Systemic lupus erythematosus: unmasking the masquerader

Systemic lupus erythematosus (SLE) is a multisystem, autoimmune disease that is challenging to diagnose because of its heterogenous clinical manifestations. Treatment for SLE is multifaceted and focuses on controlling disease activity, managing symptoms and preventing organ damage.
- Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterised by chronic inflammation, with B-cell hyperactivity playing a significant role in the immune dysregulation.
- SLE is rare, affecting less than 0.1% of the global population, but its prevalence is higher in specific demographic groups, such as Indigenous Australians and Australians with South-East Asian ancestry.
- The diagnosis of SLE can be challenging because of its diverse clinical manifestations, and it often relies on clinical expertise and appropriate laboratory testing. The 2019 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) classification criteria can be a useful guide for the diagnostic process.
- Treatment for SLE aims to control disease activity, reduce flares and prevent organ damage. It includes a combination of anti-inflammatory agents, immunosuppressants, immunomodulatory agents and nonpharmacological measures.
- Long-term follow-up and monitoring are essential to assess disease activity, manage symptoms and prevent damage accrual in patients with SLE, with awareness of cardiovascular risk management and addressing complications during pregnancy.
Systemic lupus erythematosus (SLE) is a complex, multisystem autoimmune disease characterised by chronic inflammation. Immunologically, a key feature of SLE is the finding of B-cell hyperactivity leading to aberrant antibody production.1 This immune dysregulation leads to chronic inflammation and organ dysfunction, with the potential for permanent damage. Its diverse clinical manifestations can sometimes make diagnosis challenging, but early recognition and treatment can prevent long-term morbidities and mortality.2
The worldwide prevalence of SLE is estimated at just over four million, demonstrating the rarity of the condition, which is reported to affect less than 0.1% of the global population.3 Epidemiological studies have shown an increased risk in certain demographics in Australia, such as Indigenous Australians and Australians with South-East Asian ancestry. These groups tend to have more severe forms of disease compared with Caucasian populations. Poor socioeconomic status is also associated with poor disease-related outcomes.4
This article provides a clinical framework for the identification, diagnosis and subsequent management of SLE in general practice, highlighting the most recent classification criteria and some novel therapeutics that are on the horizon.
Pathophysiology of SLE
Our understanding of the pathophysiology of SLE continues to evolve, with major scientific breakthroughs in the past few years. It is appreciated that both innate and adaptive immune dysfunction contribute to SLE development.1,5 In addition to the well-established phenomenon of B-cell hyperactivity, there are key molecular pathways that serve to drive the inflammatory cascade associated with SLE.6 These pathways can contribute to the breakdown of self-tolerance and perpetuation of autoimmunity.1,5
One of the hallmarks of a diagnosis of SLE is the detection of antinuclear antibodies (ANAs). The presence of ANAs is often considered a surrogate marker for the presence of autoreactive B cells that produce these antibodies.7 Diminished clearance of damaged cells may provide the source of cellular debris and self-antigens that can trigger the sustained activation of B cells and immune complex formation. These immune complexes can activate other immune cells, such as neutrophils and macrophages, resulting in the production of pro-inflammatory cytokines and other inflammatory mediators that can cause injury to specific organs.8
The type I interferon (IFN) response is an antiviral host defence mechanism mediated by the innate immune system.9 There is sustained activation and dysregulation of this pathway in SLE, which is coined the ‘IFN signature’. Type I IFNs activate natural killer cells, macrophages, monocytes and dendritic cells, while amplifying CD4+ and CD8+ T-cell survival and promoting Th1 differentiation to Th17 helper cells. The IFN signature is determined by an upregulation of chemokines and cytokines that are downstream effects of IFN activation.
Clinical presentation and diagnosis of SLE
One of the major difficulties reported by primary care physicians is the challenging nature of diagnosing SLE. This relates to the heterogeneity of the immunological and clinical manifestations.
