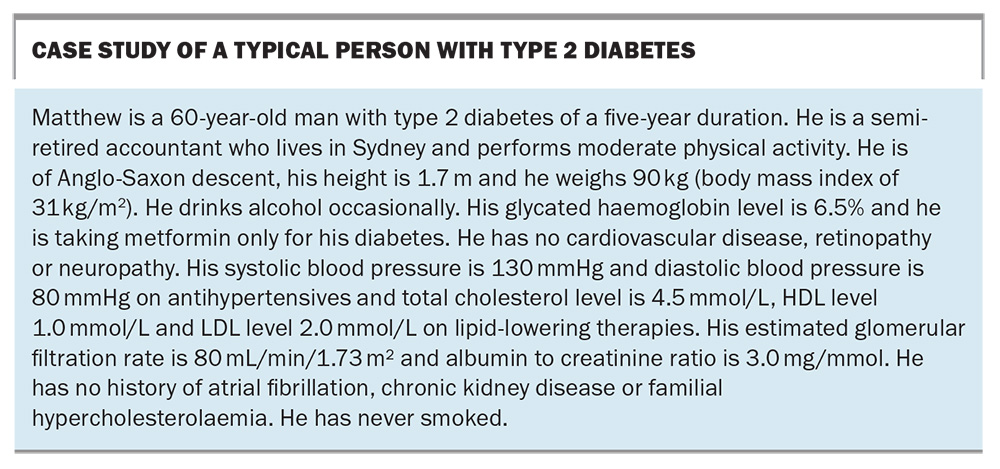
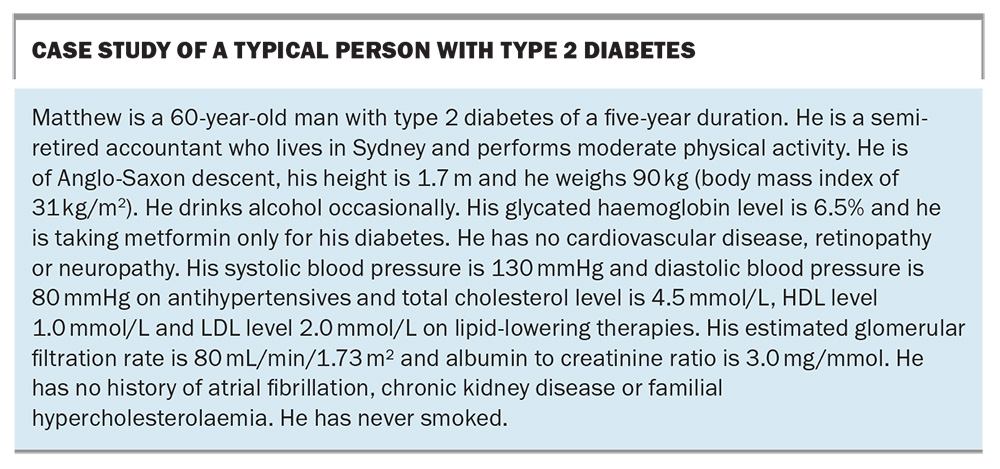
We are, of course, well into the 21st century, and the dust is only just beginning to settle on this flurry of new treatments. There is very little doubt that one of the most impactful classes of medications to have been introduced are the SGLT-2 inhibitors. Clinical, regulatory and PBS guidelines have changed rapidly over time to reflect the increasing body of evidence around their use. To understand the impact of recent changes, it is helpful to consider a case study of a typical person with type 2 diabetes (see Box). At first glance, most clinicians would view Matthew as having well-controlled diabetes. His glycated haemoglobin (HbA1c) is 6.5% and he has little in the way of complications, and no cardiovascular (CV) history. There seems to be little reason to add or change his diabetes treatments at this point. Indeed, even if we wanted to, the prior PBS listings (which have existed in a fairly static form for the past 10 years) would not have allowed us from using some of the newer classes of medications, such as DPP-4 inhibitors, GLP-1RAs or SGLT-2 inhibitors, unless the HbA1c was over 7.0%. For all intents and purposes, the PBS was also telling clinicians that Matthew was ‘well-controlled’ from a diabetes perspective.
