The dawning of a new era in RSV prevention
Respiratory syncytial virus (RSV) infection causes significant morbidity and mortality, particularly in children, Aboriginal and Torres Strait Islander Australians, older persons and immunocompromised people. After many decades of research, preventives against RSV may soon be available, in the form of long-acting monoclonal antibodies and vaccines, which will provide an opportunity to prevent severe RSV disease in infants and older persons. This marks a milestone in the battle against lower respiratory tract infections, and adds to the list of achievements in the management of other key respiratory pathogens, including vaccines for influenza, pneumococcus and COVID-19.
The problem
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract infection (LRTI) hospitalisation and is commonly associated with the clinical syndromes of bronchiolitis and pneumonia. Global estimates performed before the COVID-19 pandemic, indicated that in children aged younger than 5 years, there were over 33 million cases of LRTI caused by RSV, resulting in 3.4 million hospital admissions. Notably, 79% of these infections occurred in previously healthy children. Additionally, there is a significant mortality associated with RSV infections in young children that is almost exclusively found in low- and middle-income countries, estimated to vary between 66,000 and 190,000 deaths per year.1
In Australia, the national RSV-coded hospitalisation rate for 2006 to 2015 was 29 per 100,000 population for all ages, rising substantially in children under 5 years (418 per 100,000), with even higher rates in children younger than 6 months of age (2468 per 100,000).2 Although relatively few RSV-attributable deaths occur in children in Australia compared with those in low- and middle-income countries, deaths have been reported in children with comorbidities, in particular, cardiopulmonary and neurological diseases, or in people who are immunocompromised.3 The costs associated with RSV hospitalisations are high, with a 2018 national estimate for children younger than 5 years of age ranging from $59 million to $121 million, inclusive of the index admission and six-month all-cause readmissions.4
RSV is also one of the most common LRTIs in adults, particularly in older persons and the immunocompromised.5 In industrialised countries (2015), it was estimated that 1.5 million episodes of acute respiratory RSV illness occurred in older adults with 14.5% (214,000) resulting in hospitalisation and 14,000 deaths in hospital.6
