Mavacamten: a novel treatment for obstructive hypertrophic cardiomyopathy
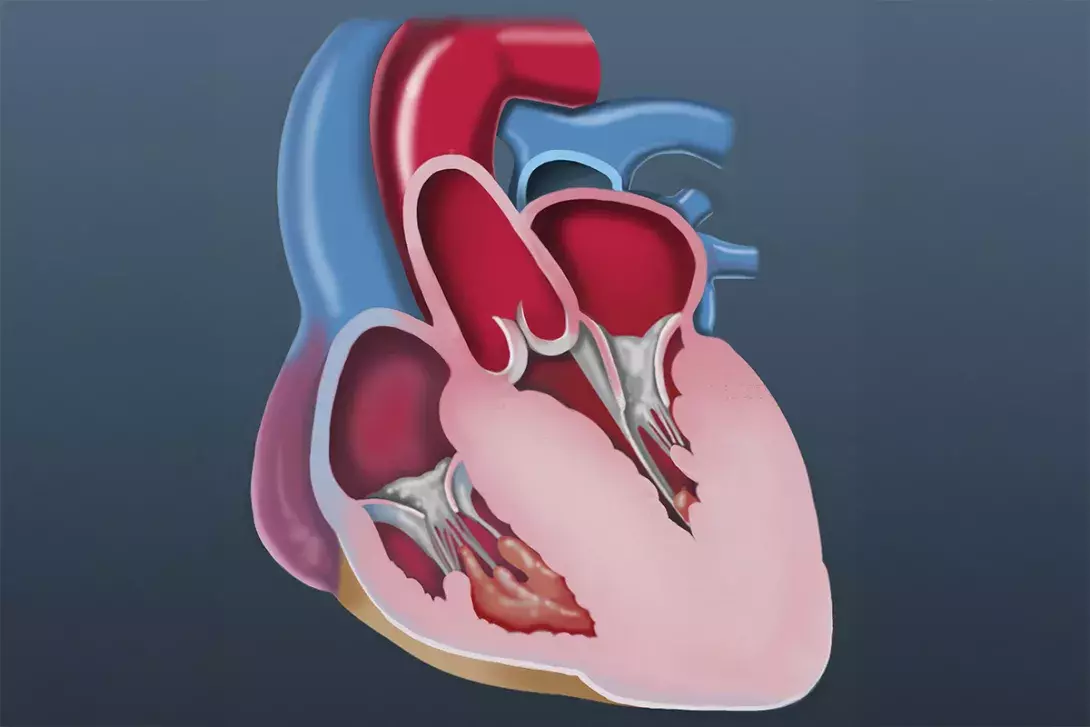
- Hypertrophic cardiomyopathy (HCM) is characterised by left ventricular (LV) hypertrophy that is not explained by associated loading conditions (e.g. hypertension or aortic stenosis) or systemic disorders.
