Management of open angle glaucoma: medicines and laser

An awareness of a patient’s risk profile for glaucoma and the importance of early detection can help prevent vision loss that is irreversible once it occurs. Knowledge of the algorithms guiding glaucoma treatment options, the medications used and factors leading to nonadherence, as well as the increasing interest in laser therapy and lifestyle and nutrition interventions, can further aid disease management.
Glaucoma is a leading cause of preventable blindness worldwide and poses a growing concern due to the ageing population, as prevalence increases with age. Chronic open angle glaucoma, the most common subtype, earned the moniker ‘silent thief of sight’ for its asymptomatic nature until the very late stages of significant and irreversible visual field loss. Human peripheral vision is extensive (about 60 degrees nasally and superiorly, 95 degrees temporally and 75 degrees inferiorly) and essential for survival. Early visual loss requires perimetric detection. By the time open angle glaucoma becomes symptomatic, even with modern automated and sensitive visual field analysers, loss of visual field does not manifest until at least 30% of the retinal ganglion cells that comprise the optic nerve have been lost.1-3 The consequences of this visual loss include an increased risk of falls and motor vehicle accidents and reduced quality of life. Thus, early detection and ongoing monitoring are vital for timely intervention. GPs can help to ensure this takes place and that ongoing management is maintained as, although there is no cure, the ‘thief’ can be stopped from causing blindness. Furthermore, as diabetes and blood pressure control increase the risk of glaucoma, optimisation of general health may play a role in management.4,5
Pathogenesis
The pathogenesis of glaucoma is multifaceted and not completely understood, despite advances in its genetics and pathophysiology. Glaucoma is defined as characteristic visual field defects with matching optic nerve changes. The most common form of glaucoma is primary open angle glaucoma (POAG), typically associated with elevated intraocular pressure (IOP). Although high IOP is an important risk factor for POAG and