The Australian context
In Australia, RSV-coded hospitalisations data from 2006 to 2015 suggest that older Australians (≥65 years of age) were 6.6 times more likely to be hospitalised compared with people aged 5 to 64 years, and that Aboriginal and Torres Strait Islander Australians were 3.3 times more likely to be hospitalised compared with nonindigenous Australians. Most RSV-coded hospitalisations ending in death occurred in Australian adults aged 65 years and older.2
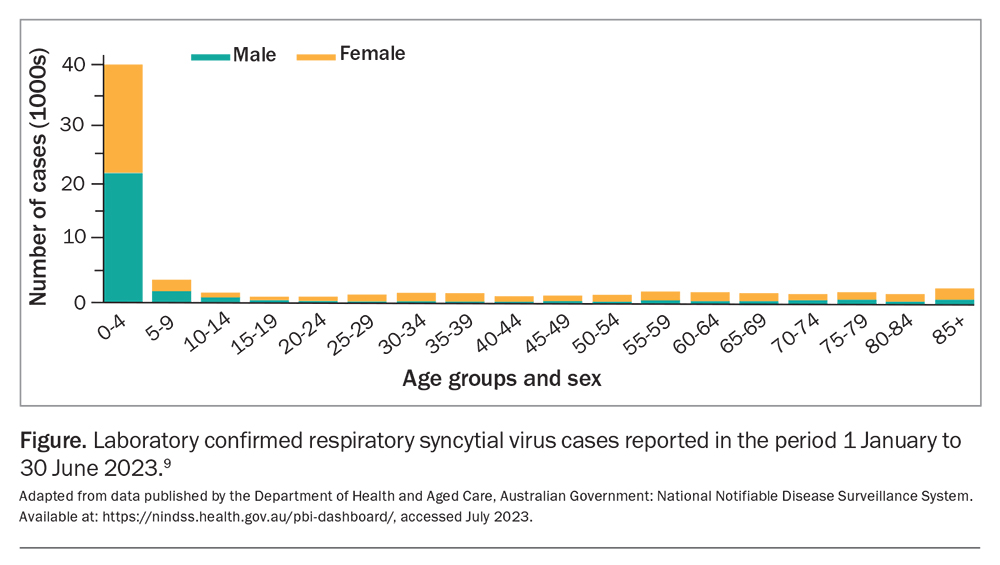
Each year, an RSV epidemic occurs in Australia, typically during autumn and winter in temperate regions, with variations in the tropical north and central Australia.7,8 From mid-2021, RSV was added to the Australian National Notifiable Diseases Surveillance System (NNDSS) and this timely capture of data will be critical in supporting the timing and targeting of future RSV prevention strategies. The establishment of syndromic clinical RSV surveillance is also needed and may be achieved through existing platforms such as The Australian Sentinel Practices Research Network, The Influenza Complications Alert Network and Paediatric Active Enhanced Disease Surveillance. The prominent disease burden in children aged 0 to 5 years is clearly reflected in laboratory confirmed cases of RSV recorded by the NNDSS database so far this year (Figure).9
Limitations in current preventives
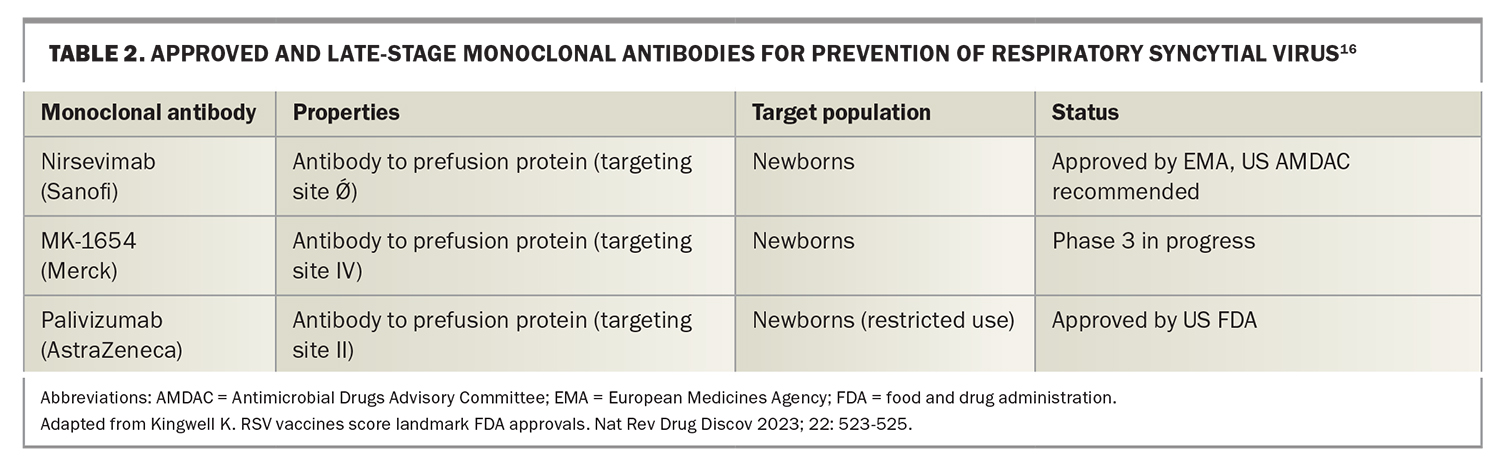
The substantial burden of RSV has long been recognised; however, preventive strategies to date have been extremely limited. The prophylactic humanised monoclonal antibody (mAb) palivizumab has been the only measure available, which is indicated for newborns at highest risk of RSV disease, such as premature infants (≤35 weeks at birth) with chronic lung disease and infants with haemodynamically significant congenital heart disease. Its high cost, coupled with the need for monthly intramuscular doses, has limited its acceptability and made its wider use prohibitive.
For other newborns, children, adults and older persons, vaccines and other preventives remain unavailable, and no treatments are available for patients with severe RSV disease. The antiviral drug ribavirin was licensed by the FDA but is no longer recommended, due to its apparent ineffectiveness.10 Clinical support measures, such as low-flow and high-flow oxygen, noninvasive ventilation, intubation, mechanical ventilation and extracorporeal membrane oxygenation, are the only treatments available for severe cases.
