Estradiol and micronised progesterone: a new oral menopausal hormone therapy

A new menopausal hormone therapy preparation containing estradiol combined with micronised progesterone is now available in Australia. The oral preparation is indicated for use during continuous combined hormone replacement therapy for oestrogen deficiency symptoms in postmenopausal women with an intact uterus and with at least 12 months since last menses.
Correction
A correction for this article appears in the May 2023 issue of Medicine Today (see link here). The online version and the full text PDF of this article have been corrected.
On average, a 51-year-old woman in Australia can expect to live for another 32 years. This means that postmenopause will comprise around one-third of a woman’s life. Up to 80% of women will experience troublesome menopausal symptoms for at least some of that time.1 It is important that women have access to a range of therapeutic options for menopausal symptoms so that treatment can be tailored to their individual needs and preferences.
A new combined oral preparation, containing 17β-estradiol (E2) 1 mg (as hemihydrate) and micronised progesterone (P4) 100 mg, offers an additional option for women considering menopausal hormone therapy (MHT) in Australia. At the time of writing, the preparation is not PBS subsidised and costs about $52 per month at discount pharmacies.
More about estradiol and micronised progesterone
The Women’s Health Initiative trial in 2002 identified potential risks for women using an MHT preparation that combined conjugated equine estrogen with medroxyprogesterone acetate, a synthetic progestogen.2 Subsequently, there was an increasing demand for MHT that used more ‘natural’ hormones (i.e. bioidentical hormone therapy), since many women believed that these might be a safer option, although this belief was not based on firm evidence.3-5 This resulted in the widespread use of compounded bioidentical hormone therapy in many countries despite a lack of efficacy and safety data. It was not until the commercial manufacture of E2 and P4 combinations that clinical trials provided efficacy and safety data, which are a prerequisite to registration by drug regulatory bodies.
Components of the preparation
Estradiol
The E2 used in this preparation is chemically identical to that produced by the ovaries during the reproductive years. Most MHT preparations (both oral and transdermal) available in Australia use E2 as their oestrogen component. The E2 1 mg contained in this preparation places it within the category of low-dose MHT preparations.6
The oestrogen component of MHT alleviates the symptoms of oestrogen deficiency, such as hot flushes and night sweats. However, women with an intact uterus also require the addition of an adequate dose of a progestogen to reduce the risk of endometrial hyperplasia.
Micronised progesterone
P4, the hormone produced by the corpus luteum during the reproductive years, is poorly absorbed when administered orally and must be micronised to increase its absorption. Although most combined oral MHT preparations available in Australia contain a synthetic progestogen, oral micronised P4 has been available since the 1980s in Europe, where it was often paired with transdermal estrogen. Several longitudinal French studies suggest that micronised P4 may be associated with a lower risk of breast cancer than synthetic progestogens when used in combined MHT.7,8 A micronised P4 capsule has been marketed in Australia for several years, both as a standalone product and in combination with an estrogen gel.
Combining E2 and micronised P4 in a single capsule to produce an effective MHT preparation is technically challenging because of differences in their structure and solubility. This new preparation of E2 1 mg with micronised P4 100 mg is the first to combine the two. Suspension of both components in a lipid carrier ensures that they are readily absorbed and reach steady-state plasma concentration within seven days of initial oral administration.9
Efficacy for the relief of menopausal symptoms
The information on the effectiveness of this MHT preparation for the control of menopausal symptoms comes largely from the REPLENISH trial.10 In this 52-week multicentre phase 3 trial, postmenopausal women with an intact uterus were randomised to receive E2 and P4 in one of four different dose combinations (n = 1684) or placebo (n = 151). The age range of the women in the trial was 40 to 60 years, with a mean age of 55 years.