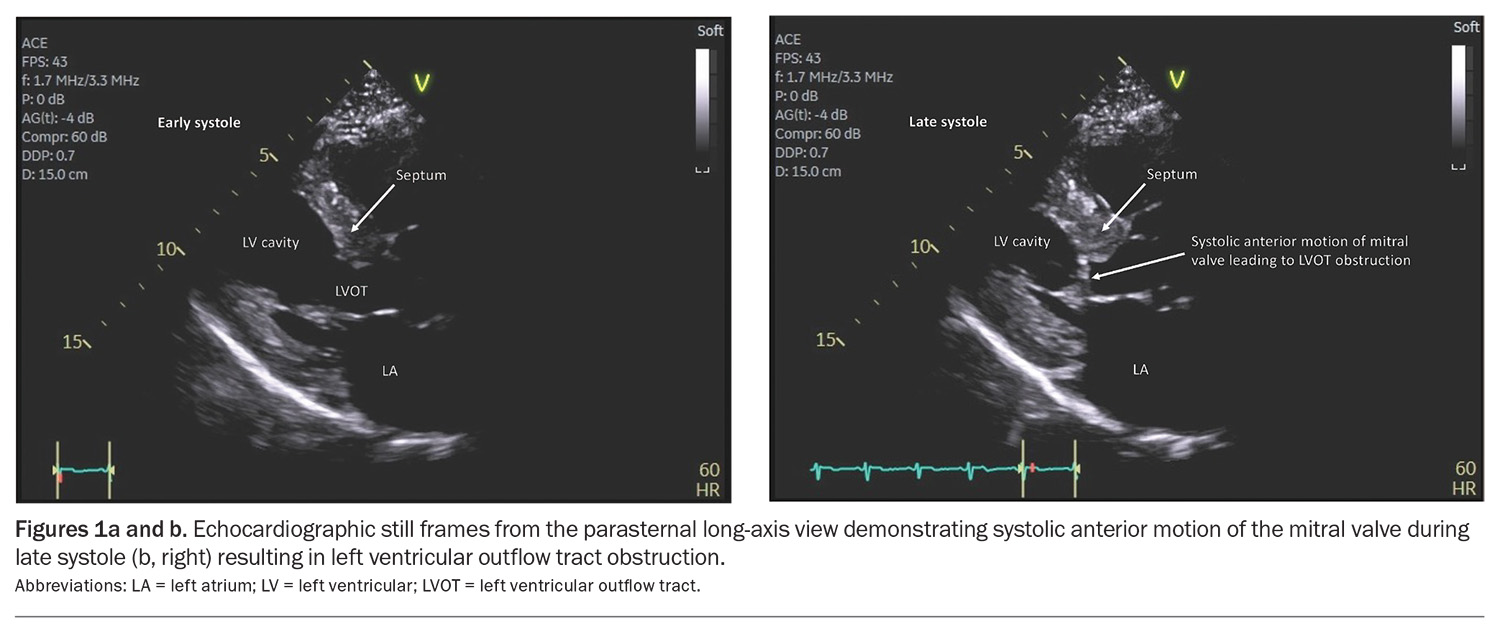
- Most patients with HCM have LV outflow tract obstruction present at rest, following Valsalva manoeuvre or with exercise.
- Mavacamten is a first-in-class, selective, allosteric and reversible cardiac myosin inhibitor that is now available in Australia as a second-line treatment for adults with New York Heart Association class II and III symptomatic obstructive HCM.
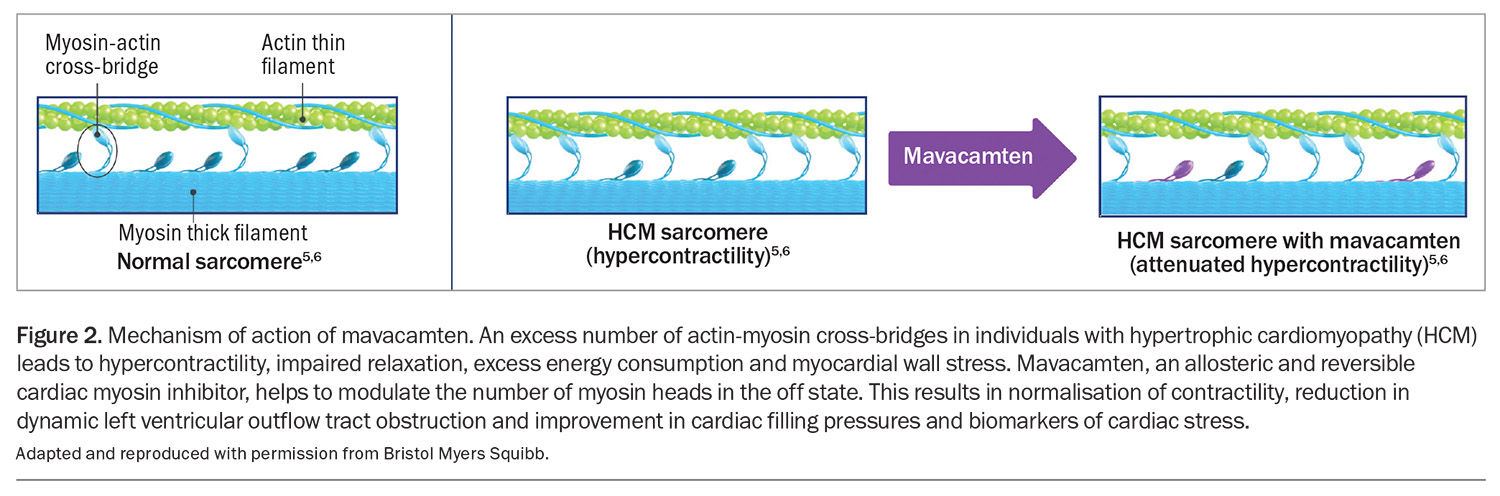
- Mavacamten modulates the number of cardiac myosin heads that can enter the power-generating state, thus reducing the probability of force-producing systolic and residual diastolic cross-bridge formation.