Musculoskeletal manifestations
Ninety-five percent of patients with SLE experience intermittent arthritis or arthralgia, most commonly presenting as a symmetrical polyarthritis affecting the hands and knees.10 Joint deformity is uncommon; however, Jaccoud’s arthropathy may indicate chronicity. Tenosynovitis is relatively common in patients with SLE; however, the degree of swelling is less than that observed in patients with other inflammatory arthropathies.11 Myalgia is frequent, whereas myositis is rare.12 Fibromyalgia can suggest a noninflammatory complication of the disease.13
Cutaneous manifestations
SLE can present with many cutaneous manifestations, with the butterfly malar rash being the most recognisable. This classic facial rash is an erythematous eruption over both cheeks, sparing the nasolabial folds.14 Worsening rash typically goes hand in hand with flaring of systemic disease. This rash is a localised, acute, cutaneous manifestation, in contrast to rashes in other chronic photosensitivity conditions, such as rosacea.14 It is also important to note that not all patients with SLE develop this rash.15
The second-most recognised cutaneous manifestation is discoid lupus erythematosus (DLE), which is characterised by the presence of raised, scaly lesions. The extent of this can be variable, and the lesions can be found all over the body but are most classically found on the scalp and face. These lesions have a propensity for scarring.14 DLE is its own entity: only 5% of patients with DLE have SLE, whereas 20% of patients with SLE have DLE.16
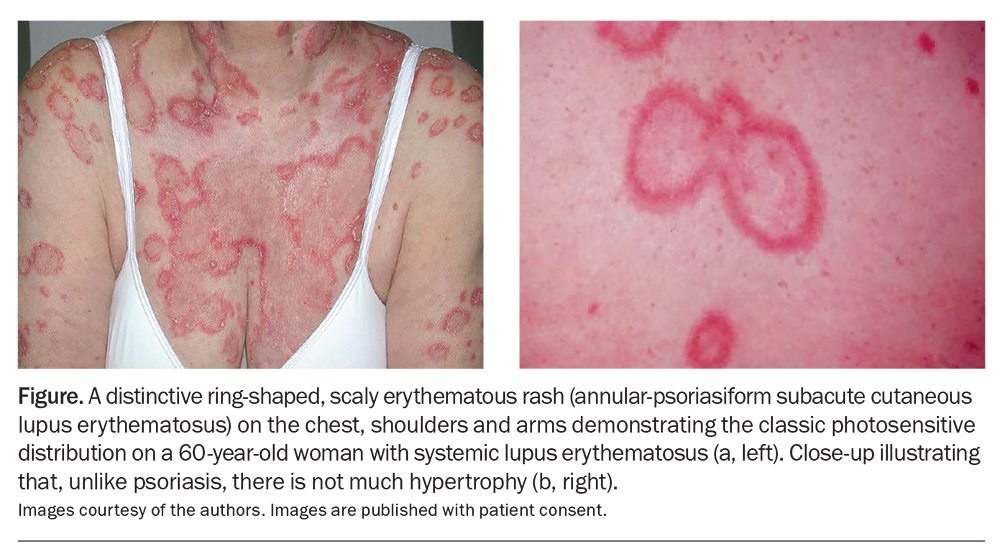
Subacute cutaneous lupus erythematosus can present as annular or psoriasiform erythematous rashes that are typically photosensitive (Figure). These lesions are usually found on sun-exposed areas, such as the upper back, chest and arms. They may be accompanied by systemic symptoms.14
Many other cutaneous and mucosal manifestations can occur in patients with SLE that require evaluation and management, often in collaboration with dermatologists or rheumatologists. The broad spectrum includes Raynaud’s phenomenon, digital ischaemia, alopecia, urticaria, lichen planus, vasculitis and nail-fold infarcts.14,17
Constitutional symptoms