contributes to optic nerve damage, it is not the condition’s defining feature. Indeed, glaucoma can occur at normal IOP levels (normal tension glaucoma [NTG]) and, conversely, elevated IOP may not always lead to optic nerve damage (ocular hypertension [OHT]). Research focuses on factors such as impaired optic nerve perfusion, ocular biomechanics and mitochondrial dysfunction in the disease process. Nonetheless, lowering IOP remains the primary modifiable risk factor and the only proven means to slow disease progression; thus, it is the focus of all current treatments.
With regard to glaucoma screening, GPs have an important role in identifying high-risk patients and facilitating formal optometric assessment, including automated visual field testing and optic nerve scanning, where available. Box 1 summarises the main risk factors for glaucoma.
Glaucoma management
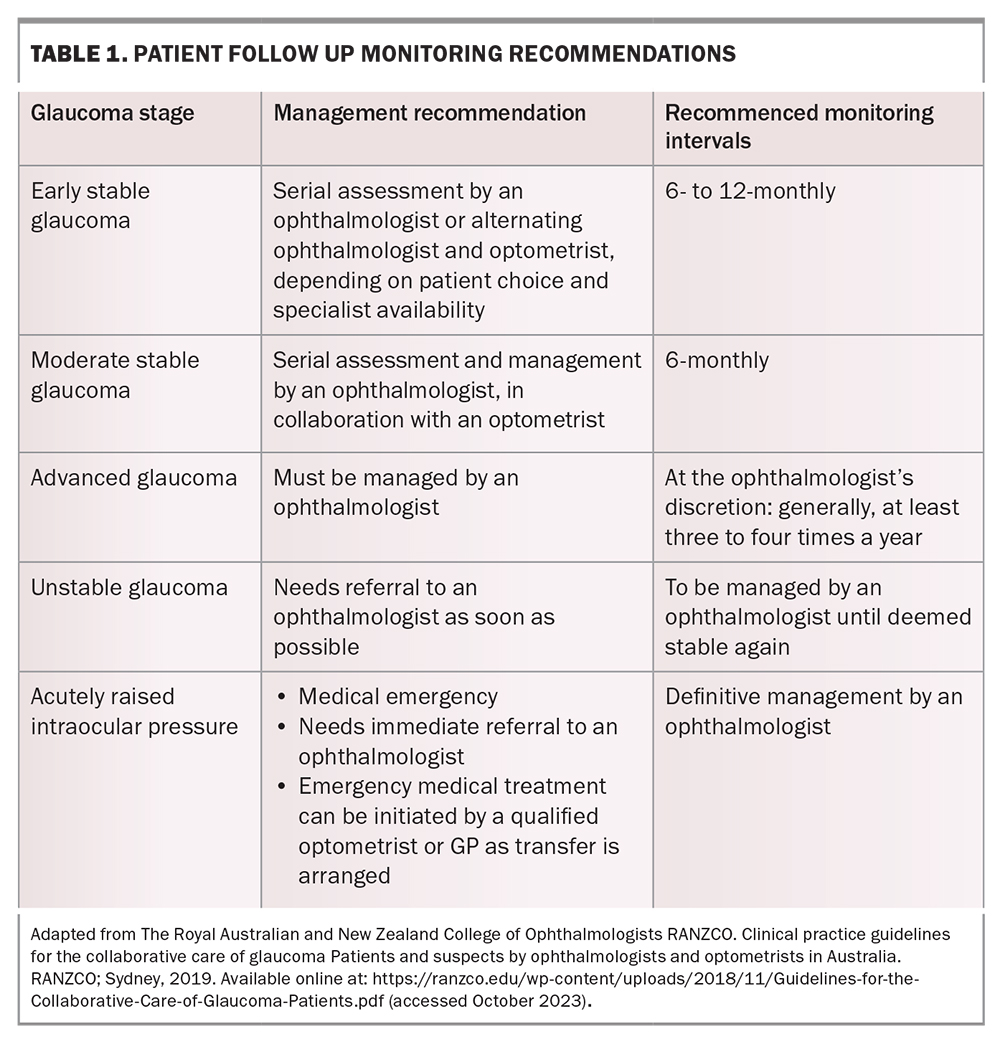
The goal of treatment is to prevent visual field loss that will impact a patient’s quality of life in their lifetime. Patients may have a ‘target IOP’ set – an estimated IOP upper limit at which progression of glaucoma is unlikely to occur. This target is based on the individual’s pretreatment IOP, the severity of their visual field defect and other risk factors, such as their corneal thickness, age and family history. Glaucoma can often progress despite seemingly satisfactory IOP. In such cases, the target pressure is lowered further, and treatment is escalated to achieve this. The prime criterion for whether therapy is adequate is when stability of the optic nerve structure and visual function have been achieved. Patients therefore need regular long-term monitoring with their ophthalmologist or optometrist (Table 1).
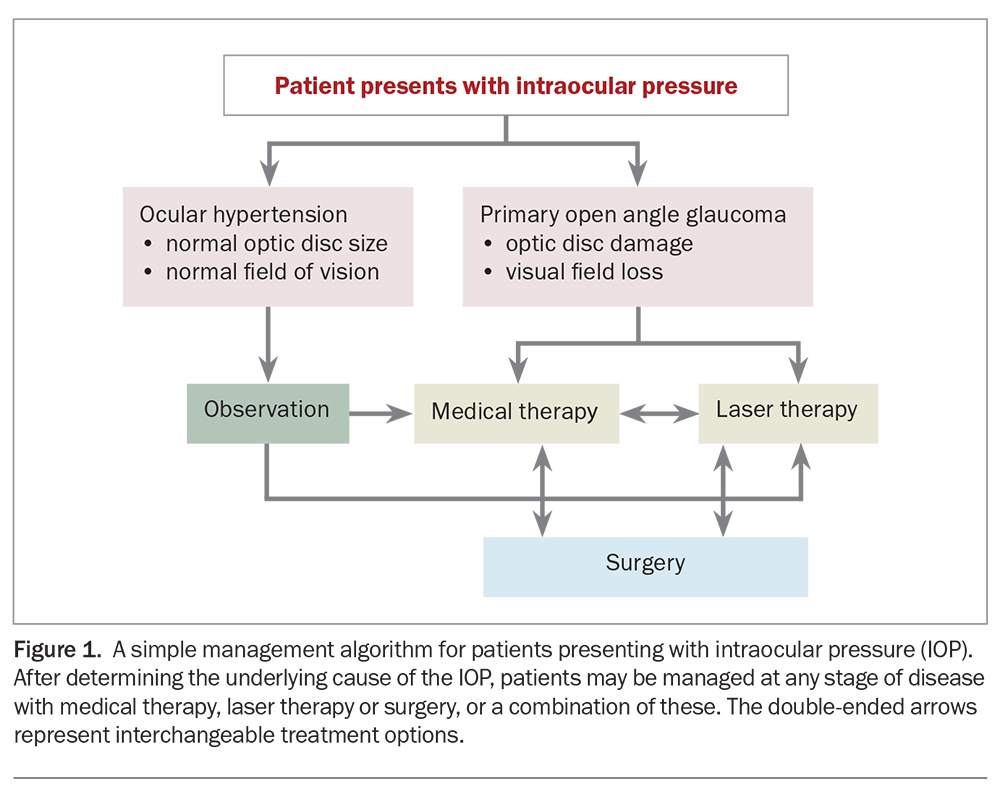
Glaucoma management has traditionally commenced with medical pharmacotherapy (eye drops), followed by laser therapy and, finally, surgical procedures. However, the patient’s treatment must be individualised for their disease severity and take into account other medical, as well as social, factors. Thus, any of these three management options can be initiated at any stage of the disease, and may often be done so in combination with each other (Figure 1).