However, this is not taking into consideration Matthew’s overall CV risk. It would be easy to assume that Matthew is at fairly low, or perhaps medium, CV risk: his diabetes and blood pressure are very well controlled, he has never smoked, he is not in the high-risk category for chronic kidney disease (CKD), and he lives in a major city. And yet, using the latest Australian cardiovascular disease risk calculator (available online at www.cvdcheck.org.au),1 his clinical features actually yield an 11% risk of a CV event in the next five years, which puts him in the high-risk category. Thus, although Matthew’s HbA1c may be below 7%, there is more that can be done to lower his CV risk. This has been facilitated by changes to the PBS.
What has changed on the PBS in the past year?
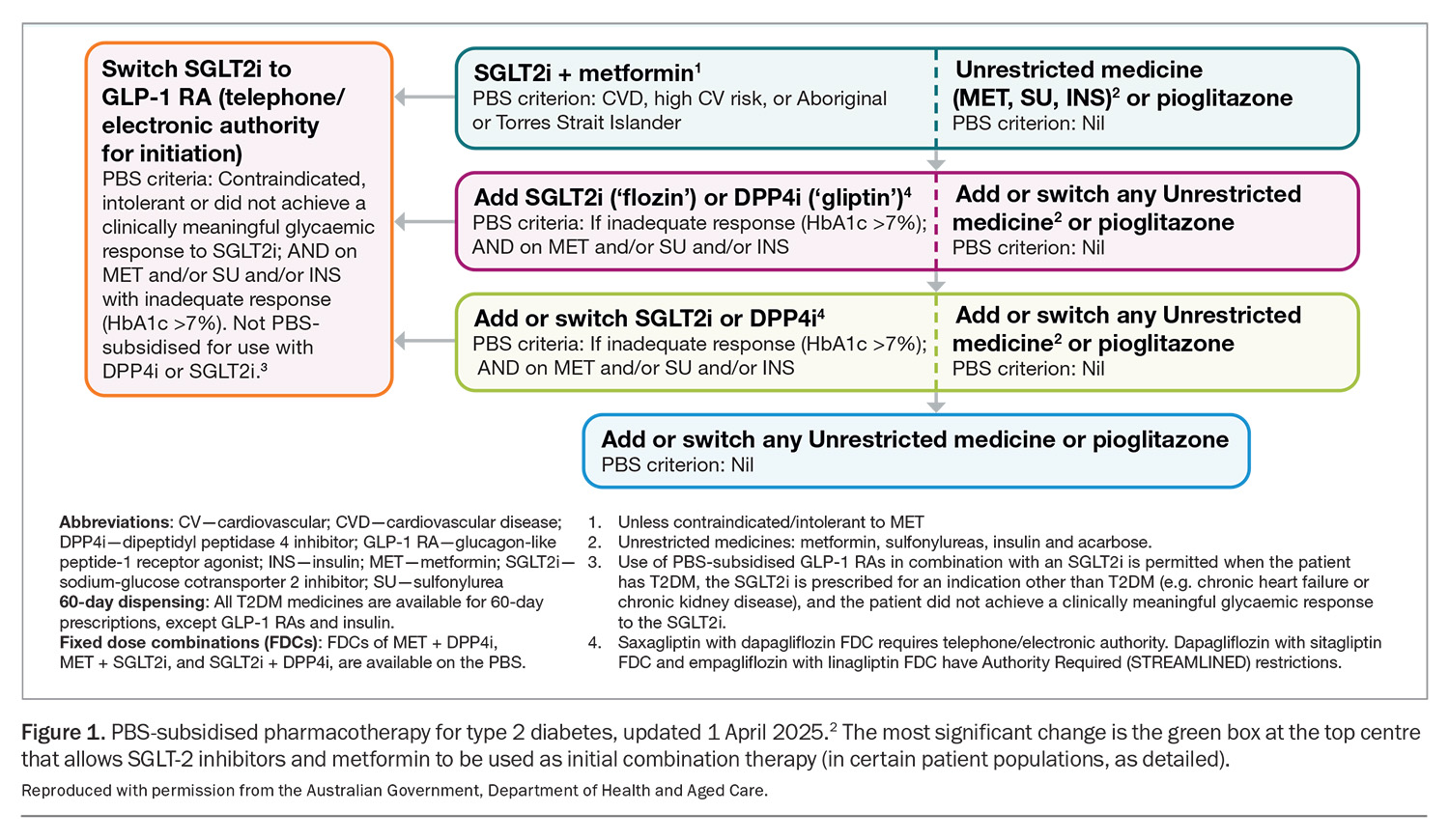
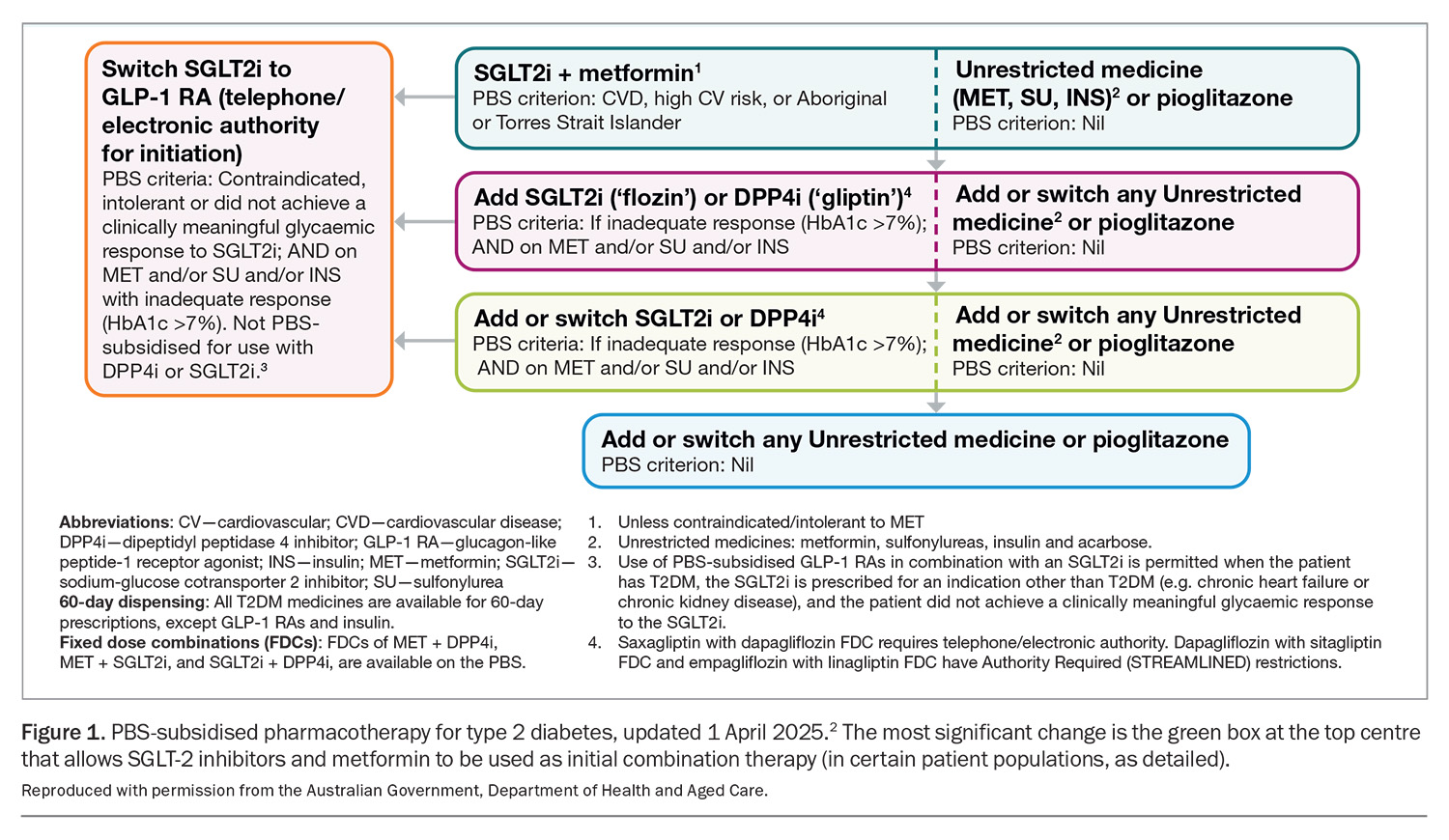