Fatigue is a common concern in patients with SLE, with 53 to 80% of patients identifying this as a primary manifestation of disease.18 The aetiology of fatigue is multifactorial; disease activity, treatment side effects, mood and external factors all play a role. Similarly, fever (usually low grade) and weight loss are common complaints.19 These symptoms are nonspecific to SLE and warrant a systematic workup to rule out other potential causes before attributing them to SLE. Sometimes, certain systems, such as the haematological or renal systems, can be clinically silent until the more advanced stage of end-organ disease and may present predominantly as constitutional symptoms.19
Haematological manifestations
Normocytic anaemia is the most common haematological manifestation of SLE.20 This is typically the result of chronic inflammation but may also stem from autoimmune haemolytic anaemia, a well-recognised complication of the disease. Lymphopenia, neutropenia and thrombocytopenia are observed in patients with SLE and may reflect disease activity or, less commonly, treatment side effects. Neutropenia secondary to SLE is not associated with an increased infection risk.21
Renal effects
Lupus nephritis occurs in around 50% of patients with SLE and is a serious, sometimes life-threatening, complication of the disease.22 Urinalysis should be performed at regular intervals to assess for glomerular haematuria, proteinuria or casts. Left untreated, lupus nephritis poses significant risks to morbidity and mortality. Definitive diagnosis and treatment protocols are based on histological sampling at the time of renal biopsy.23
Neuropsychiatric syndromes
The term ‘neuropsychiatric SLE’ refers to a broad range of neurological and psychiatric syndromes that can occur in patients with SLE. The exact pathogenesis of each of these syndromes is complex, and both neuroinflammatory and ischaemic mechanisms have been proposed.24 Chronic headache and ‘brain fog’ are reported frequently, but attributing SLE as their primary cause is difficult.24 Cognitive dysfunction is common in patients with SLE, but this does not correlate well with the symptoms of ‘brain fog’.25 Recent Australian data have validated screening tools, such as the Montreal Cognitive Assessment, for use in detecting cognitive dysfunction.26
Some neuropsychiatric syndromes are rare but more specifically associated with the disease, such as seizures or acute psychosis. These are usually acute presentations, frequently associated with other systemic features of the disease requiring aggressive immunosuppression. It is also important to consider concurrent antiphospholipid syndrome in individuals presenting with stroke features.24