Do lifestyle factors play a role in glaucoma management?
Several important lifestyle factors can impact disease progression. Treatment compliance is the most important of these.
Certain hobbies can increase the risk of disease progression. Activities that result in a prolonged valsalva manoeuvre, strain or increased venous return to the head can lead to IOP spikes, which if sustained, may impact a patient’s glaucoma. Examples include inversions during yoga, wearing tight goggles when swimming, playing brass or wind instruments, glass blowing and weightlifting. Systemic diseases, such as diabetes, hypertension and hypotension, are also risk factors for glaucoma development.
Diet and nutrition may affect the risk of glaucoma. Although there is no scientific evidence that any specific diet has a beneficial effect on glaucoma, randomised controlled trials (RCTs) are underway to investigate whether nutritional supplementation with oral nicotinamide (vitamin B3) offers neuroprotection against glaucoma progression.6 Nonvalidated research suggests that deficiencies in vitamin B12, folate and vitamin D may be associated with NTG and its progression. Patients with NTG also often present as a particular demographic that includes having a low BMI, being vigilantly health conscious, and more likely to be taking many supplements and consuming a low-salt diet. In these patients, achieving a healthy BMI, avoiding dehydration and consuming an adequate amount of salt is recommended to avoid hypotension and low cerebrospinal fluid pressure that may worsen glaucoma.
Early data suggest that stress management and relaxation techniques, including meditation, may have a complementary role in glaucoma management.7 An RCT showed that mindfulness-based stress reduction by meditation reduced IOP, positively affected stress biomarkers and improved quality of life of patients with POAG.7