- Treatment with mavacamten should be initiated and supervised by a specialist cardiologist or consultant physician with experience in the management of obstructive HCM and requires regular monitoring of the patient’s symptoms, LV outflow tract gradient with Valsalva manoeuvre and LV ejection fraction using regular echocardiographic assessment.
Hypertrophic cardiomyopathy (HCM) is a primary cardiac disorder characterised by left ventricular (LV) hypertrophy (LVH) not explained by associated loading conditions (e.g. hypertension or aortic stenosis) or systemic disorders.1 Although HCM was initially considered a rare disease, more recent studies have suggested that it is not uncommon.2 Mechanistic studies suggest that LVH in HCM is a secondary phenomenon, at least partly related to excess contractility with increased actin-myosin cross-bridging.3 Most patients with HCM have LV outflow tract obstruction present at rest, following Valsalva manoeuvre or with exercise (Figures 1a and b).
Patients with symptomatic LV outflow tract obstruction are initially treated with beta blockers or nondihydropyridine calcium channel blockers, the primary goal being to improve quality of life.4 However, these treatments have limited efficacy and are often not well tolerated. Additional treatment options in patients with refractory symptoms despite these first-line treatment options include disopyramide or septal reduction therapy (either septal myectomy or alcohol septal ablation). Mavacamten is a novel therapy that is now available in Australia as a second-line treatment for adults with New York Heart Association (NYHA) class II and III symptomatic obstructive HCM.
What is mavacamten?
Mavacamten is a first-in-class, selective, allosteric and reversible cardiac myosin inhibitor. It modulates the number of cardiac myosin heads that can enter the power-generating state, thus reducing the probability of force-producing systolic and residual diastolic cross-bridge formation (Figure 2).5 Mavacamten has therefore been investigated as a therapy to reduce excess hypercontractility, which would be expected to improve symptoms, especially in patients with obstructive HCM.3 It is available in 2.5 mg, 5 mg, 10 mg and 15 mg capsules and is taken orally once daily. The capsule should be swallowed whole with water and can be taken with or without food.
What is the clinical trial evidence?
Mavacamten has been shown to improve symptoms and quality of life, and decrease the LV outflow tract gradient despite first-line treatments in patients with symptomatic, obstructive HCM in two randomised controlled trials.6,7 Furthermore, in the VALOR-HCM trial (A Study to Evaluate Mavacamten in Adults With Symptomatic Obstructive HCM Who Are Eligible for Septal Reduction Therapy), mavacamten significantly reduced eligibility for invasive septal reduction therapy.7 These clinical benefits were accompanied by reductions in biomarkers (N-terminal pro-B-type natriuretic peptide and cardiac troponin I) and significant improvements in echocardiographic indices (LV mass index, left atrial volume index and LV filling pressure [E/e’ ratio]).6-8
When and in whom is mavacamten used?
Mavacamten is for use in patients with NYHA class II and III symptomatic obstructive HCM despite treatment with beta blockers or nondihydropyridine calcium channel blockers.
The PBS criteria to commence treatment with mavacamten are outlined below.