Serositis
Inflammation of the serous membranes, including pleurisy and pericarditis, can occur in individuals experiencing acute lupus flares. Patients may present with pleuritic chest pain or dyspnoea. Appropriate investigations such as ECG, chest x-ray or echocardiography should be considered to confirm the diagnosis.17,27
Diagnosis
The diagnosis of SLE is largely based on evidence of a multisystem autoimmune disease supported by appropriate laboratory test results. There are, however, overlapping syndromes and mimics; therefore, the diagnosis relies on physician expertise and experience. There is no single gold-standard test to confirm the diagnosis of SLE, and both overdiagnosis and delays in diagnosis are encountered in practice.28
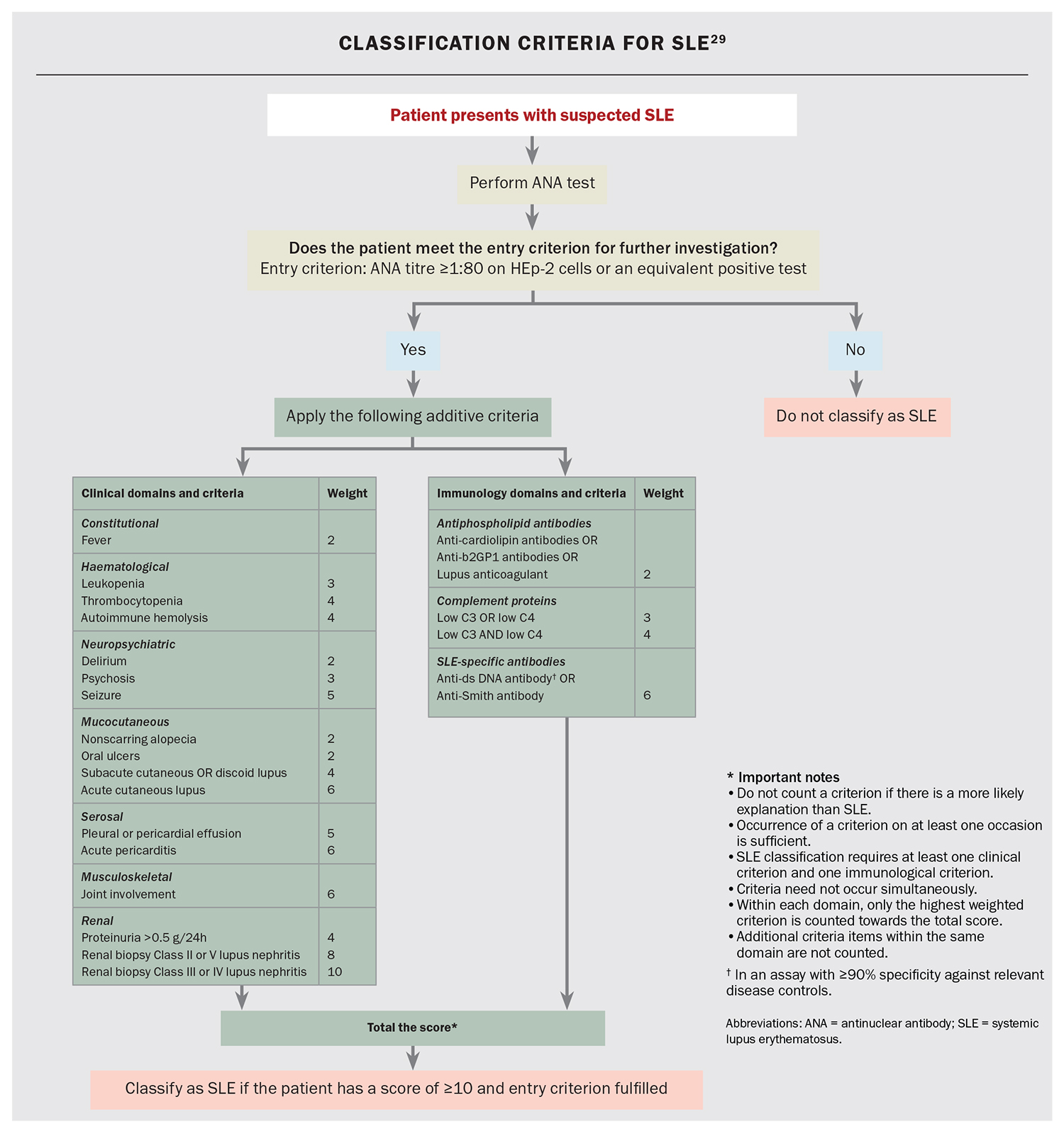
Although originally designed for research purposes to establish a homogeneous cohort of patients, classification criteria can be used to support the diagnostic process, with the most recent being the 2019 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) classification criteria for SLE (Flowchart).29 ANA is the serological gatekeeper for a SLE diagnosis. With up to 98% of patients with SLE having an elevated ANA level, a positive result raises the first suspicion of the presence of an autoimmune disease, whereas a negative result should prompt consideration of an alternative diagnosis.30 The ANA test is an excellent screening tool for SLE and forms the entry criterion in the 2019 EULAR/ACR classification criteria.29 The titre of the ANA refers to the concentration of the autoantibodies, and although a titre of 1:80 can be considered an entry criterion for SLE, ANA levels are typically moderately high (e.g. 1:320 or higher). The titre, however, does not necessarily indicate more active or severe disease but simply refers to the concentration of ANA present in that individual.
Diagnosing SLE can be difficult, as the symptoms may mimic those of other common presentations in general practice. If SLE is suspected, consider risk factors with symptoms and signs suggestive of an inflammatory process (i.e. young age, female sex). It is important to note that up to 15% of healthy people will have ANA positivity that is not associated with SLE or other connective tissue disease.31 As such, ANA test results should be interpreted in the clinical context with an appropriate level of suspicion.