Medical management
Topical therapy
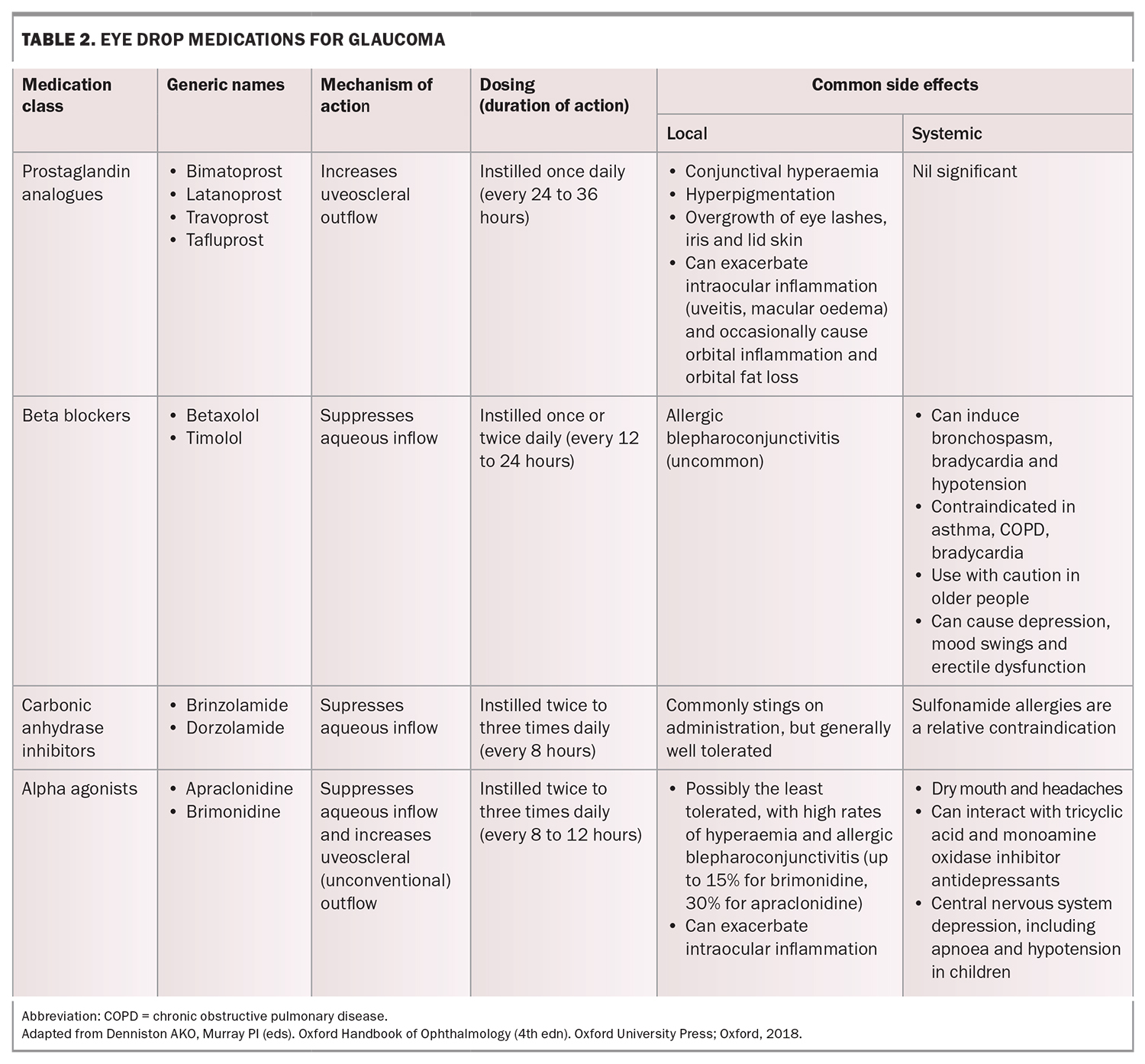
Topical pharmacological therapies for glaucoma, namely eye drops, reduce IOP by increasing the outflow of aqueous humour, decreasing aqueous humour production, or both. The four main classes of glaucoma eye drops used today are prostaglandin analogues, beta blockers, carbonic anhydrase inhibitors and alpha agonists. Their doses, mechanisms of action and common side effects are summarised in Table 2.
Prostaglandin analogues are generally considered first-line treatment because of their excellent efficacy and once-a-day dosing (usually nightly). Miotics such as pilocarpine are no longer in common use for open angle glaucoma because of their poor side effect profile. If a second agent is required, several options exist for combination formulations that optimise convenience and adherence for patients (e.g. latanoprost/timolol, bimatoprost/timolol, brinzolamide/timolol, dorzolamide/timolol, brimonidine/timolol and brinzolamide/brimonidine).
Poor treatment adherence
Strict adherence with eye drop use is critical to achieving glaucoma stability. Poor adherence is poses a significant challenges to glaucoma management, as patients do not have symptoms from the disease and drops may be poorly tolerated. Studies show nonadherence with glaucoma treatment to be as high as 60%.8 Patients tend to use their drops more reliably in the lead up to an appointment, resulting in a falsely low IOP reading that does not reflect their usual control level. A recent study explored factors affecting glaucoma eye drop adherence in 145 patients in Australia through a questionnaire. Lower adherence rates were correlated with:9
- difficulty in applying drops
- a history of depression
- poor memory
- low self-reported motivation rating.