After a decade of stability, the PBS has changed no less than three times in the recent past in regards to the treatment of type 2 diabetes: June 2024, December 2024 and most recently on 1 April 2025.2 Although the June 2024 changes were covered in an article published in Endocrinology Today in August 2024,3 the April 2025 changes are significant enough to require another review. The latest change allows the use of any SGLT-2 inhibitor as initial combination therapy with metformin, provided the patient is either at high CV risk (defined as ≥10% risk of event in the next five years), has established CV disease or is of Aboriginal or Torres Strait Islander origin (Figure 1).
This change highlights two important philosophical shifts. Firstly, metformin is now no longer the ‘default’ initial choice, and the combination of SGLT-2 inhibitor and metformin can be first-line therapy in the right patient. Secondly, there is no longer an HbA1c restriction attached to this option. Previously, the HbA1c level had to be more than 7.0% after the initiation of metformin before an SGLT-2 inhibitor could be prescribed on the PBS. This latter point means that the PBS has actually simplified things in removing one of the key considerations (the HbA1c criteria) in first prescribing an SGLT-2 inhibitor in individuals with type 2 diabetes. Of course, all the old PBS listings for SGLT-2 inhibitors remain in place, namely the ability to prescribe in people with uncontrolled diabetes, heart failure (HF) and CKD.
The GLP-1RAs are now not available on the PBS until an SGLT-2 inhibitor has been tried first and the patient has been shown to be intolerant of, or did not achieve a clinically meaningful glycaemic response to, an SGLT-2 inhibitor (Figure 1).
Consistency with guidelines
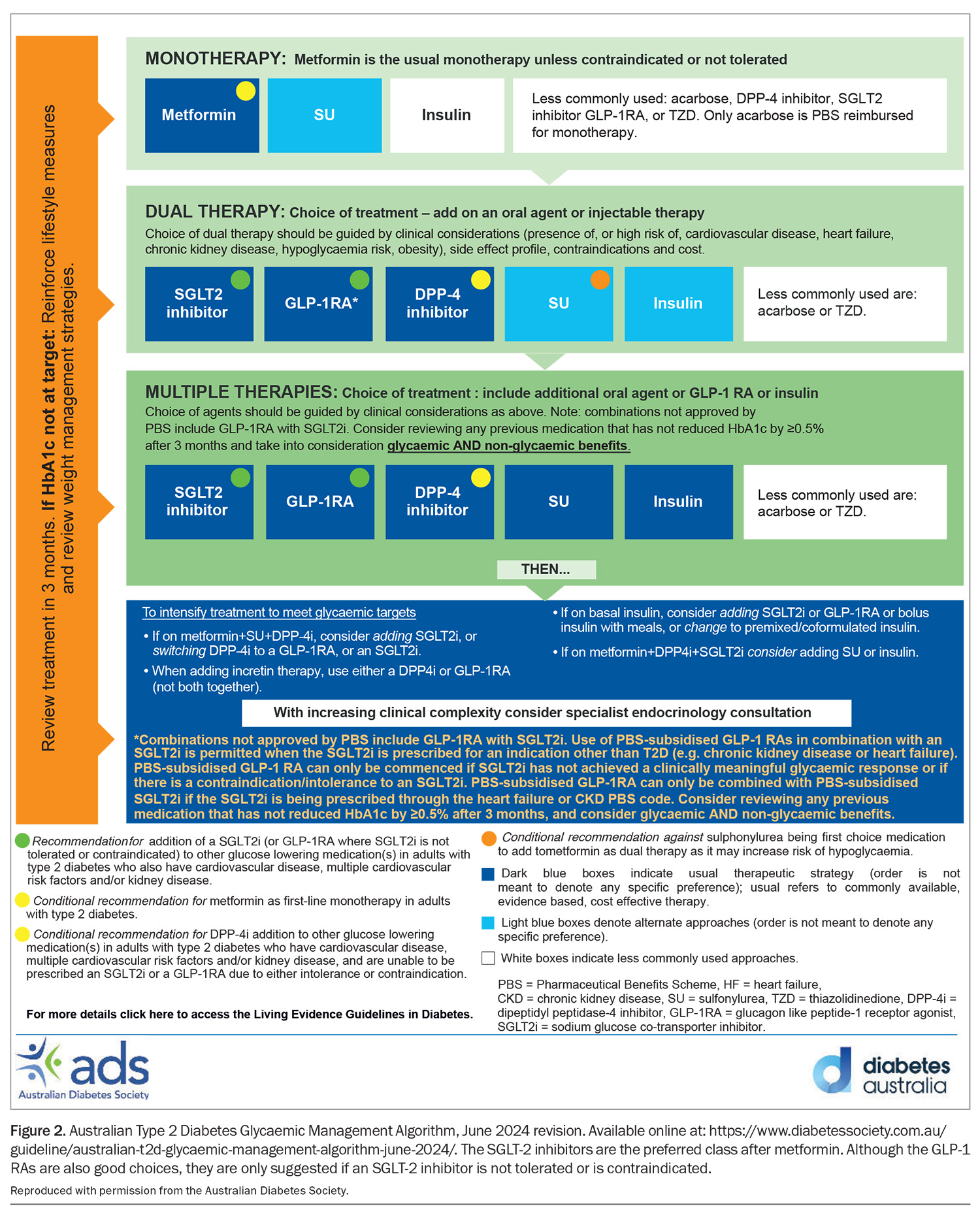
This update to the PBS aligns it with the major national and international guidelines, although with a few minor differences. For example, the latest Royal Australian College of General Practitioner’s guidelines on Management of Type 2 Diabetes: A handbook for general practice published in June 2024 had SGLT-2 inhibitors as the usual and preferred treatment option after metformin, but with metformin still as the first line of therapy.4 Furthermore, GLP-1RAs should only be used when SGLT-2 inhibitors are not tolerated or indicated, which clearly influenced the latest revision of the PBS (Figure 2).
Similarly, the other major Australian guideline, the Living Evidence for Diabetes Consortium: Australian Evidence-Based Clinical Guidelines for Diabetes also advocates for the use of SGLT-2 inhibitors as the first option after metformin (and before GLP-1RA), especially if the patient also has CV disease, multiple CV risk factors and/or kidney disease.5 This again is very similar to the current PBS criteria.