The slow development of RSV vaccines for children stems from failed early trials in the 1960s using formalin-inactivated whole virus vaccines, which resulted in RSV-enhanced disease in RSV-naive children with 80% hospitalisation rate and two deaths following natural infection.11
The solutions
Great advancements have been made in recent years in both mAbs and vaccines against RSV, with various products across all stages of development.12 The development of these products has been made possible largely by a better understanding of the structure and function of the RSV fusion (F) protein, as well as better understanding of immune responses to infection and the causes of RSV vaccine-enhanced disease.13 The F protein sequence is highly conserved, compared with the G protein sequence of RSV. The F protein is solely responsible for the fusion of the cell and viral membranes, which leads to the formation of syncytia (a single cell or cytoplasmic mass containing several nuclei, and also the pathogen’s namesake), and is a critical part of RSV’s infectious cycle. Neutralising antibodies to RSV are mainly targeted to the prefusion form of the F protein, not to the postfusion F form, and so solving the structure and stabilisation of the F protein in its prefusion form was critical to the development of the current RSV vaccines and mAbs.14,15
Clinical trials for upcoming vaccines
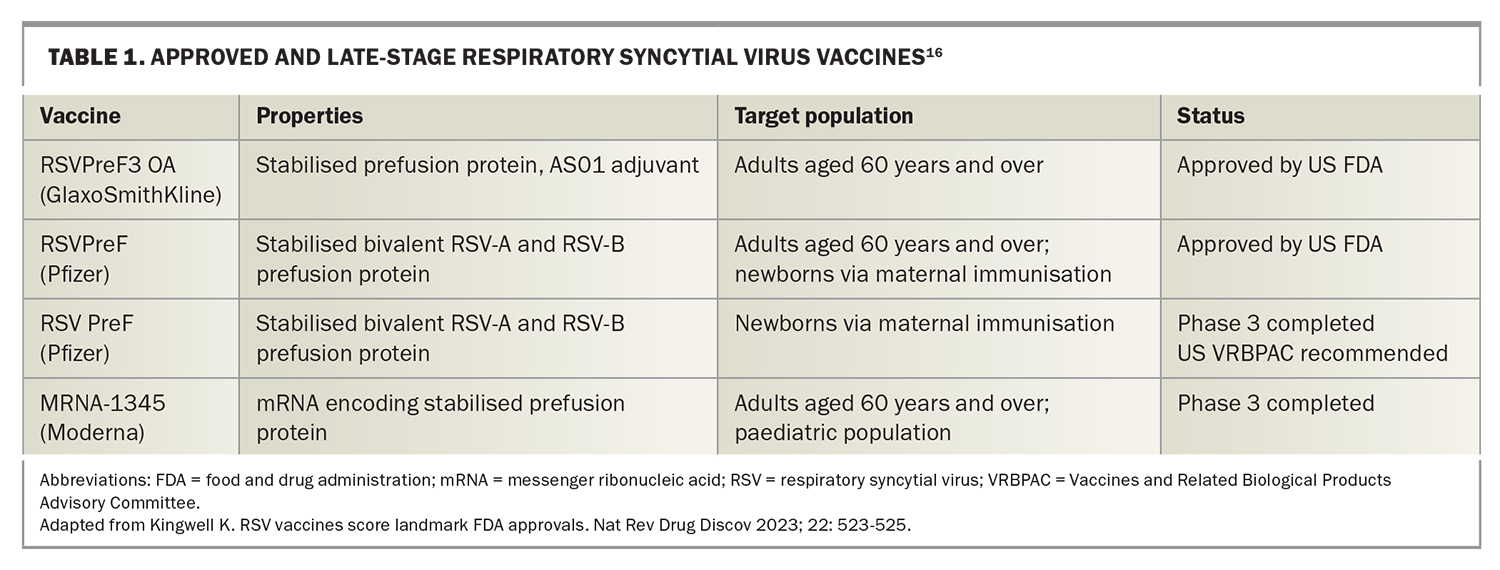
The most advanced products are RSV vaccines for older persons (≥60 years of age), three of which have had successful phase 3 trials (against both RSV-A and RSV-B viruses) and are under consideration for licensure in several countries. Both Pfizer’s and GlaxoSmithKline’s (GSK) RSV vaccines for older persons have recently been approved by the US Food and Drug Administration (FDA), and use the stabilised recombinant pre-F protein, whereas the Moderna’s RSV vaccine for older adults is based on mRNA encoding the stabilised prefusion F protein (Table 1).16-18
A trial of Pfizer’s prefusion F protein-based vaccine in older adults reported that ‘RSV-associated lower respiratory tract illness with at least two signs or symptoms occurred in 11 participants in the vaccine group (1.19 cases per 1000 person-years of observation) and 33 participants in the placebo group (3.58 cases per 1000 person-years of observation) (vaccine efficacy, 66.7%; 96.66% confidence interval [CI], 28.8 to 85.8); 2 cases (0.22 cases per 1000 person-years of observation) and 14 cases (1.52 cases per 1000 person-years of observation), respectively, occurred with at least three signs or symptoms (vaccine efficacy, 85.7%; 96.66% CI, 32.0 to 98.7).’19
GSK reported ‘efficacy against severe RSV-LRTD [lower respiratory tract disease], defined as LRTD with at least two lower respiratory signs or assessed as severe by the investigator and confirmed by the external adjudication committee, was 94.1% (95% CI, 62.4–99.9, 1 of 12,466 vs 17 of 12,494). In participants with pre-existing comorbidities, such as underlying cardiorespiratory and endocrinometabolic conditions, vaccine efficacy was 94.6% (95% CI, 65.9–99.9, 1 of 4,937 vs 18 of 4,861), with 93.8% (95% CI, 60.2–99.9, 1 of 4,487 vs 16 of 4,487) efficacy observed in adults aged 70-79 years.’20