A substudy was also conducted within the larger trial. This included 726 women experiencing moderate to severe hot flushes (seven or more per day, or 50 or more per week) to observe the effect of the various doses of E2 and P4 on vasomotor symptoms over a 12-week period. The highest dose combination used in the trial, E2 1 mg and micronised P4 100 mg, was found to be more effective at reducing the frequency and severity of moderate to severe vasomotor symptoms than lower doses or placebo. This result was significant by week three and maintained throughout the 12-week substudy.10
The Menopause-Specific Quality of Life questionnaire is a validated self-administered questionnaire often used to measure global improvement in menopause-related trials.11 Based on the findings of this questionnaire, women in the REPLENISH trial receiving active treatment showed improvements in vasomotor symptoms, as well as in other psychosocial, physical and sexual domains.12
An interesting finding of the REPLENISH trial was the effect that the preparation had on sleep.10 Sleep disturbance is a common menopausal symptom, causing difficulties in initiating and maintaining sleep and more frequent nocturnal and early-morning awakening.13 A lack of good-quality sleep is also linked to other common menopausal symptoms, such as mood disturbance and reduced concentration.14
The women receiving active treatment in the REPLENISH trial showed significant improvements in the validated Medical Outcomes Study Sleep Scale Survey scores.15 An improvement in total scores was noted from baseline through to week 12, and these improvements were sustained until the end of the trial.10 It is likely that these improvements in sleep measures resulted primarily from the reduction in the frequency and severity of nocturnal vasomotor symptoms, but P4 might have also had a direct effect, as observed in other studies.16,17 As such, MHT preparations containing micronised P4 should be taken at night.
Safety and tolerability
Side effects
The most common side effects reported by the women taking E2 1 mg and micronised P4 100 mg in the REPLENISH trial were breast tenderness (10.4%), headache (3.4%), nausea (2.2%), pelvic pain (3.1%), vaginal haemorrhage (3.4%) and vaginal discharge (3.4%).18 These findings are comparable to the range and incidence of side effects seen in other MHT trials.
Metabolic effects
No clinically significant changes in lipid or glucose parameters were observed over the course of the REPLENISH trial in the women receiving active treatment and between baseline and month 12. No clinically meaningful changes in body weight and systolic or diastolic blood pressure were observed.10
Endometrial hyperplasia
To ensure that the various doses of E2 and P4 provided adequate endometrial protection, women in the REPLENISH trial underwent endometrial biopsies at baseline and at month 12 (or alternatively at early trial discontinuation). No cases of endometrial cancer were reported during the trial. One case of simple endometrial hyperplasia (without atypia) was detected in a woman who received E2 1 mg and P4 100 mg (n = 1/268, 0.37%; two-sided 95% confidence interval, 1.83%).10,19 This is consistent with the background incidence of up to 1% associated with endometrial hyperplasia seen in postmenopausal women of this age in the USA.10,19
Four cases of disordered proliferative endometrium that did not fulfil the criteria of hyperplasia were reported among women taking the E2 1 mg and P4 100 mg combination.10
Bleeding control
Bleeding and spotting were reported by 30% of women in the REPLENISH trial receiving E2 1 mg and P4 100 mg during the first three months of treatment. At months 10 to 12, 83% of these women reported amenorrhoea.10
Stroke, coronary heart disease and deep vein thrombosis
No cases of stroke were reported in the REPLENISH trial.20 Two coronary heart disease events were observed in women receiving active treatment during the 12-month trial period; this rate (2/1684) is marginally lower than the expected annual rate of two to three per 1000 in women in the USA of this age.20,21 No clinically meaningful changes in coagulation parameters were observed in the women receiving active treatment. The observed rate of deep vein thrombosis (DVT) of 1/1684 was lower than the expected annual rate of 1.7/1000 reported for women of a similar age in the USA.20,22
Breast cancer
Over the 12 months of the REPLENISH trial, six women (0.36%) receiving active treatment had a diagnosis of breast cancer.23 The annual incidence rate for breast cancer in the USA in women aged 50 to 60 years is 0.25%, which is comparable to the rate of breast cancer in the REPLENISH trial.24
Practical considerations
Women considering systemic MHT have the choice of receiving an oral or a transdermal preparation. Transdermal delivery of MHT has been increasingly considered first-line because it may be associated with a lower incidence of serious adverse events.25 However, transdermal delivery is not without side effects, and some women simply prefer oral therapy to transdermal therapy.