There are several useful additional serological tests to assist with the diagnostic process. Other autoantibodies associated with SLE include those against double-stranded DNA and those found on extractable nuclear antigen testing, including anti-Smith, which is highly specific for SLE, and anti-Ro, anti-La and anti-U1RNP, which may indicate crossover syndromes with Sjögren’s syndrome or a mixed connective tissue disease.32 Levels of complement component (C) 3 and C4 are often reduced in patients with SLE due to tissue deposition of immune complexes disrupting the classical pathway. Low complement levels are a classification criterion and can also be used to assess disease activity.33 The presence of antiphospholipid antibodies or antiglobulin is also associated with SLE, and counts towards the classification criteria scoring.29,34
A careful medical history and physical examination, as well as relevant investigations, can help clarify a patient’s symptoms and assess any organ involvement. Patients should be referred to a rheumatologist for further evaluation and management when there is a clinical suspicion of SLE based on symptoms, signs or laboratory findings. Some common indications may be:
- symptoms or presentations that suggest multiorgan involvement, such as persistent joint pain and skin rash
- the presence of multiple autoantibodies or serological abnormalities
- persistent unexplained systemic inflammation.
Treatment
The overall aims of therapy for SLE are to control disease activity, reduce flares and ultimately prevent organ damage. A combination of anti-inflammatory agents and immunosuppressants is used to reduce disease activity, achieve remission or lower disease activity and establish long-term disease control.35
Hydroxychloroquine
Consensus from international colleges recommends the use of hydroxychloroquine in all patients with SLE from the time of diagnosis unless contraindicated.28,36 The drug has disease-modifying effects in reducing disease activity, preventing flares and improving long-term outcomes. It also has anti-inflammatory properties useful for skin and joint disease.37 In some circumstances, hydroxychloroquine is used for its positive influence on lipid and glucose metabolism and antiplatelet effects, therefore potentially lowering cardiovascular and thrombotic risk in individuals with antiphospholipid syndrome. Current recommendations for the optimal hydroxychloroquine dose to minimise the risk of retinopathy is 5.0 mg/kg/day (actual body weight) or less in the long-term, combined with regular screening after five years of use.38
Glucocorticoids
Glucocorticoids have been used for the treatment of active SLE for many years. As potent anti-inflammatory agents, glucocorticoids have been leveraged to rapidly control disease activity, induce remission and treat SLE flares. However, the significant morbidity associated with chronic glucocorticoid use is now well established.39 Short-term, high-dose glucocorticoid use may be required in patients with severe organ manifestations or difficult-to-treat disease; however, this should be supplemented by a clear plan for glucocorticoid weaning to avoid harmful metabolic and infective side effects.35 EULAR guidelines recommend the prompt initiation of immunomodulatory agents to increase the chance of successful tapering.28 There is no role for glucocorticoid monotherapy in SLE. Careful monitoring of mood disturbances, blood pressure, blood sugar levels and bone mineral density to assess glucocorticoid side effects should be a part of regular follow up and addressed with the assistance of the patient’s primary care physician.39
Immunosuppressants
Early initiation of immunosuppressants can improve disease control and expedite glucocorticoid tapering.35 Conventional immunosuppressants, such as mycophenolate, methotrexate and azathioprine, are commonly used for various SLE manifestations, such as lupus nephritis and joint pain and swelling. Calcineurin inhibitors, such as ciclosporin and tacrolimus, have proven efficacy for lupus nephritis.40 The choice of agent is usually based on the organ manifestation, severity of disease and patient factors, including the previous tolerance profile and pregnancy considerations. Monitoring is required while patients are on immunosuppressants, and this may include routine blood counts and biochemistry assessments, as well as measurements of lupus-related serum and urine parameters. Long-term immunosuppression can be associated with increased risks of infection and malignancy; these should be discussed with patients during their routine review, and any vaccinations or relevant screening should be optimised for these comorbidities.