- The patient must have a confirmed diagnosis of HCM with an end-diastolic LV wall thickness which is at least 15 mm (in the absence of a family history of HCM) or at least 13 mm (in the presence of a family history of HCM), AND
- The patient must have a confirmed peak LV outflow tract gradient of no less than 50 mmHg which is measured either: (i) at rest OR (ii) after provocation with at least one of (a) Valsalva manoeuvre or (b) exercise, AND
- The patient must have a current LV ejection fraction (LVEF) of no less than 55%, AND
- The patient must have had prior treatments with each of a (i) beta blocker and (ii) nondihydropyridine calcium channel blocker unless contraindicated (as listed in the TGA approved Product Information) or not tolerated, AND
- The patient must be undergoing concomitant treatment with at least one of (i) beta blocker, (ii) nondihydropyridine calcium channel blocker unless contraindicated (as listed in the TGA approved Product Information) or not tolerated AND
- The patient must be symptomatic (NYHA class II or III), AND
- The patient must be treated by a cardiologist, or must be treated by a consultant physician with experience in the management of HCM, AND
- The patient must be at least 18 years of age.
How is mavacamten used?
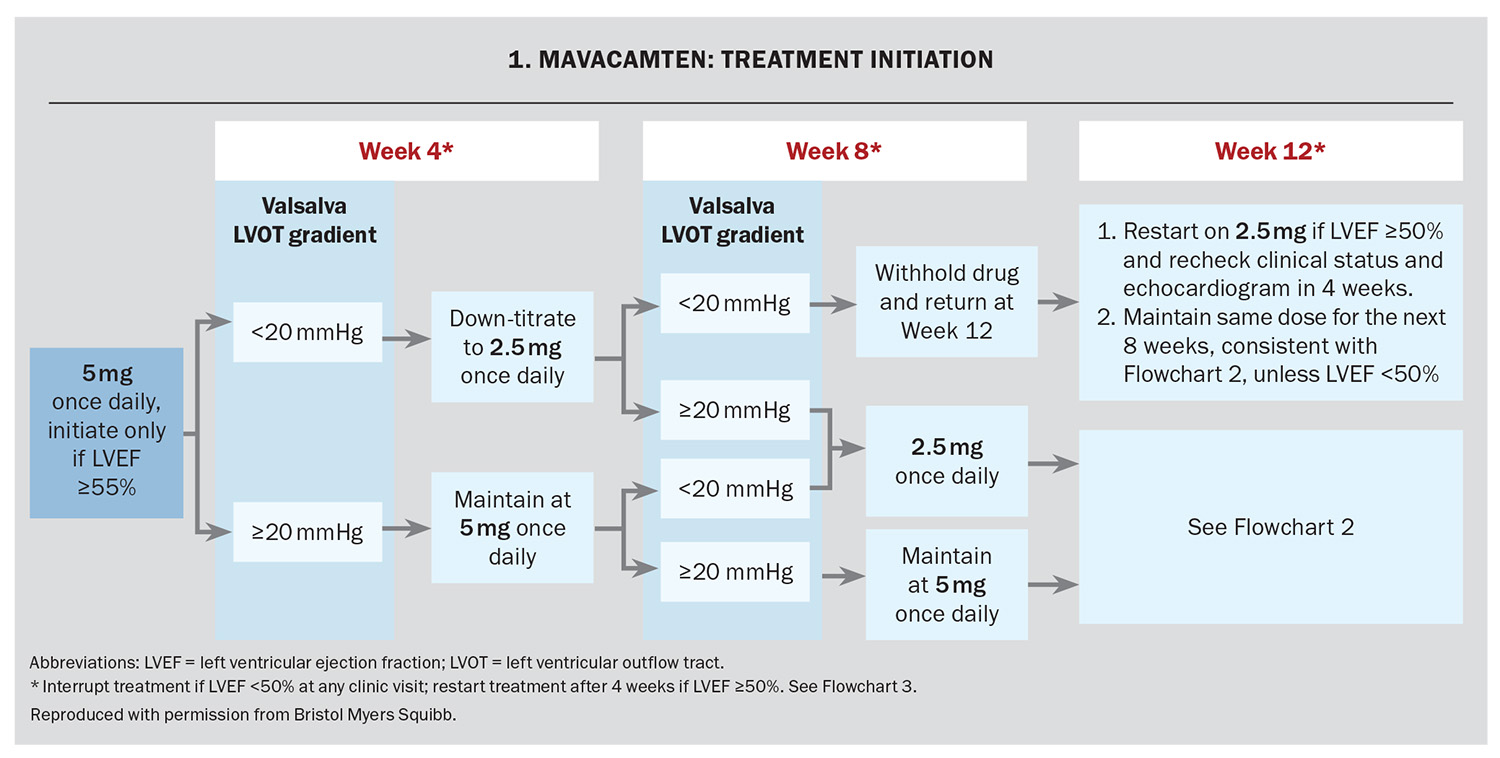
Mavacamten treatment should be initiated and supervised by a specialist cardiologist or consultant physician with experience in the management of obstructive HCM. It requires regular monitoring of the patient’s symptoms, LV outflow tract gradient with Valsalva manoeuvre and LVEF using regular echocardiographic assessment. Before initiating treatment, the patient’s LVEF measured by echocardiography must be 55% or higher. The recommended starting dose is 5 mg orally once daily.