The American Diabetes Association and European Association for the Study of Diabetes Consensus Statement on diabetes management is a widely recognised guideline globally. Its December 2024 update went one step further, no longer stating that metformin is the automatic first line of medical treatment in type 2 diabetes.6 Instead, SGLT-2 inhibitors (and GLP-1RAs) are now emphasised, particularly in patients with atherosclerotic CV disease, high CV risk, HF and/or CKD. The PBS takes a clear step in this direction, with the combination of SGLT-2 inhibitors and metformin now being the initial choice of treatment in people with type 2 diabetes and established CV disease, at high risk of a CV event, or in those of Aboriginal or Torres Strait Islander origin. Local guidelines may need to be updated soon to reflect both the PBS changes and shifts in overseas guidance.
What is the evidence for this change?
The last time diabetes medications were significantly realigned on the PBS was a little over 10 years ago, when the SGLT-2 inhibitors were introduced. In the decade since, there has been much in the way of empirical evidence that has informed the large shifts in the guidelines as well as the PBS.
It was almost 10 years ago that the landmark Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG Outcome trial) started this change in thinking.7 This CV outcome trial demonstrated for the first time that a diabetes medication (in this case empagliflozin) reduced three-point major CV events over that of standard of care and independent of HbA1c lowering. Other positive CV outcome trials for SGLT-2 inhibitors followed,8,9 and in a meta-analysis the class clearly shows a significant 10% reduction of major CV events related to atherosclerotic CV disease, which has informed the changes to the PBS.10 It should be noted that the still popular DPP-4 inhibitor class of agents do not show change in major CV outcomes, either in their individual trials or in meta-analysis. However, the EMPA-REG Outcome trial stood out not only as the first, but the only one to also show a significant reduction in CV death in patients with type 2 diabetes and established CV disease.7 This is recognised by empagliflozin’s unique TGA indication, which is for use in patients with type 2 diabetes and established CV disease to reduce the risk of CV death.11
As well as just narrowly looking at atherosclerotic CV endpoints, these CV outcome trials examined other outcomes, with HF showing particularly strong signals. Dedicated outcomes studies were then performed with the Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) trial and the Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPEROR- Reduced), proving the positive effects of both dapagliflozin and empagliflozin (respectively) in patients with reduced ejection fraction HF.12,13 Soon after, the EMPEROR-Preserved with empagliflozin trial, and Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) trial demonstrated positive outcomes in patients with HF with preserved ejection fraction (HFpEF).14,15 These were especially prominent results, as the SGLT-2 inhibitors became the first pharmacological agents to prove any benefit in HFpEF. Both SGLT-2 inhibitors thus gained indications for the treatment of HF across the entire spectrum of ejection fraction. No other medication has this indication.
Further, CKD outcome studies were undertaken. The Dapagliflozin and Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) study found that dapagliflozin reduced composite renal endpoints in people with albuminuria CKD.16 The Study of Heart and Kidney Protection With Empagliflozin (EMPA- KIDNEY) trial extended this result even more broadly by demonstrating a similar effect, but in a wider range of baseline estimated glomerular filtration rate, and also in people both with and without albuminuria.17 As these two studies were conducted in people with and without type 2 diabetes, both SGLT-2 inhibitors are now TGA indicated to reduce the risk of progressive decline in kidney function in adults with CKD (stages 2, 3 or 4) with proteinuria (defined as ≥30 mg/g).11 Additionally, empagliflozin can be used in CKD stages 3B, 4 and 5 irrespective of urine albumin to creatinine ratio.11
Lastly, it should be noted that all SGLT-2 inhibitors are contraindicated in type 1 diabetes. All of the studies detailed above specifically exclude people with type 1 diabetes, yet it is well known that SGLT-2 inhibitors increase the risk of diabetic ketoacidosis in this population. In essence, there is little evidence of benefit while there is known evidence of harm. Thus, SGLT-2 inhibitors should not be routinely prescribed in people with type 1 diabetes, even if they have atherosclerotic CV disease, HF or CKD.
Implications for Matthew and summary
The change from metformin as default first-line therapy in type 2 diabetes to the new advice of considering initial combination of SGLT-2 inhibitor and metformin is one of the biggest changes in diabetes care for decades. Although it pertains to specific populations only (people with established CV disease, at high risk of a CV event or of Aboriginal or Torres Strait Islander origin), it encompasses a surprisingly large proportion of people with type 2 diabetes.