Immunomodulatory agents
New immunomodulatory treatments for SLE have been explored, but their access is limited because of a lack of funding. Belimumab is a monoclonal antibody that targets and inhibits the activity of
B-lymphocyte stimulator.41 It is generally used as add-on therapy for patients with active SLE who have not achieved adequate disease control with standard treatments.42 Anifrolumab, another monoclonal antibody that targets the type I IFN receptor, has been shown to improve disease activity control in clinical trials involving patients with persistently active disease despite standard of care with or without alternative immunosuppression.43 Rituximab, a monoclonal antibody that targets anti-CD20, has been shown to deplete B cells effectively and has been extensively used in observational studies of refractory SLE.44 These therapies are generally only used at specialised lupus centres, with rituximab used off label.
Nonpharmacological measures
Patients with cutaneous manifestations of SLE should pay particular attention to avoiding overexposure to sunlight. Photoprotection with a broad-spectrum sunscreen of sun protection factor 50 or higher is vital.45 Cigarette smoking is a well-recognised risk factor for SLE development, and is particularly associated with poorly controlled cutaneous disease. Continued smoking after diagnosis is associated with a higher disease burden, leading to more frequent flares, worsened disease control and poor response to therapy. Early referral to smoking cessation services is recommended.46
Early studies have demonstrated that exercise improves psychological function and reduces fatigue in patients with SLE. Exercise is safe and tolerated by most patients.47 Although many symptoms can be improved with pharmacotherapy, some noninflammatory symptoms may be more refractory and, instead, more amenable to nonpharmacological measures, such as exercise therapy and stress management. Appropriate self care and lifestyle modifications can help promote emotional wellbeing. Sometimes, specific psychological intervention or counselling may be required to manage anxiety, depression and stress.48
Other considerations
Pregnancy
SLE is associated with an increased risk of maternal and fetal complications, but most patients with SLE experience a healthy pregnancy. The current aim is for disease remission at least six months prior to conception to avoid antenatal complications.49
Women who test positive for SSA (anti-Ro60) and SSB (anti-La) antibodies should be referred for weekly fetal echocardiography starting at week 16 of gestation to monitor for congenital heart block.49
Women with lupus have higher rates of preeclampsia and are recommended to take low-dose aspirin from week 12 to mitigate this risk. This recommendation is the same for those with known antiphospholipid syndrome. Intensified anticoagulation in the ante- and postpartum periods depends on the prior thromboembolic and obstetric history.49
Management of cardiovascular risk
Cardiovascular disease is a leading cause of morbidity and mortality in patients with SLE; thus, special attention should be paid to decelerating atherosclerotic disease and managing cardiovascular risk. With the assistance of the patient’s GP, blood pressure and lipid profiles should be assessed at semiregular intervals. Expert lupus guidelines recommend tight blood pressure control (target lower than 130/80 mmHg) and commencement of statin therapy if low-density lipoprotein levels are greater than 2.6 mmol/L.50,51
Conclusion
SLE is a complex disease that can run a variable clinical course, and its diagnosis can be challenging because of the diverse organ manifestations. Primary care physicians will be familiar with common presentations and some indications for rheumatology referral.
Although the prognosis in patients with SLE has dramatically improved in the last decade, there is still an unacceptably high rate of morbidity and mortality in the young population. Long-term follow up of patients with SLE is essential to assess for disease activity, manage symptoms and prevent damage accrual. Regular monitoring, ideally in partnership between the patient, primary care physician and treating rheumatologist, will allow for optimal engagement and improved patient outcomes. MT
COMPETING INTERESTS: Dr Igel has received support from Novartis to attend EULAR 2023. Associate Professor Hoi has received sponsorships for the Australian Lupus Registry and Biobank from GSK, UCB and AstraZeneca; received funding for contracted research from BMS, Merck Serono, GSK, Eli Lilly, UCB and Janssen; received speaker fees from Novartis and Janssen; participated on the advisory boards for UCB, Recordati and Janssen; and is an Arthritis Australia and Perpetual IMPACT fund grant recipient.