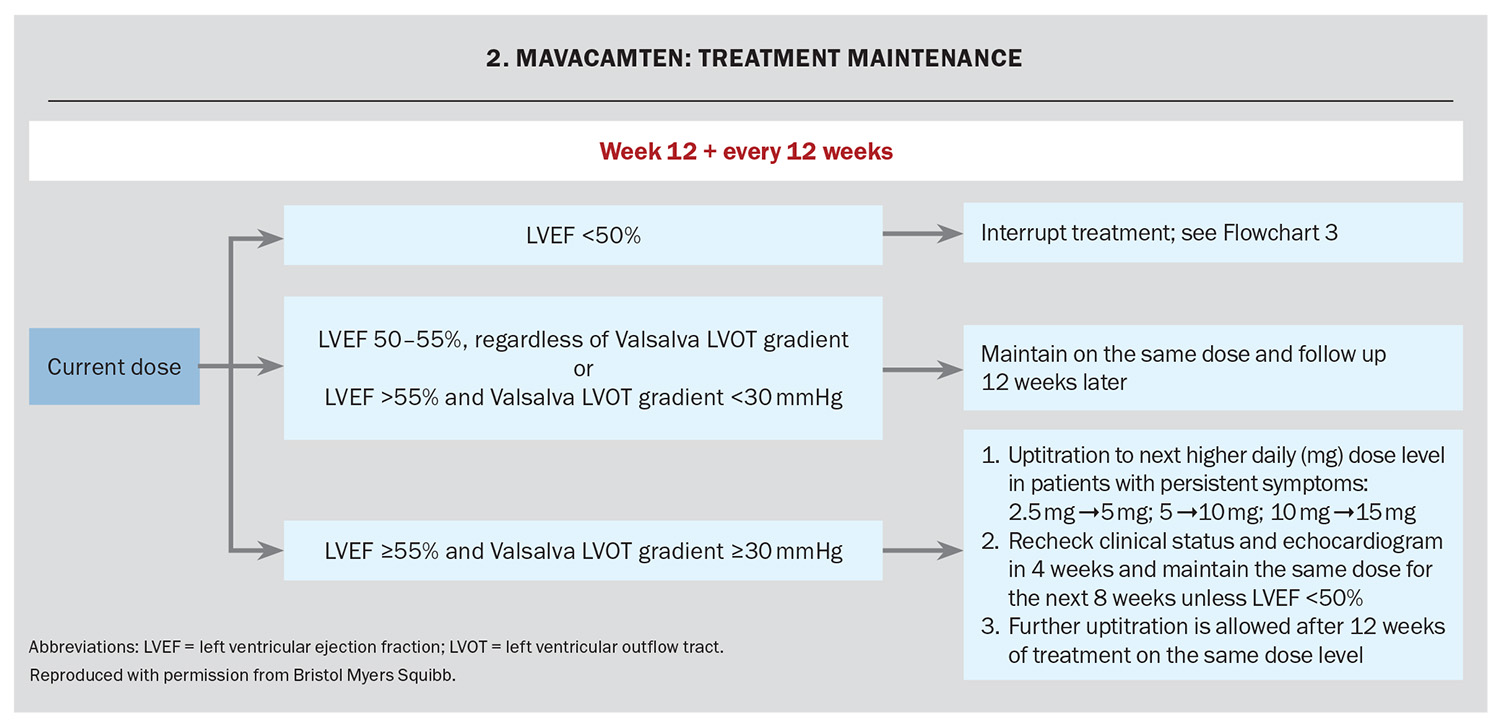
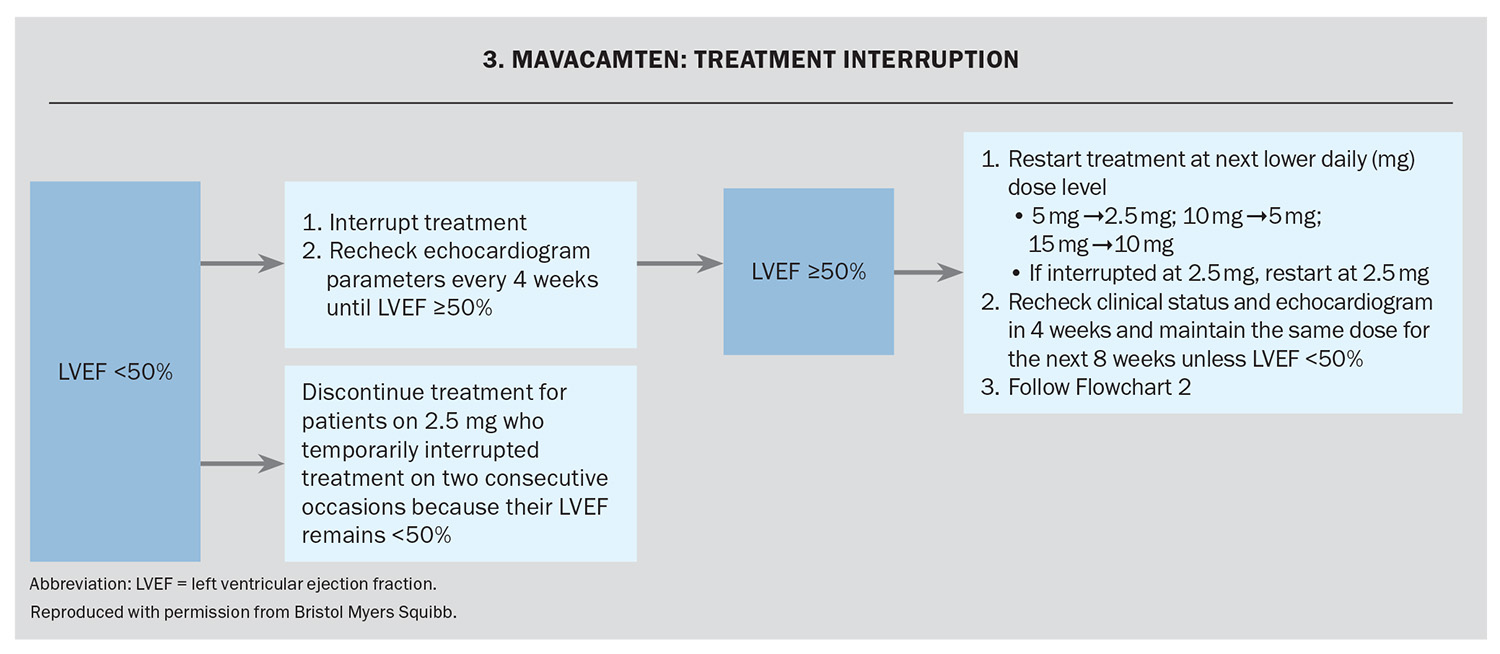
The response to treatment, including assessment of LV outflow tract gradient with Valsalva manoeuvre and LVEF using echocardiography, occurs at Weeks 4, 8 and 12 and every 12 weeks thereafter (unless the dose is changed). The dose is adjusted based on the LV outflow tract gradient with Valsalva and the LVEF on echocardiography (Flowchart 1 and Flowchart 2). If at any visit the LVEF on the echocardiogram is less than 50% the treatment should be interrupted for four weeks and until the LVEF returns to 50% or higher (Flowchart 3).