In their interim analysis, Moderna announced that ‘the primary efficacy endpoints have been met, including vaccine efficacy (VE) of 83.7% (95.88% CI: 66.1%, 92.2%; p<0.0001) against RSV-associated lower respiratory tract disease (RSV-LRTD) as defined by two or more symptoms. Based on these results, Moderna intends to submit for regulatory approval in the first half of 2023.’21
Pfizer has also completed a phase 3 trial of its maternal RSV vaccine (using the same vaccine as for older persons), which is designed to protect newborns for the first 6 months of life from RSV infection and is awaiting FDA’s licensure. The vaccine has already been recommended by the Center for Biologics Evaluation and Research Vaccines and Related Biological Products Advisory Committee (VRBPAC).
The results of the phase 3 trial found that ‘medically attended severe lower respiratory tract illness occurred within 90 days after birth in 6 infants of women in the vaccine group and 33 infants of women in the placebo group (vaccine efficacy, 81.8%; 99.5% CI, 40.6 to 96.3); 19 cases and 62 cases, respectively, occurred within 180 days after birth (vaccine efficacy, 69.4%; 97.58% CI, 44.3 to 84.1). Medically attended RSV-associated LRTI occurred within 90 days after birth in 24 infants of women in the vaccine group and 56 infants of women in the placebo group (vaccine efficacy, 57.1%; 99.5% CI, 14.7 to 79.8).’22
Particle-based and subunit-based vaccines developed for older persons or maternal immunisation are unlikely to be suitable for children, due to the risk of vaccine-enhanced disease in RSV-naive infants. Live-attenuated, chimaeric, vectors and nucleic acid vaccines are the preferred product types for children; however, these are still in the early stages of development.12
In addition to vaccines, AstraZeneca (in conjunction with Sanofi) have developed a second generation anti-RSV human monoclonal antibody, nirsevimab, which has been approved for use in Europe and is under assessment at the FDA, following recommendations from the FDA’s Antimicrobial Drugs Advisory Committee (Table 2). This antibody is directed to the site Ǿ of the prefusion F protein and is aimed at preventing serious outcomes from RSV infections in the first 6 months of life. It has been engineered to be long-acting, requiring only a single administration to cover the entire RSV season. This should greatly reduce the cost and increase the effectiveness of nirsevimab, compared with its predecessor, palivizumab. The cost of nirsevimab has not yet been released; however, cost-benefit analyses have been presented, using figures of US$300 to US$500 (AUD$451.33 to AUD$751.83) per treatment at the US Advisory Committee on Immunization Practices meeting in February 2023. Merck also has a mAb in phase 3 clinical trials for use in newborns and infants called clesrovimab (MK-1654) that targets site IV of the prefusion F protein (Table 2).