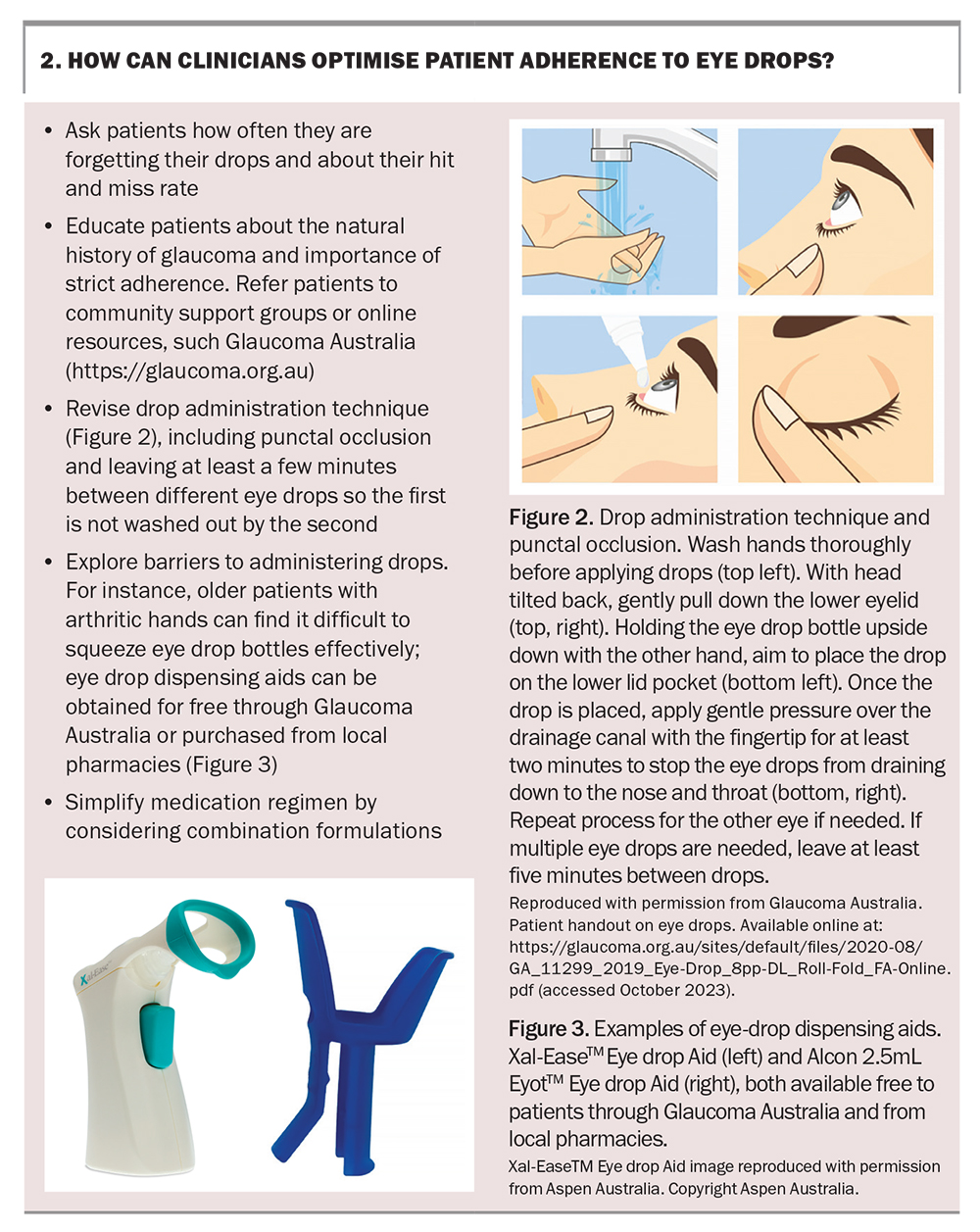
Recognising these risk factors, especially a history of mental health problems, can help identify and overcome compliance barriers. Encouraging adherence is one of the important ways GPs can contribute to the care of patients with glaucoma. Key points on how GPs can help optimise compliance are summarised in Box 2.
Eye drop intolerance
Eye drops can be poorly tolerated, leading to the exacerbation of dry eye symptoms and causing irritation, redness and, occasionally, a more severe follicular conjunctivitis. This can be a result of intolerance to the medications themselves, as well as the preservatives contained in eye drop formulations. Benzalkonium chloride is the most commonly used preservative and has toxic effects to the ocular surface.