Assessment of LVEF is recommended if clinical status changes or in patients with a serious intercurrent illness such as infection or arrhythmia (including atrial fibrillation or other uncontrolled tachyarrhythmia).
Side effects
Mavacamten is generally well tolerated. Up to 5 to 6% of patients experienced reductions in LVEF below 50% in the clinical trials; however, these were generally reversible and patients were usually able to safely restart mavacamten.6,7 Longer term follow up of patients enrolled in the clinical trials and real-world data have been reassuring, with no new safety concerns identified.9 Nonetheless, as with all new therapies, clinicians should be alert to any potential newly recognised side effects and should report any suspected adverse events to the TGA.
Important precautions and interactions
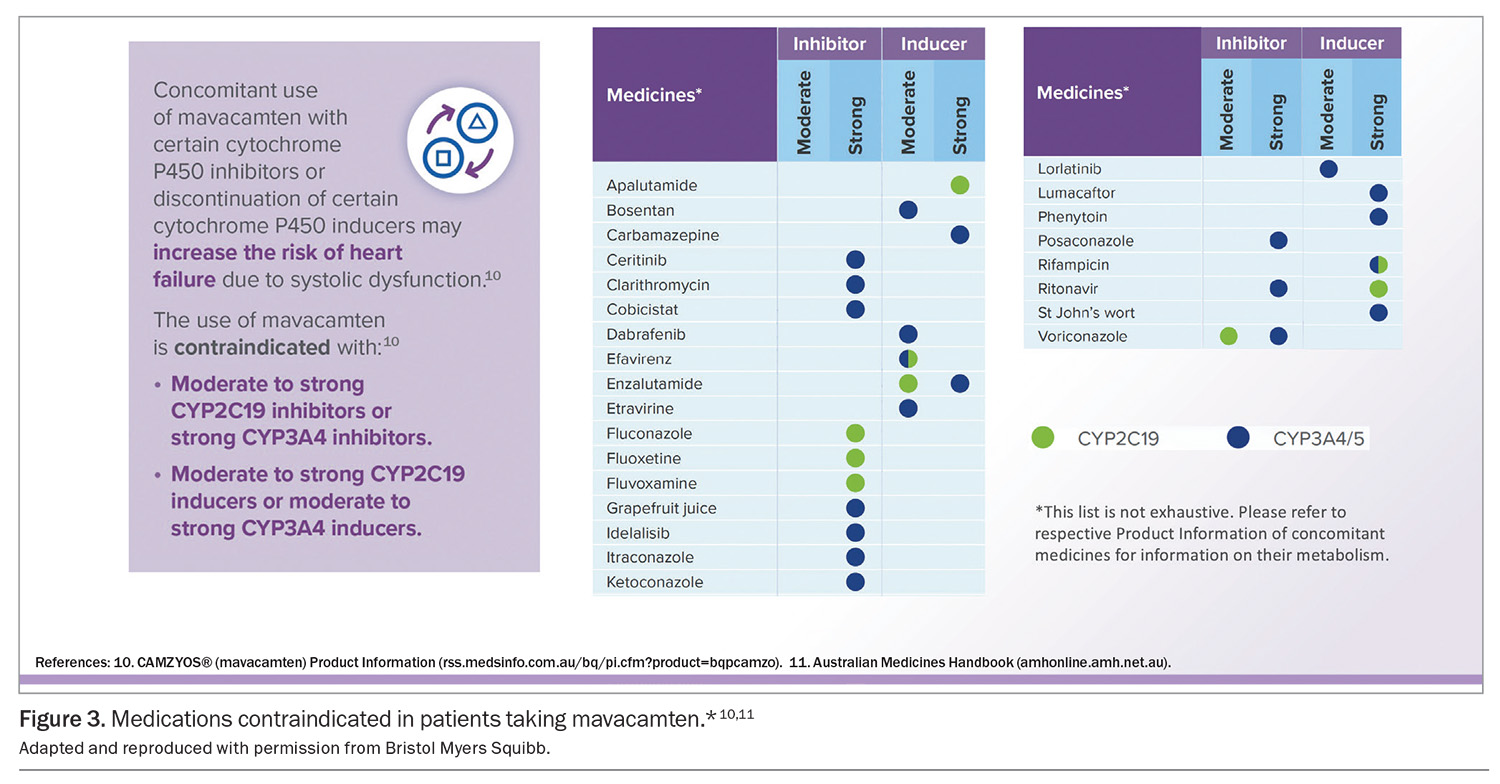
Mavacamten is primarily metabolised by cytochrome P450 (CYP)2C19 and to a lesser extent by CYP3A4 enzymes. Concomitant use of mavacamten with moderate to strong CYP2C19 inhibitors, strong CYP3A4 inhibitors, moderate to strong CYP2C19 inducers and moderate to strong CYP3A4 inducers is contraindicated (Figure 3).10,11 Involving pharmacists to review potential medication interactions for patients taking mavacamten may be considered.
Mavacamten should not be administered to patients who are pregnant. Women of reproductive potential who undergo treatment with mavacamten should be informed of the potential hazard to the fetus and advised to avoid becoming pregnant for at least four months after discontinuing treatment. Women of reproductive age should be advised to use highly effective contraception during treatment.
Conclusion
Mavacamten is a novel therapy for patients with symptomatic obstructive HCM despite beta blocker or nondihydropyridine calcium channel blocker treatment that decreases hypercontractility and LV outflow tract obstruction and has been shown to improve symptoms and functional capacity. MT