In Sanofi’s phase 3b trial, Preventing Hospitalizations Due to Respiratory Syncytial Virus in Infants (HARMONIE), a single intramuscular dose of nirsevimab (<5 kg, 50 mg; ≥5 kg, 100 mg) reduced RSV-associated LRTI hospitalisation by 83.2% (95% CI, 67.77–92.04; p<0.001) and very severe medically-attended RSV-associated LRTI by 75.71% (95% CI, 32.75–92.91; p<0.001), with a reduction in the incidence of all-cause LRTI hospitalisations of 58.04% (95% CI, 39.69–71.19; p<0.001), during the 2022 to 2023 RSV season in France, Germany and the UK.23 The previous phase 3 trial, Monoclonal Antibody With an Extended Half-Life Against Respiratory Syncytial Virus, in Healthy Late Preterm and Term Infants (MELODY), reported similar adverse events in the nirsevimab group and the placebo group up to 360 days after injection.24
The road ahead
Some questions remain unanswered for both RSV mAbs and vaccines. For mAb immunoprophylaxis, the timing of administration, cost-benefit and effect on other newborn to childhood vaccinations will need to be determined. Vaccines for older persons and for maternal immunisation will need to consider potential rare side effects. In particular, the rates of Guillain–Barré syndrome in adult vaccinees, and preterm births for maternal immunisation will need to be closely monitored in postmarketing studies and through established vaccine safety surveillance, such as AusVaxSafety (https://ausvaxsafety.org.au/).
Both mAbs and vaccines will need to be monitored for their effectiveness and for the emergence of antibody escape variants, once these products are in wide use, through epidemiological tracking and virological means using viral gene sequencing. Other impacts that may occur with use of these products includes the potential shifting in the seasonal disease curve, impacts on recurrent wheeze and asthmatic rates and changes in the epidemiology of other respiratory pathogens.
The optimal timing for vaccine administration for older persons will need to be determined, and should consider the existing vaccine schedules and possible vaccine interactions, virus seasonality, and consumer acceptability. Maternal RSV vaccination will need to be delivered alongside other important maternal vaccinations for pertussis, tetanus, influenza and COVID-19. In older persons, timely administration alongside vaccinations for influenza, pneumococcal COVID-19 and shingles will be important.
Preliminary data from GSK indicated that coadministration of influenza and RSV vaccines was safe and effective. For older persons, the longevity of RSV vaccines will also need to be considered in determining whether annual, biennial or longer periods of effectiveness can be achieved without repeat vaccination.
Lastly, these new RSV preventative products must be factored into existing regulatory and funding models. This is of particular importance for new mAbs that lack precedent as population-level preventives and may not fit in the current immunisation regulatory framework in Australia and other countries.25
Conclusion
We are at the dawn of a new era in RSV prevention, with a range of products to prevent the most serious consequences of RSV infections in both infants and older persons, which is a notable achievement. Although these preventives are very welcome, further work is required to ensure that these products meet optimal effectiveness and safety targets in their intended populations. There is also a continuing need to develop safe and effective RSV vaccines for children, and RSV antiviral drug therapies against RSV, for patients of all ages. Practice points for GPs are presented in the Box. MT
COMPETING INTERESTS: None.
References
1. Li Y, Wang X, Blau DM, et al. Respiratory Virus Global Epidemiology Network; Nair H; RESCEU investigators. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022; 399: 2047-2064.
2. Saravanos GL, Sheel M, Homaira N, et al. Respiratory syncytial virus-associated hospitalisations in Australia, 2006-2015. Med J Aust 2019; 210: 447-453.