The baseline risk for severe adverse events, such as DVT and stroke, in women taking an oral estrogen is very low in young, otherwise healthy, postmenopausal women. The micronised P4 used in this E2 1 mg and micronised P4 100 mg preparation may confer some benefits over other oral MHT preparations in terms of reduced effects on mood and beneficial effects on sleep quality.26
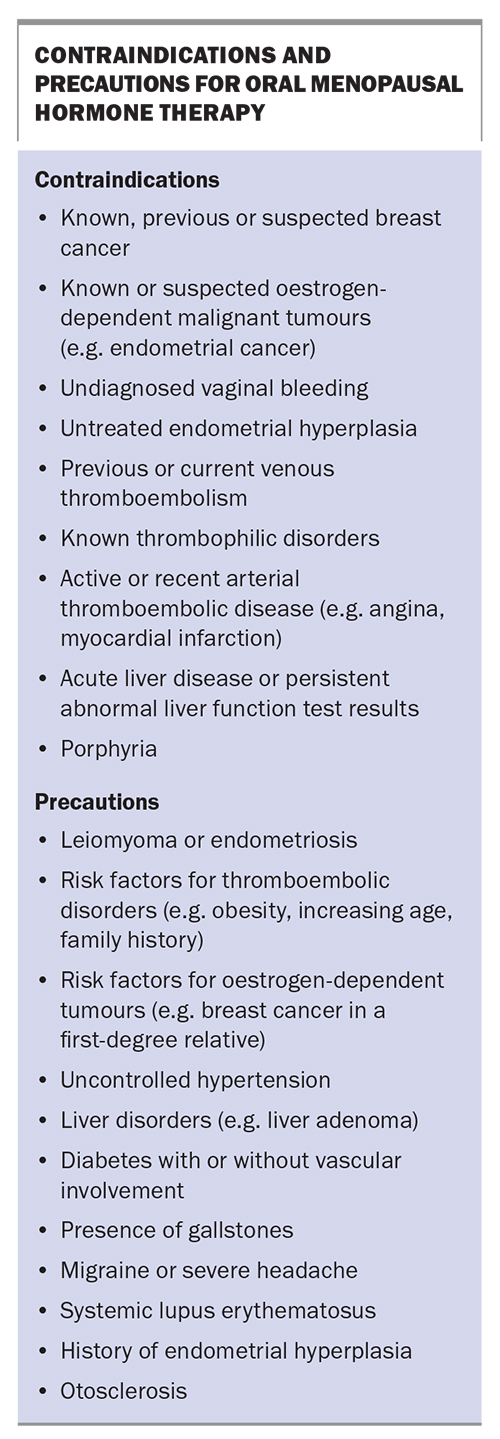
The specific contraindications and precautions of oral menopausal hormone therapy are listed in the Box. An individualised risk–benefit analysis should be undertaken for women with conditions listed as precautions. Transdermal estrogens are a safer choice for women who have risk factors for cardiovascular disease or DVT (e.g. history of provoked DVT, being overweight, presence of migraines).
Drug interactions
No formal drug interaction studies have been conducted with the E2 1 mg and micronised P4 100 mg preparation. For an extensive list of potential interactions of this preparation, clinicians should consult its Product Information.
Both oestrogens and progestogens are partly metabolised in the liver by the CYP3A4 enzymes in the cytochrome P450 pathway. Inducers of CYP3A4, such as St John’s wort (Hypericum perforatum) and most antiepileptic and all antituberculosis medications, may lower plasma concentrations of oestrogens and progestogens.27 This may then reduce symptom control and increase irregular uterine bleeding. Inhibitors of CYP3A4, such as erythromycin, clarithromycin, ketoconazole and itraconazole may increase plasma concentrations of oestrogens and progestogens, potentially increasing the incidence of side effects, such as breast tenderness.28
Oral estrogens are associated with an increased production of sex hormone-binding globulin in the liver, resulting in lower free testosterone levels. Although the impacts of this on libido, sexual function and wellbeing for most postmenopausal women remain uncertain, the use of transdermal estrogen is preferred in women who take exogenous testosterone therapy to improve sexual dysfunction.29
Oral estrogens also increase thyroxine-binding globulin levels more than transdermal preparations, which results in lower bioavailable thyroxine levels. Therefore, in women receiving thyroid hormone replacement therapy, the use of an oral MHT preparation may increase thyroid hormone dose requirements.30
Practice points
The E2 1 mg and micronised P4 100 mg preparation is available in a blister pack containing 30 same-dose capsules, one to be taken each day. This continuous combined MHT preparation is indicated for oestrogen deficiency symptoms in postmenopausal women with an intact uterus who are at least 12 months since their last menses.27 As with all systemic MHT preparations, it is recommended that therapy be initiated before the age of 60 years or within 10 years of the last menstrual period.