COMPETING INTERESTS: Dr McCormack: None. Professor Atherton has received payments to his employer for consultancy or honoraria and conference sponsorship from Bristol Myers Squibb.
This article is for general information purposes only, and the full Product Information should be consulted before prescribing any of the mentioned medications.
References
1. Ommen SR, Ho CY, Asif IM, et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024; 149: e1239-e1311.
2. Playford D, Strange GA, Atherton JJ, Harris S, Chan YK, Stewart S. Clinical to population prevalence of hypertrophic cardiomyopathy phenotype: insights from the National Echo Database Australia. Heart Lung Circ 2024; 33: 212-221.
3. Kawana M, Spudich JA, Ruppel KM. Hypertrophic cardiomyopathy: mutations to mechanisms to therapies. Front Physiol 2022; 13: 975076.
4. Prasad SB, Atherton JJ. Quality first in obstructive hypertrophic cardiomyopathy. Lancet 2021; 397: 2440-2441.
5. Anderson RL, Trivedi DV, Sarkar SS, et al. Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci USA 2018; 115: E8143-E8152.
6. Olivotto I, Oreziak A, Barriales-Villa R, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020; 396: 759-769.
7. Desai MY, Owens A, Geske JB, et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy. J Am Coll Cardiol 2022; 80: 95-108.
8. Hegde SM, Lester SJ, Solomon SD, et al. Effect of mavacamten on echocardiographic features in symptomatic patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2021; 78: 2518-2532.
9. Desai MY, Hajj-Ali A, Rutkowski K, et al. Real-world experience with mavacamten in obstructive hypertrophic cardiomyopathy: observations from a tertiary care center. Prog Cardiovasc Dis 2024; 86: 62-68.
10. Australian Product Information Camzyos® (mavacamten). Melbourne: Bristol Myers Squibb. Approved 19 September 2022, revised 8 October 2024. Available online at https://rss.medsinfo.com.au/bq/pi.cfm?product=bqpcamzo (accessed January 2025).
11. Australian Medicines Handbook. Adelaide: Australian Medicines Handbook Pty Ltd; 2023.