For mild adverse reactions, conservative measures are recommended, such as switching to preservative-free formulations, using regular lubricant artificial tears and treating any coexisting blepharitis. If the reaction is severe, identifying and stopping the culprit drop is essential; alternative drops can be trialled and selective laser trabeculoplasty (SLT) should be considered instead.
Although it can be tempting to consider a course of weak topical corticosteroids to treat the inflammatory component of dry eye, these should be used with caution in patients with glaucoma and only under the guidance of an ophthalmologist because of the high prevalence of corticosteroid-induced IOP spikes (known as a steroid response) that occur in one of 10 patients, which can be detrimental to glaucoma progression.
Glaucoma can be associated with dry eye syndrome, and aggressive treatment may improve both IOP control and quality of life. Treatment may include the use of topical immunomodulatory agents, such as ciclosporin or lifitegrast, and treatment options, such as trying to reduce reliance on topical treatment by the use of SLT.
Oral therapy
Acetazolamide is an oral agent that is extremely effective at lowering IOP but is poorly tolerated and can cause paraesthesia in the fingers, toes and around the mouth, gastrointestinal upset, altered taste, depression and malaise. It is not suitable for long-term use as it can lead to hypokalaemia, metabolic acidosis and renal impairment. Its use is, therefore, usually reserved for temporising situations, for example, in acute glaucoma or while awaiting surgical intervention. Kidney function and electrolyte levels should be monitored during use.
On the horizon
The following agents show promise in the management of glaucoma.
Nicotinamide (vitamin B3) – has been shown to reduce glaucomatous and metabolic damage in animal models.10,11 Promising findings in human studies support a potential neuroprotective effect, independent of IOP lowering.12,13 Several major international RCTs are underway to determine whether its use slows glaucoma progression.14-16
Implantable depot prostaglandin – a number of innovative sustained delivery platforms for glaucoma medications are under development. The bimatoprost sustained-release implant is a biodegradable, polymer-based delivery system that slowly releases the medication into the aqueous humour over a period of four to six months, with promising results shown in phase 3 studies.17,18
Rho kinase inhibitors – these are a fifth class of eye drops for pressure-lowering that increase aqueous outflow via the trabecular meshwork. Introduced in Japan and gaining FDA approval in 2017, they have been recently rolled out in Europe and the UK; however, they are not yet PBS listed in Australia.
Selective laser trabeculoplasty
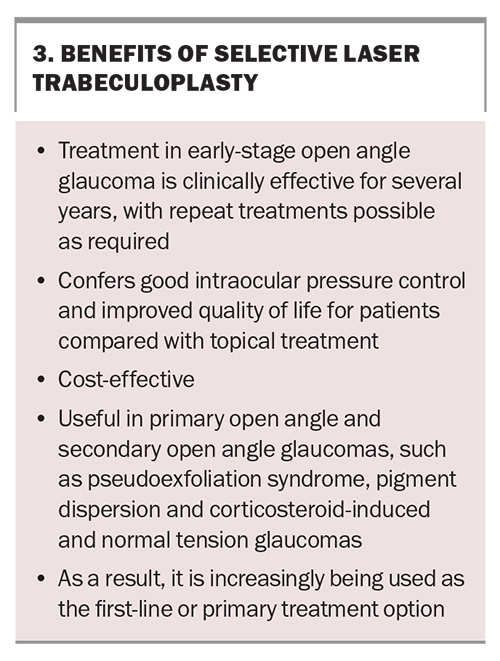
Laser trabeculoplasty is an alternative to topical pharmacotherapy. SLT, currently the treatment of choice for open angle glaucoma, was endorsed by the FDA in 2001 and has since been increasingly adopted as an alternative to eye drop use. It can be utilised as first-line therapy in eye drop-naïve patients, as additional therapy to existing drops when further IOP reduction is required or as a replacement for eye drops when intolerance is an issue.