In the first 12 months after menopause, cyclical MHT preparations are indicated because the rates of irregular bleeding are unacceptably high in women who start continuous therapy. If a woman has had surgically induced menopause, or if she has previously used another continuous combined preparation, she may start taking E2 1 mg and micronised P4 100 mg at any time. Women taking a cyclical preparation should complete their current 28-day pack and then change to the E2 1 mg and micronised P4 100 mg preparation.27
The preparation should be taken in the evening to optimise the sleep benefits.27 Taking it with food enhances absorption.27 If a dose is missed, it should be taken as soon as possible, as erratic dosing increases the risk of irregular bleeding.27
The suitability of any MHT preparation should be evaluated initially at three months and then at least annually. At each review visit, attention should be paid to the risk–benefit analysis of continued MHT therapy and to the patient’s needs and preferences. There is no absolute limit to the time that MHT can be prescribed, although clinical experience in treating women over the age of 65 years with E2 1 mg and micronised P4 100 mg is limited.
Conclusion
A new oral E2 1 mg and micronised P4 100 mg MHT preparation is now available in Australia, widening the range of options for women who prefer an oral MHT preparation and increasing the possibility for women to choose a preparation that suits them best. It has an acceptable safety profile, but clinicians should remain mindful of the contraindications and risks associated with all MHT preparations. MT
COMPETING INTERESTS: Dr Foran has attended several advisory board meetings for Theramex, which distributes Bijuva in Australia. She has also presented at educational events for healthcare providers that were sponsored by Theramex and for which an honorarium was accepted.
This article is for general information purposes only, and the full Product Information should be consulted before prescribing any of the mentioned medications.
References
1. Guthrie JR, Dennerstein L, Taffe JR et al. Hot flushes during the menopause transition: a longitudinal study in Australian-born women. Menopause 2005; 12: 460-467.
2. Rossouw JE, Anderson GL, Prentice RL, et al.; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288: 321-333.
3. MacLennan AH, Gill TK, Broadbent JL, et al. Continuing decline in hormone therapy use: population trends over 17 years. Climacteric 2009; 12: 122-130.
4. Iftikhar S, Shuster LT, Johnson RE, et al. Use of bioidentical compounded hormones for menopausal concerns: cross-sectional survey in an academic menopause center. J Womens Health 2011; 20: 559-565.
5. Boothby LA, Doering PL, Kipersztok S. Bioidentical hormone therapy: a review. Menopause 2004; 11: 356-367.
6. Australasian Menopause Society. AMS guide to equivalent MHT/HRT doses Australia only. Australasian Menopause Society Ltd; 2022. Available online at: https://www.menopause.org.au/hp/information-sheets/ams-guide-to-equivalent-mht-hrt-doses (accessed March 2023).
7. Fournier A, Fabre A, Mesrine S, et al. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol 2008; 26: 1260-1268.
8. Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among post-menopausal women in France. PLoS One 2013; 11: 1-9.
9. Lobo RA, Liu J, Stanczyk FZ, et al. Estradiol and progesterone bioavailability for moderate to severe vasomotor symptom treatment and endometrial protection with the continuous-combined regimen of TX-001HR (oral estradiol and progesterone capsules). Menopause 2019; 26: 720-727.
10. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2018; 132: 161-170.
11. Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas 1996; 24: 161-175.
12. Simon JA, Kaunitz A, Kroll R, et al. Oral 17β-estradiol-progesterone (TX-001HR) and quality of life in postmenopausal women with vasomotor symptoms. Menopause 2019; 26: 506-512.
13. Kagan R, Constantine G, Kaunitz AM, et al. Improvement in sleep outcomes with a 17β-estradiol-progesterone oral capsule (TX-001HR) for postmenopausal women. Menopause 2019; 26: 622-628.
14. Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG). Managing menopausal symptoms. RANZCOG; 2020. Available online at: https://ranzcog.edu.au/wp-content/uploads/2022/05/Managing-menopausal-symptoms.pdf (accessed March 2023).
15. Medical Outcomes Study Sleep Scale Survey Instrument. RAND Corporation; 1986. Available online at: https://www.rand.org/health-care/surveys_tools/mos/sleep-scale.html (accessed March 2023).
16. Söderpalm AH, Lindsey S, Purdy RH, et al. Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology 2004; 29: 339-354.
17. Caufriez A, Leproult R, L’Hermite-Balériaux M, et al. Progesterone prevents sleep disturbances and modulates GH, TSH, and melatonin secretion in postmenopausal women. J Clin Endocrin Metab 2011; 96: E614-E623.
18. Archer DF, Bernick BA, Mirkin S. A combined, bioidentical, oral, 17β-estradiol and progesterone capsule for the treatment of moderate to severe vasomotor symptoms due to menopause. Expert Rev Clin Pharmacol 2019; 12: 729-739.
19. Mirkin S, Goldstein SR, Archer D, et al. Endometrial safety and bleeding profile of a 17β-estradiol/progesterone oral softgel capsule (TX-001HR). Menopause 2020; 27: 410-417.
20. Lobo RA, Kaunitz AM, Santoro N, et al. Metabolic and cardiovascular effects of TX-001HR in menopausal women with vasomotor symptoms. Climacteric 2019; 22: 610-616.
21. Lobo RA. Evaluation of cardiovascular event rates with hormone therapy in healthy, early postmenopausal women: results from 2 large clinical trials. Arch Intern Med 2004; 164: 482-484.
22. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004; 292: 1573-1580.
23. Liu JH, Black DR, Larkin L, et al. Breast effects of oral, combined 17β-estradiol, and progesterone capsules in menopausal women: a randomized controlled trial. Menopause 2020; 27: 1388-1395.
24. Breast Cancer – SEER incidence rates by age at diagnosis, 2015-2019 (by sex, observed SEER incidence rate, all races). National Cancer Institute; 2023. Available online at: https://seer.cancer.gov/statistics-network/explorer/application.html?site=55&data_type=1&graph_type=3&compareBy=sex&chk_sex_3=3&rate_type=1&race=1&advopt_precision=1&advopt_show_ci=on&hdn_view=1&advopt_show_apc=on&advopt_display=2#resultsRegion1 (accessed March 2023).
25. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015; 100: 3975-4011.
26. Sherif K. Hormone therapy: A clinical handbook. New York: Springer; 2013.
27. Australian Register of Therapeutic Goods (ARTG). Australian PI – Bijuva® 1/100 (estradiol (as hemihydrate) and progesterone) soft capsules. ARTG; 2022. Available online at: https://www.tga.gov.au/resources/artg/367690 (accessed March 2023).
28. Martin J, Fay M. Cytochrome P450 drug interactions: are they clinically relevant? Aust Prescr 2001; 24: 10-12.
29. Stomati M, Hartmann B, Spinetti A, et al. Effects of hormonal replacement therapy on plasma sex hormone-binding globulin, androgen and insulin-like growth factor-1 levels in postmenopausal women. J Endocrinol Invest 1996; 19: 535-541.
30. Kaminski J, Junior CM, Pavesi H, et al. Effects of oral versus transdermal estradiol plus micronized progesterone on thyroid hormones, hepatic proteins, lipids, and quality of life in menopausal women with hypothyroidism: a clinical trial. Menopause 2021; 28: 1044-1052.