References
1. Hoi A, ed. Pathogenesis of systemic lupus erythematosus: insights from translational research. Switzerland: Springer Cham; 2021.
2. Kernder A, Richter JG, Fischer-Betz R, et al. Delayed diagnosis adversely affects outcome in systemic lupus erythematosus: cross sectional analysis of the LuLa cohort. Lupus 2021; 30: 431-438.
3. Tian J, Zhang D, Yao X, Huang Y, Lu Q. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study. Ann Rheum Dis 2023; 82: 351-356.
4. Barber MRW, Drenkard C, Falasinnu T, et al. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol 2021; 17: 515-532.
5. Crow MK. Pathogenesis of systemic lupus erythematosus: risks, mechanisms and therapeutic targets. Ann Rheum Dis 2023; 82: 999-1014.
6. Golder V, Hoi A. Systemic lupus erythematosus: an update. Med J Aust 2017; 206: 215-220.
7. Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol 2020; 16: 565-579.
8. Mahajan A, Herrmann M, Munoz LE. Clearance deficiency and cell death pathways: a model for the pathogenesis of SLE. Front Immunol 2016; 7: 35.
9. Mesev EV, LeDesma RA, Ploss A. Decoding type I and III interferon signalling during viral infection. Nat Microbiol 2019; 4: 914-924.
10. Bello N, Birt JA, Workman J, Zhou X, Ross-Terres JA, Petri M. Treatment patterns and clinical characteristics of patients with systemic lupus erythematosus and musculoskeletal symptoms: a retrospective, observational study. Adv Ther 2022; 39: 3131-3145.
11. Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol 2009; 23: 495-506.
12. Bitencourt N, Solow EB, Wright T, Bermas BL. Inflammatory myositis in systemic lupus erythematosus. Lupus 2020; 29: 776-781.
13. Wolfe F, Petri M, Alarcon GS, et al. Fibromyalgia, systemic lupus erythematosus (SLE), and evaluation of SLE activity. J Rheumatol 2009; 36: 82-88.
14. Stull C, Sprow G, Werth VP. Cutaneous involvement in systemic lupus erythematosus: a review for the rheumatologist. J Rheumatol 2023; 50: 27-35.
15. Rad SN, Vashisht P. Malar rash. Treasure Island, FL: StatPearls; 2023.
16. Provost T. The relationship between discoid lupus erythematosus and systemic lupus erythematosus. A hypothesis. Am J Dermatopathol 1979; 1: 181-184.
17. Apostolopoulos D, Hoi AY. Systemic lupus erythmatosus - when to consider and management options. Aust Fam Physician 2013; 42: 696-700.
18. Ahn GE, Ramsey-Goldman R. Fatigue in systemic lupus erythematosus. Int J Clin Rheumtol 2012; 7: 217-227.
19. Kawka L, Schlencker A, Mertz P, Martin T, Arnaud L. Fatigue in systemic lupus erythematosus: an update on its impact, determinants and therapeutic management. J Clin Med 2021; 10: 3996.
20. Fayyaz A, Igoe A, Kurien BT, et al. Haematological manifestations of lupus. Lupus Sci Med 2015; 2: e000078.
21. Meyer A, Guffroy A, Blaison G, et al. Systemic lupus erythematosus and neutropaenia: a hallmark of haematological manifestations. Lupus Sci Med 2020; 7: e000399.
22. Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol 2017; 12: 825-835.
23. Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. Manifestations of systemic lupus erythematosus. Maedica (Bucur) 2011; 6: 330-336.
24. Sarwar S, Mohamed AS, Rogers S, et al. Neuropsychiatric systemic lupus erythematosus: a 2021 update on diagnosis, management, and current challenges. Cureus 2021; 13: e17969.
25. Raghunath S, Glikmann-Johnston Y, Vincent FB, Morand EF, Stout JC, Hoi A. Patterns and prevalence of cognitive dysfunction in systemic lupus erythematosus. J Int Neuropsychol Soc 2023; 29: 421-430.