The procedure
SLT is a safe, well-tolerated treatment performed in the clinic setting under topical anaesthesia. Patients may be pretreated with pilocarpine 2% eye drops if their angles are assessed as narrow for optimised visualisation. The laser is focused through a mirrored lens onto the trabecular meshwork: the drainage area in the angle between the cornea and iris. SLT uses a 532 nm frequency, double q-switched Nd:Yag laser with a 400 micrometer beam. Compared with its predecessor argon laser trabeculoplasty (ALT), SLT minimises collateral tissue damage through an ultrashort pulse duration of three nanoseconds, an order of magnitude that is 109 times lower than that of ALT. Thus, the laser is only absorbed by the pigmented meshwork cells and spares the adjacent nonpigmented tissue.
The procedure takes less than five minutes to perform and is well tolerated, with the main side effects being mild redness, discomfort and glare that resolves within a few days. Mild topical anti-inflammatory eye drops may be prescribed for up to a week after the procedure. Patients may be asked to stay for 30 minutes after the procedure for an IOP check; however, there are no post-procedure restrictions.
Several studies have investigated whether 90, 180 or 360 degrees of the angle need to be treated for optimal pressure reduction. Success rates were 34% in 90 degree-treated patients, 65% in 180 degree-treated patients and 82% in 360 degree-treated patients, suggesting that the whole angle should be treated for optimal success.19
Efficacy of SLT
The Laser in Glaucoma and Ocular Hypertension (LiGHT) trial was the first multicentre RCT to show that SLT is both clinically effective and cost saving compared with medication therapy in POAG.20 Since then, at least eight RCTs have been performed, and a meta-analysis of 35 studies concluded that SLT results in an average IOP reduction of 20 to 30% for up to five years.21-23 A recent 10-year follow-up study confirmed that a pressure reduction of more than 20% or an IOP below 19 mmHg was still achieved in 72% of patients at 10 years.24
Several studies have confirmed the use of SLT in secondary open angle glaucomas. Treatment of pseudo-exfoliation glaucoma, the most common form accounting for 25% of all open angle glaucomas worldwide, with SLT equally as successful as POAG. Other secondary open angle glaucomas, such as pigment dispersion or steroid-induced glaucoma, have equivalent pressure-lowering success with SLT but an increased risk of inflammation, discomfort or pressure spikes and, thus, patients may need treatment over two sessions. SLT is usually avoided in patients with uveitic glaucoma due to the higher risk of inflammation. In addition to an overall pressure reduction, SLT can reduce IOP fluctuations to below 3 mmHg, an important mechanism of disease progression, especially in NTG.25
The IOP-lowering effect of SLT does wear off over time, with a wide variability in the range of effect duration ranging from a few months to several years. In the authors’ experience, most patients will have sustained pressure reduction for three to four years, although there are some reports of success from a single treatment for over eight years. With a sound safety profile, SLT is repeatable, with equal efficacy extending the duration of pressure control that can be achieved with the laser.
One of the major advantages of SLT is the benefit to the patient’s quality of life through the reduction of ocular surface disease, the lack of need for daily adherence and a reduced need for surgical intervention for up to six years. Other benefits are listed in Box 3.
Conclusion
Helping patients maintain functional vision throughout their lives is one of the most powerful healthcare interventions that GPs help manage. Glaucoma vision loss is silent, gradual and irreversible. Early detection is therefore crucial and relies on awareness of patient risk factors, screening tools and recommended surveillance. Management has traditionally relied on topical IOP-lowering pharmacological therapies; however, because of high rates of nonadherence, SLT therapy is increasingly utilised. As with other ophthalmic diseases, holistic approaches to management, such as a focus on lifestyle and nutrition, may play a role in ensuring optimal vision in glaucoma. Practice points for GPs on glaucoma management are summarised in Box 4. MT
COMPETING INTERESTS: None.
References
1. Broadway DC. Visual field testing for glaucoma - a practical guide. Community Eye Health 2012; 25: 66-70.
2. Montana CL, Bhorade AM. Glaucoma and quality of life: fall and driving risk. Curr Opin Ophthalmol 2018; 29: 135-140.
3. Zhao YX, Chen XW. Diabetes and risk of glaucoma: systematic review and a meta-analysis of prospective cohort studies. Int J Ophthalmol 2017; 10: 1430-1435.