One such person is Matthew, the person described in the case study in the Box. He appears superficially to be a fairly low-risk patient with short duration of type 2 diabetes. However, even with a relatively benign looking set of clinical indices, he is classed as a high CV risk patient. This emphasises that many people with type 2 diabetes seen routinely in primary care are actually at high CV risk and that this may not be apparent unless the risk factors are properly assessed (ideally by an appropriate risk calculator).1 Someone like Matthew would ideally have an SGLT-2 inhibitor added on top of his metformin, even though his HbA1c is below 7.0%. The new changes to the PBS now allow this. Even if he were newly diagnosed with type 2 diabetes, he would still qualify for initial combination of SGLT-2 inhibitor and metformin on the PBS. This is a paradigm shift in thinking: metformin alone is no longer the automatic sole choice as first-line therapy in type 2 diabetes.
This change to the PBS has come about from the ever-increasing mountain of evidence of the benefits from the SGLT-2 inhibitors class, from reducing atherosclerotic CV disease, CV death (for empagliflozin), HF at any ejection fraction and CKD. Although change always causes initial confusion as familiar therapeutic patterns shift, this new listing for SGLT-2 inhibitors actually make things easier for clinicians: we no longer have to take HbA1c thresholds into consideration when prescribing SGLT-2 inhibitors for our patients most at risk of CV disease. This opens up the use of this highly beneficial class of agents to a much wider proportion of our patients with type 2 diabetes. MT
COMPETING INTERESTS: Dr Wu has received grants from Novo Nordisk, Abbott, Boehringer Ingelheim (paid to hospital); payment or honoraria from Boehringer Ingelheim, Abbott, Eli Lilly, Novo Nordisk, Astra Zeneca, GSK and Bayer; and participated on Advisory boards for Boehringer Ingelheim, Abbott, Eli Lilly, GSK, Roche and Bayer.
References
1. Australian Government Department of Health and Aged Care. Australian guideline for assessing and managing cardiovascular disease risk, 2023. Canberra: Commonwealth of Australia; 2023. Available online at: https://www.cvdcheck.org.au/ (accessed May 2025).
2. Pharmaceutical Benefits Scheme, Australian Government. PBS-subsidised pharmacotherapy for T2DM. Available online at: https://m.pbs.gov.au/reviews/pbs-restriction-changes-to-type-2-diabetes-mellitus/Attachment-2-PBS-Pharmacotherapy-diagram-April-2025.PDF (accessed May 2025).
3. Dover T, Phillips L. Type 2 diabetes management – what’s new? Endocrinology Today 2024; 13: 6-13.
4. Royal Australian College of General Practitioners (RACGP). Management of Type 2 Diabetes: A handbook for general practice; RACGP, 2024.
5. The Living Evidence for Diabetes Consortium. Living Evidence Guidelines in Diabetes – Medications for blood glucose management in adults with type 2 diabetes, 2023. Available online at: https://app.magicapp.org/#/guideline/E5AbPE (accessed May 2025).
6. American Diabetes Association – Professional Practice Committee. Pharmacologic approaches to glycemic treatment: Standards of care in diabetes – 2025. Diabetes Care 2025; 48 Suppl 1: S181-S206.
7. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117-2128.
8. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644-657.
9. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347-357.
10. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021; 6: 148-158.
11. Therapeutic Goods Administration (TGA). Jardiance Australian Product Information. Available online at: https://www.tga.gov.au/sites/default/files/auspar-empagliflozin-171026-pi.pdf (accessed May 2025).
12. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995-2008.
13. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413-1424.
14. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021; 385: 1451-1461.
15. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022; 387: 1089-1098.
16. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436-1446.
17. EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023; 388: 117-127.