26. Raghunath S, Glikmann-Johnston Y, Golder V, et al. Clinical associations of cognitive dysfunction in systemic lupus erythematosus. Lupus Sci Med 2023; 10: e000835.
27. Man BL, Mok CC. Serositis related to systemic lupus erythematosus: prevalence and outcome. Lupus 2005; 14: 822-826.
28. Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update on the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis 2021; 80: 14-25.
29. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol 2019; 71: 1400-1412.
30. Olsen NJ, Karp DR. Finding lupus in the ANA haystack. Lupus Sci Med 2020; 7: e000384.
31. Li X, Liu X, Cui J, et al. Epidemiological survey of antinuclear antibodies in healthy population and analysis of clinical characteristics of positive population. J Clin Lab Anal 2019; 33: e22965.
32. Dema B, Charles N. Autoantibodies in SLE: specificities, isotypes and receptors. Antibodies (Basel) 2016; 5: 2.
33. Sandhu V, Quan M. SLE and serum complement: causative, concomitant or coincidental? Open Rheumatol J 2018; 12: 171.
34. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol 2016; 3: 75-84.
35. Hoi AY, Morand EF. Treatment update in systemic lupus erythematous. Rheum Dis Clin North Am 2021; 47: 513-530.
36. Mok CC, Hamijoyo L, Kasitanon N, et al. The Asia-Pacific League of Associations for Rheumatology consensus statements on the management of systemic lupus erythematosus. Lancet Rheumatol 2021; 3: e517-e531.
37. Dima A, Jurcut C, Arnaud L. Hydroxychloroquine in systemic and autoimmune diseases: where are we now? Joint Bone Spine 2021; 88: 105143.
38. Melles RB, Jorge AM, Marmor MF, et al. Hydroxychloroquine dose and risk for incident retinopathy: a cohort study. Ann Intern Med 2023; 176: 166-173.
39. Ugarte-Gil MF, Mak A, Leong J, et al. Impact of glucocorticoids on the incidence of lupus-related major organ damage: a systematic literature review and meta-regression analysis of longitudinal observational studies. Lupus Sci Med 2021; 8: e000590.
40. Mok CC. Calcineurin inhibitors in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2017; 31: 429-438.
41. Hahn BH. Belimumab for systemic lupus erythematosus. N Engl J Med 2013; 368: 1528-1535.
42. Furie R, Rovin BH, Houssiau F, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med 2020; 383: 1117-1128.
43. Morand EF, Furie RA, Bruce IN, et al. Efficacy of anifrolumab across organ domains in patients with moderate-to-severe systemic lupus erythematosus: a post-hoc analysis of pooled data from the TULIP-1 and TULIP-2 trials. Lancet Rheumatol 2023; 4: e282-e292.
44. Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010; 62: 222-233.
45. Ahluwalia J, Marsch A. Photosensitivity and photoprotection in patients with lupus erythematosus. Lupus 2019; 28: 697-702.
46. Speyer CB, Costenbader KH. Cigarette smoking and the pathogenesis of systemic lupus erythematosus. Expert Rev Clin Immunol 2018; 14: 481-487.
47. Blaess J, Goepfert T, Geneton S, et al. Benefits & risks of physical activity in patients with systemic lupus erythematosus: a systematic review of the literature. Semin Arthritis Rheum 2023; 58: 152128.
48. Zhang J, Wei W, Wang CM. Effects of psychological interventions for patients with systemic lupus erythematosus: a systematic review and meta-analysis. Lupus 2012; 21: 1077-1087.
49. Petri M. Pregnancy and systemic lupus erythematosus. Best Pract Res Clin Obstet Gynaecol 2020; 64: 24-30.
50. Drosos GC, Vedder D, Houben E, et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis 2022; 81: 768-779.
51. Jha SB, Rivera AP, Monar GVF, et al. Systemic lupus erythematosus and cardiovascular disease. Cureus 2022; 14: e22027.