4. Leeman M, Kestelyn P. Glaucoma and blood pressure. Hypertension 2019; 73: 944-950.
5. Nijm LM, De Benito-Llopis L, Rossi GC, Vajaranant TS, Coroneo MT. Understanding the dual dilemma of dry eye and glaucoma: an international review. Asia Pac J Ophthalmol (Phila. 2020; 9: 481-490.
6. Hui F, Tang J, Williams PA, et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: a crossover randomized clinical trial. Clin Exp Ophthalmol 2020; 48: 903-914.
7. Dada T, Mittal D, Mohanty K, et al. Mindfulness meditation reduces intraocular pressure, lowers stress biomarkers and modulates gene expression in glaucoma: a randomized controlled trial. J Glaucoma 2018; 27: 1061-1067.
8. Guven S, Koylu MT, Mumcuoglu T. Adherence to glaucoma medication, illness perceptions, and beliefs about glaucoma: Attitudinal perspectives among Turkish population. Eur J Ophthalmol 2021; 31: 469-476.
9. Spencer SKR, Shulruf B, McPherson ZE, et al. Factors affecting adherence to topical glaucoma therapy: a quantitative and qualitative pilot study analysis in Sydney, Australia. Ophthalmol Glaucoma 2019; 2(2): 86-93.
10. Williams PA, Harder JM, John SWM. Glaucoma as a metabolic optic neuropathy: making the case for nicotinamide treatment in glaucoma. J Glaucoma 2017; 26: 1161-1168.
11. Tribble JR, Otmani A, Sun S, et al. Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol 2021; 43: 101988.
12. Hui F, Tang J, Williams PA, et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: a crossover randomized clinical trial. Clin Exp Ophthalmol 2020; 48: 903-914.
13. De Moraes CG, John SWM, Williams PA, et al. Nicotinamide and pyruvate for neuroenhancement in open-angle glaucoma: a phase 2 randomized clinical trial. JAMA Ophthalmol 2022; 140: 11-18.
14. Leung CK, Ren ST, Chan PP, et al. Nicotinamide riboside as a neuroprotective therapy for glaucoma: study protocol for a randomized, double-blind, placebo-control trial. Trials 2022; 23: 45.
15. Bhartiya S. Niacinamide and neuroprotection: the glaucoma holy grail. J Curr Glaucoma Pract 2022; 16:141-143.
16. Goulart Nacácio e Silva S, Occhiutto ML, Costa VP. The use of nicotinamide and nicotinamide riboside as an adjunct therapy in the treatment of glaucoma. Eur J Ophthalmol 2023; 33: 1801-1815.
17. Craven ER, Walters TR, Christie WC et al. Phase 3 evaluation of bimatoprost sustained-release implant in patients with glaucoma or OHT: results at primary database lock. Paper presented at 2019 Annual Meeting of the American Academy of Ophthalmology 12-15 2019; San Francisco CA. Abstract PA054.
18. Medeiros FA, Walters TR, Kolko M, et al. Phase 3, randomised, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology 2020; 127: 1627-1641.
19. Nagar M, Ogunyomade A, O’Brart DP, Howes F, Marshall J. A randomised, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. Br J Ophthalmol 2005; 89: 1413-1417.
20. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet 2019; 393(10180): 1505e1516.
21. Gracner T, Falez M, Gracner B, Pahor D. [Long-term follow-up of selective laser trabeculoplasty in primary open-angle glaucoma]. Klin Monbl Augenheilkd 2006; 223: 743-747. [Article in German].
22. Katz LJ, Steinmann WC, Kabir A, et al. Selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma 2012; 21: 460-468.
23. Wong MO, Lee JW, Choy BN, Chan JC, Lai JS. Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in open-angle glaucoma. Surv Ophthalmol 2015; 60: 36-50.
24. Ansari E. 10-year outcomes of first-line selective laser trabeculoplasty (SLT) for primary open-angle glaucoma (POAG). Graefes Arch Clin Exp Ophthalmol 2021; 259: 1597-1604.
25. Prasad N, Murthy S, Dagianis JJ, Latina MA. A comparison of the intervisit intraocular pressure fluctuation after 180 and 360 degrees of selective laser trabeculoplasty (SLT) as a primary therapy in primary open angle glaucoma and ocular hypertension. J Glaucoma 2009; 18: 157-160.