3. Pham H, Thompson J, Wurzel D, Duke T. Ten years of severe respiratory syncytial virus infections in a tertiary paediatric intensive care unit. J Paediatr Child Health 2020; 56: 61-67.
4. Brusco NK, Alafaci A, Tuckerman J, et al. The 2018 annual cost burden for children under five years of age hospitalised with respiratory syncytial virus in Australia. Commun Dis Intell 2022; 46.
5. Walsh EE. Respiratory syncytial virus infection: an illness for all ages. Clin Chest Med 2017; 38: 29-36.
6. Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-Analysis. J Infect Dis 2020; 222 (Suppl 7): S577-S583.
7. Dede A, Isaacs D, Torzillo PJ, et al. Respiratory syncytial virus infections in Central Australia. J Paediatr Child Health 2010; 46: 35-39.
8. Paynter S, Ware RS, Sly PD, Weinstein P, Williams G. Respiratory syncytial virus seasonality in tropical Australia. Aust N Z J Public Health 2015; 39: 8-10.
9. Department of Health and Aged Care, Australian Government: National Notifiable Disease Surveillance System (NNDSS). Available online at: https://nindss.health.gov.au/pbi-dashboard/ (accessed July 2023).
10. Ralston SL, Lieberthal AS, Meissner HC. et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2015; 136: 782.
11. Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89: 422-434.
12. McLellan JS, Chen M, Joyce MG, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013; 342: 592-598.
13. Battles MB, McLellan JS. Respiratory syncytial virus entry and how to block it. Nat Rev Microbiol 2019; 17: 233–245.
14. Ngwuta JO, Chen M, Modjarrad K, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 2015; 7: 309.
15. Walsh EE, Pérez Marc G, Zareba AM, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med 2023; 388: 1465-1477.
16. Kingwell K. RSV vaccines score landmark FDA approvals. Nat Rev Drug Discov 2023; 22: 523-525.
17. Press release: FDA approves first respiratory syncytial virus (RSV) vaccine. US Food & Drug Administration; 2023. Available online at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine/ (accessed July 2023).
18. Press release: US FDA approves Pfizer’s RSV vaccine. Reuters; 2023. Available online at: https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-approves-pfizers-rsv-vaccine-2023-05-31/ (accessed July 2023).
19. Walsh EE, Marc GP, Zareba AM, et al for the RENOIR Clinical Trial Group. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Eng J Med 2023; 388: 1465-1477.
20. Press release: GSK’s older adult respiratory syncytial virus (RSV) vaccine candidate shows 94.1% reduction in severe RSV disease and overall vaccine efficacy of 82.6% in pivotal trial. GlaxoSmithKline; 2022. Available online at: https://www.gsk.com/en-gb/media/press-releases/gsk-s-older-adult-respiratory-syncytial-virus-rsv-vaccine-candidate/ (accessed July 2023).
21. Press release: Moderna announces MRNA-1345, an investigational respiratory syncytial virus (RSV) vaccine, has met primary efficacy endpoints in phase 3 trial in older adults. Moderna; 2023. Available online at: https://investors.modernatx.com/news/news-details/2023/Moderna-Announces-mRNA-1345-an-Investigational-Respiratory-Syncytial-Virus-RSV-Vaccine-Has-Met-Primary-Efficacy-Endpoints-in-Phase-3-Trial-in-Older-Adults/default.aspx (accessed July 2023).
22. Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med 2023; 388:1451-1464.
23. Press release: nirsevimab delivers 83% reduction in RSV infant hospitalizations in a real-world clinical trial setting. Sanofi; 2023. Available online at: https://www.sanofi.com/en/media-room/press-releaes/2023/2023-05-12-08-50-00-2667568/ (accessed July 2023).
24. Muller WJ, Madhi SA, Seoane Nuñez B, et al. Nirsevimab for prevention of RSV in term and late-preterm infants. N Engl J Med 2023; 388: 1533-1534.
25. Weil-Olivier C, Salisbury D, Navarro-Alonso JA, et al. Immunization technologies: Time to consider new preventative solutions for respiratory syncytial virus infections. Hum Vaccin Immunother 2023; 19: 2209000.